Abstract
We had found previously that children with ragweed hay fever were somewhat less symptomatic after preseasonal immunization with large doses of ragweed pollen extract than were placebo-treated children. To study further the immunologic changes which accompany immunotherapy, these children were treated again the following year. Each patient served as his own control.
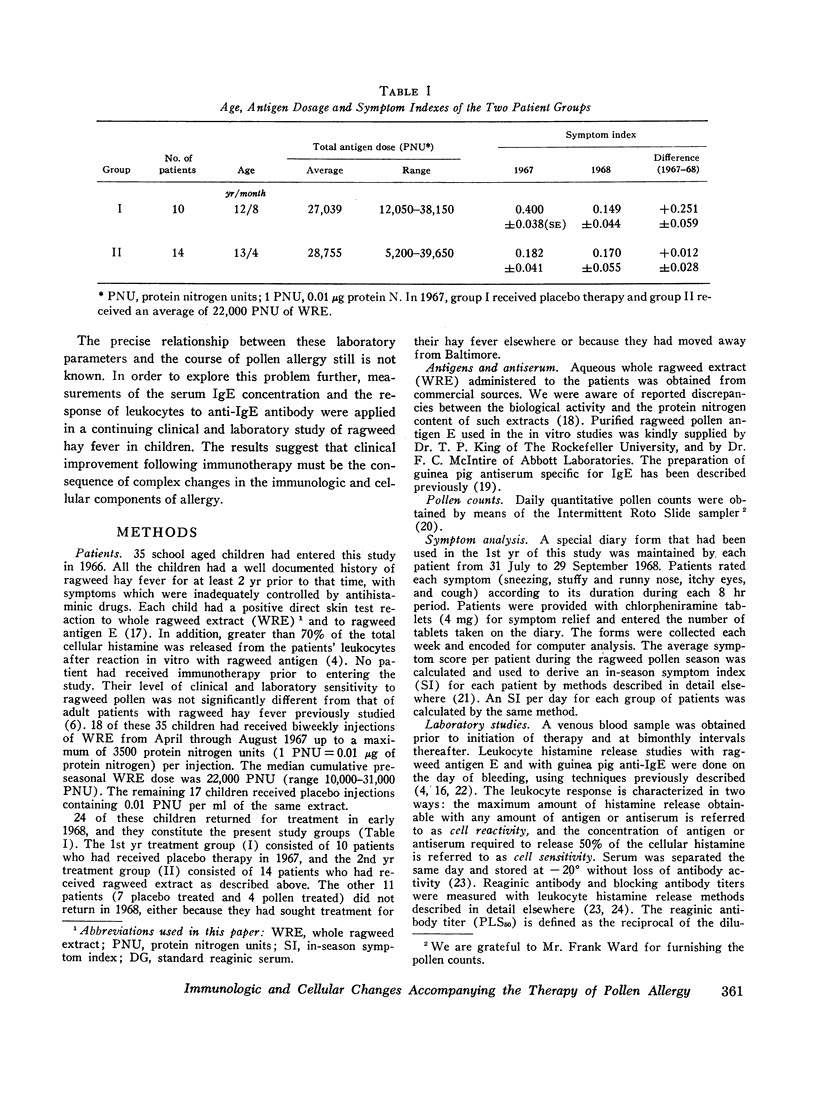
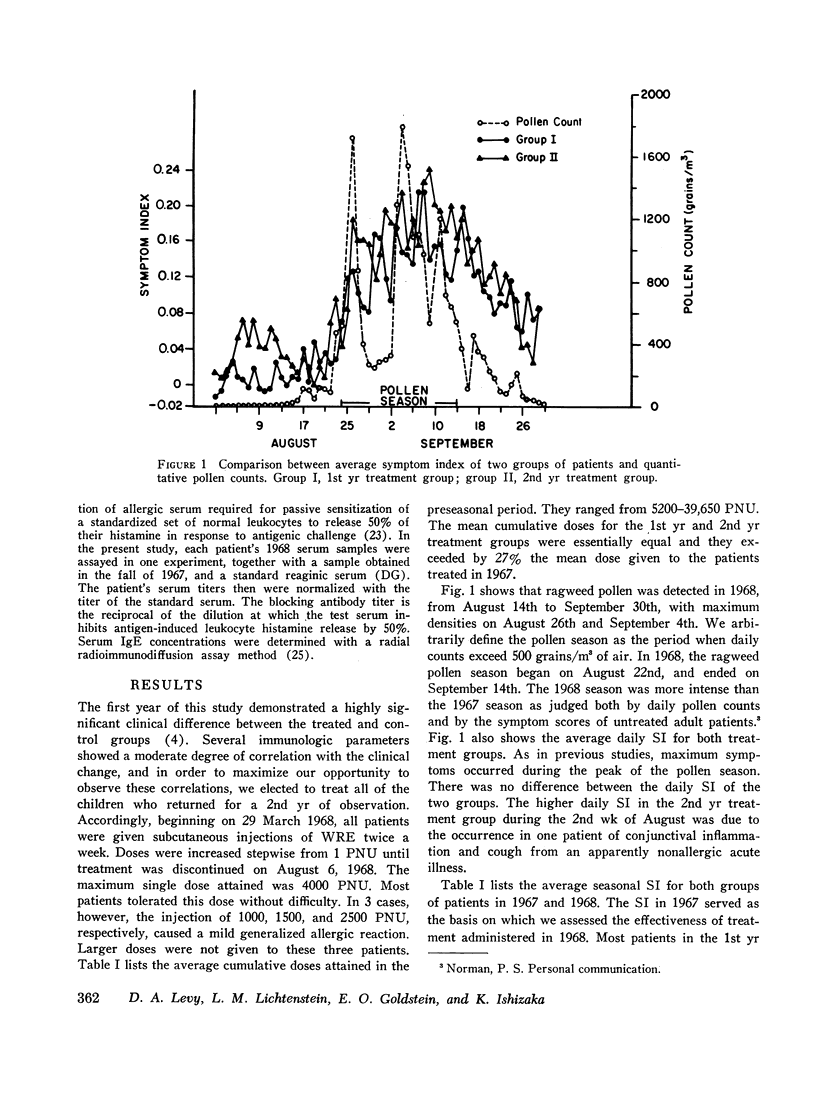
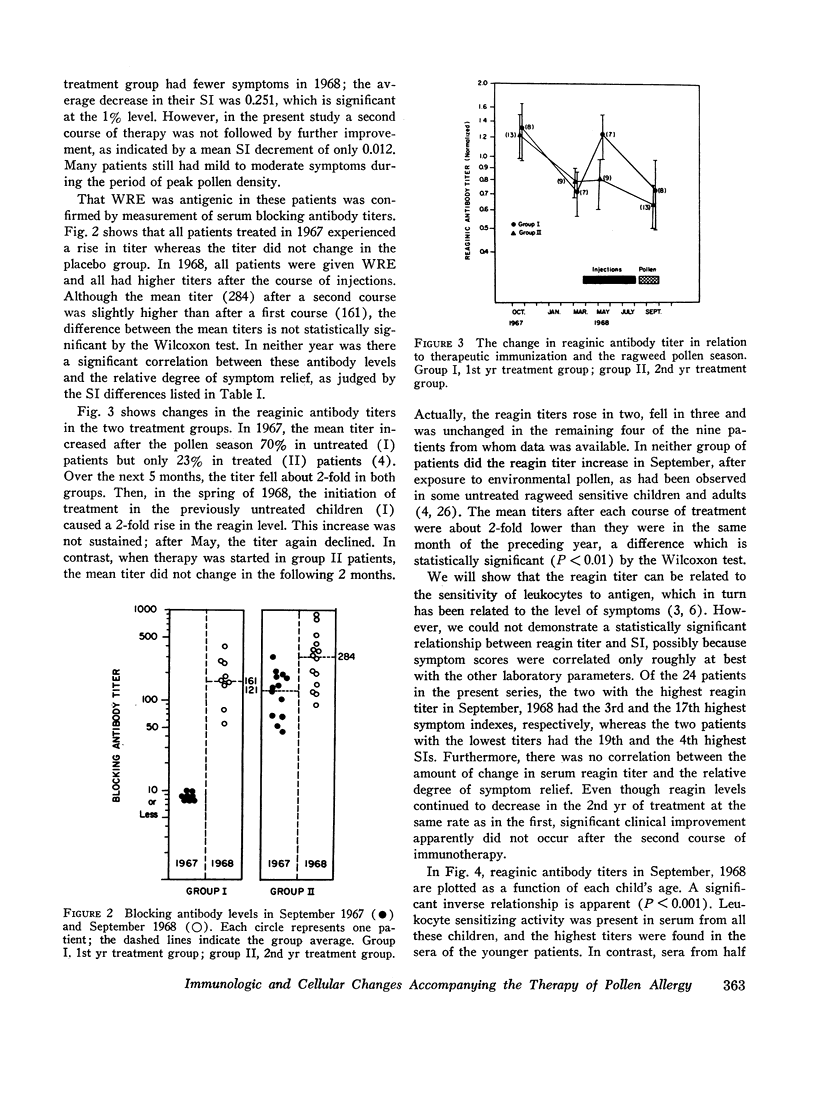
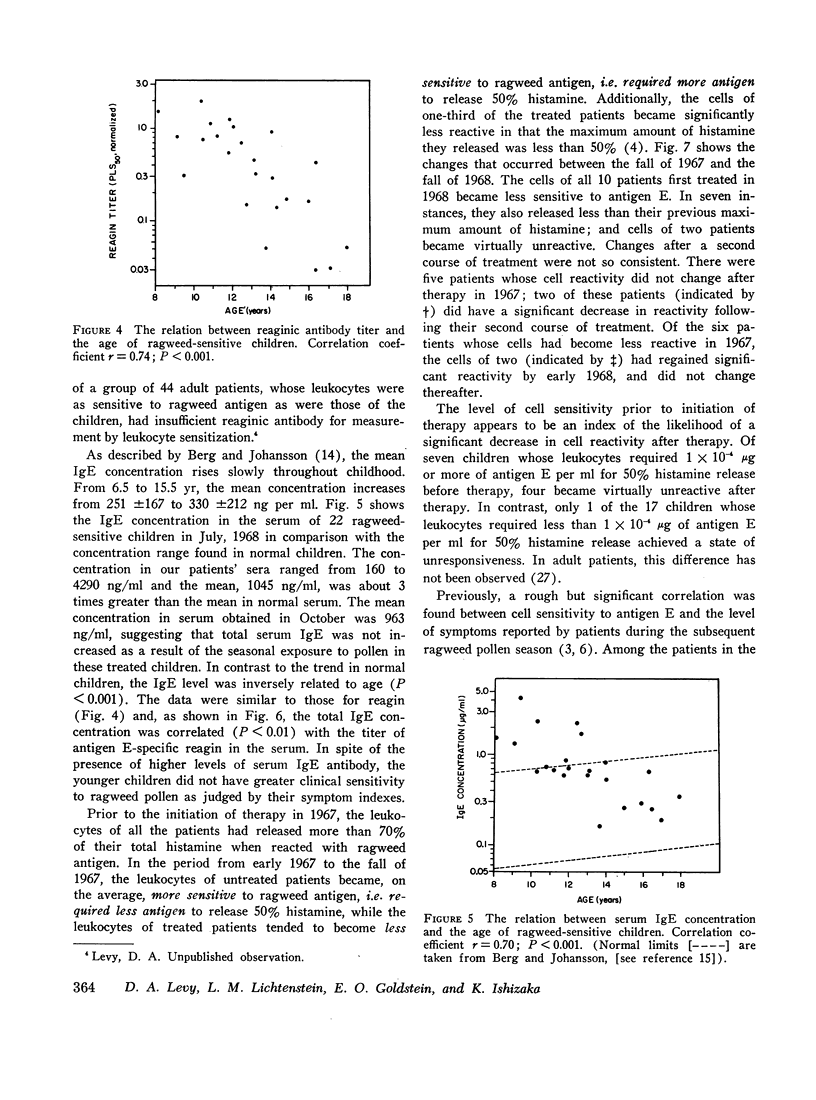
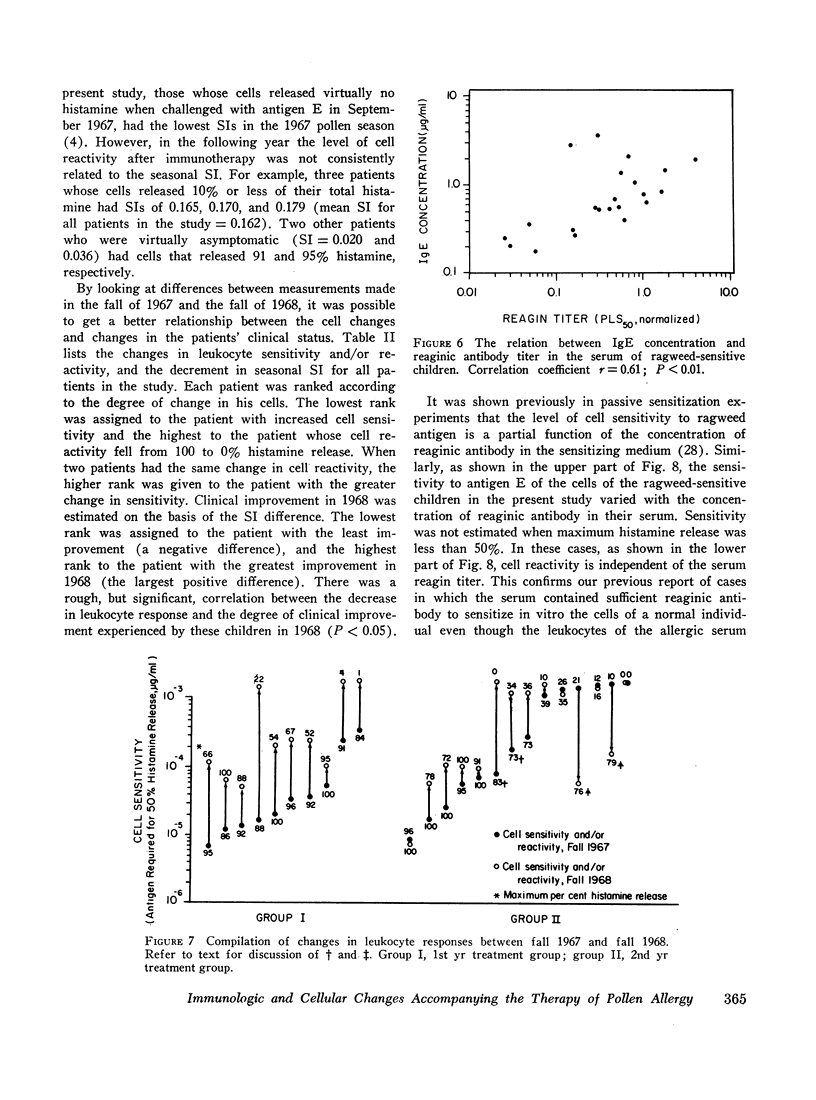
Serum blocking (IgG) antibody, measured by inhibition of antigen-induced leukocyte histamine release, was increased 20- to 40-fold after therapy. The anticipated postpollen season increase of serum reaginic (IgE) antibody, measured by passive sensitization of leukocytes from nonallergic donors, was suppressed. Instead, the mean titer was decreased after treatment. Total serum IgE levels, measured by radial radioimmunodiffusion assay, were higher than normal; were correlated with reaginic antibody titers; and also did not increase in the pollen season after treatment. The concentration of both IgE and reaginic antibody was lower in the older children, irrespective of treatment.
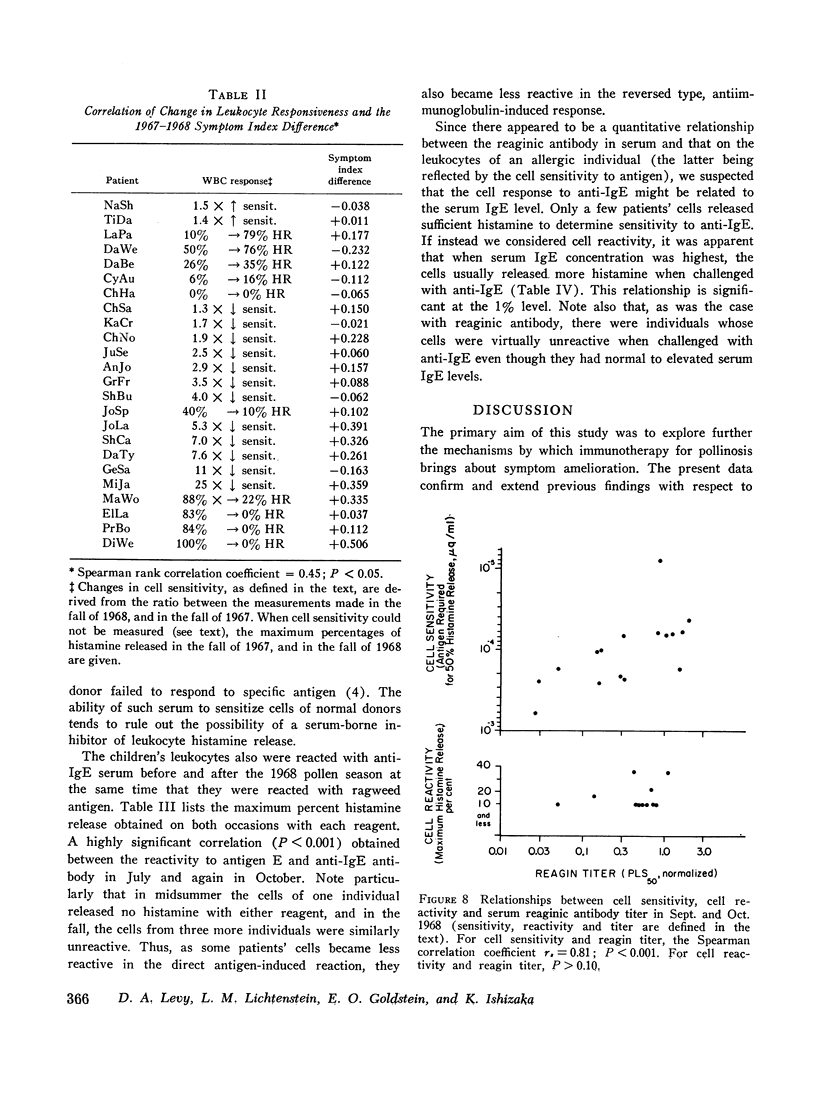
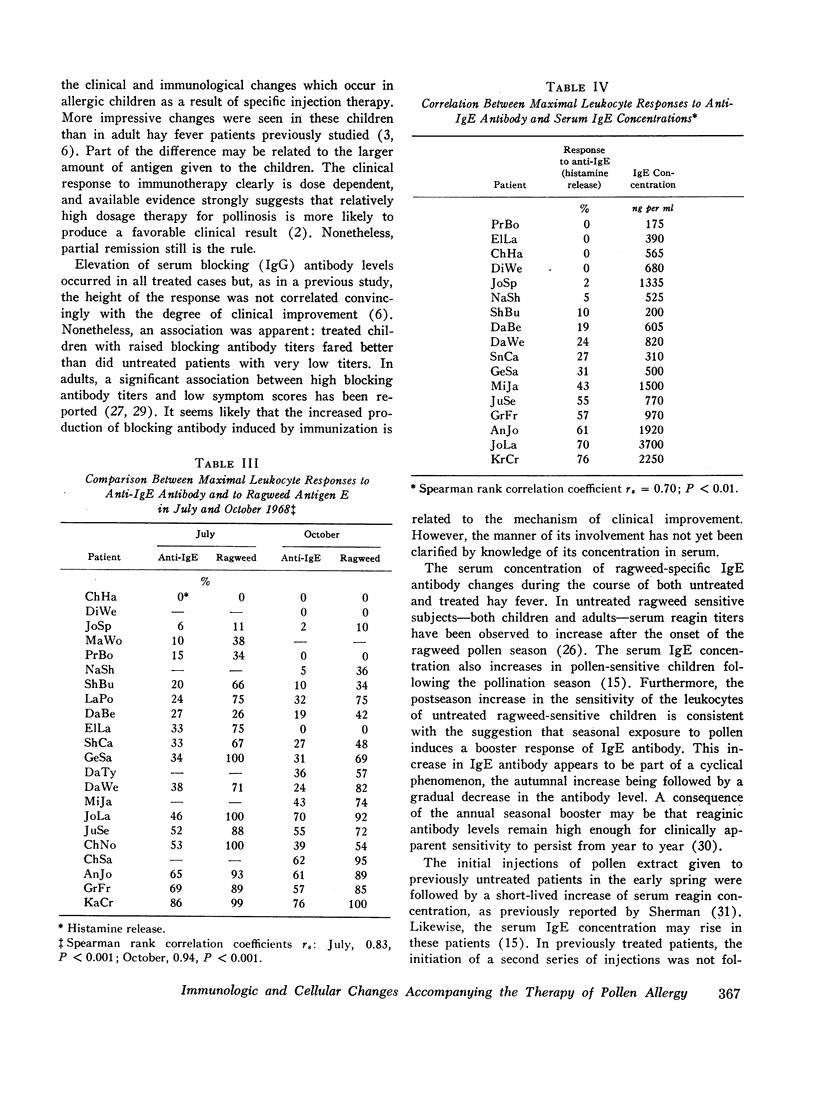
Leukocyte response to ragweed antigen E and guinea pig anti-IgE antiserum was assessed by means of in vitro histamine release techniques. After treatment, the leukocytes of 21 patients were less sensitive (11 cases), or less reactive (10 cases), to antigen E. Response to anti-IgE antibody also was diminished after treatment. In four cases, neither anti-IgE nor antigen E induced histamine release, although both IgE protein and ragweed-specific IgE antibody were present in the patients' own sera.
Clinical improvement was correlated best with decreased leukocyte sensitivity and leukocyte reactivity to ragweed antigen E. It appeared that decreased cell sensitivity was related to lower serum reaginic antibody levels. Decreased cell reactivity, in the presence of both IgE protein and IgE antibody in the serum, may indicate a change in cellular response mechanisms. These studies suggest that clinical improvement following specific immunotherapy must be the result of complex changes in the immunologic and cellular components of allergic disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer H., Godfrey H., Maloney C. J., Norman P. S., Lichtenstein L. M. The potency and antigen E content of commercially prepared ragweed extracts. J Allergy. 1970 Jun;45(6):347–354. doi: 10.1016/0021-8707(70)90043-2. [DOI] [PubMed] [Google Scholar]
- Berg T., Johansson S. G. IgE concentrations in children with atopic diseases. A clinical study. Int Arch Allergy Appl Immunol. 1969;36(3):219–232. doi: 10.1159/000230745. [DOI] [PubMed] [Google Scholar]
- Berg T., Johansson S. G. Immunoglobulin levels during childhood, with special regard to IgE. Acta Paediatr Scand. 1969 Sep;58(5):513–524. doi: 10.1111/j.1651-2227.1969.tb04753.x. [DOI] [PubMed] [Google Scholar]
- CONNELL J. T., SHERMAN W. B. SKIN-SENSITIZING ANTIBODY TITER. III. RELATIONSHIP OF THE SKIN-SENSITIZING ANTIBODY TITER TO THE INTRACUTANEOUS SKIN TEST, TO THE TOLERANCE OF INJECTIONS OF ANTIGENS, AND TO THE EFFECTS OF PROLONGED TREATMENT WITH ANTIGEN. J Allergy. 1964 Mar-Apr;35:169–176. doi: 10.1016/0021-8707(64)90031-0. [DOI] [PubMed] [Google Scholar]
- Connell J. T., Sherman W. B. Changes in skin-sensitizing antibody titer after injections of aqueous pollen extract. J Allergy. 1969 Jan;43(1):22–32. doi: 10.1016/0021-8707(69)90017-3. [DOI] [PubMed] [Google Scholar]
- Connell J. T., Sherman W. B. Hay fever symptoms related to immunological findings. Ann Allergy. 1967 May;25(5):239–250. [PubMed] [Google Scholar]
- Feldmann M., Diener E. Antibody-mediated suppression of the immune response in vitro. I. Evidence for a central effect. J Exp Med. 1970 Feb;131(2):247–274. doi: 10.1084/jem.131.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fireman P., Boesman M., Gitlin D. Disappearance of intradermally administered plasma immunoglobulins and skin-sensitizing antibodies. J Allergy. 1967 Nov;40(5):304–316. doi: 10.1016/0021-8707(67)90078-0. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Human reaginic antibodies and immunoglobulin E. J Allergy. 1968 Dec;42(6):330–363. doi: 10.1016/0021-8707(68)90095-6. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Reversed type allergic skin reactions by anti-gamma-E-globulin antibodies in humans and monkeys. J Immunol. 1968 Mar;100(3):554–562. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Johansson S. G., Bennich H. Histamine release from human leukocytes by anti-gamma E antibodies. J Immunol. 1969 Apr;102(4):884–892. [PubMed] [Google Scholar]
- Johansson S. G. Raised levels of a new immunoglobulin class (IgND) in asthma. Lancet. 1967 Nov 4;2(7523):951–953. doi: 10.1016/s0140-6736(67)90792-1. [DOI] [PubMed] [Google Scholar]
- KING T. P., NORMAN P. S. Isolation studies of allergens from regweed pollen. Biochemistry. 1962 Jul;1:709–720. doi: 10.1021/bi00910a027. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIN L. M., OSLER A. G. STUDIES ON THE MECHANISMS OF HYPERSENSITIVITY PHENOMENA. IX. HISTAMINE RELEASE FROM HUMAN LEUKOCYTES BY RAGWEED POLLEN ANTIGEN. J Exp Med. 1964 Oct 1;120:507–530. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. A., Osler A. G. Studies on the mechanisms of hypersensitivity phenomena. XIV. Passive sensitization in vitro of human leukocytes to ragweed pollen antigen. J Immunol. 1966 Aug;97(2):203–212. [PubMed] [Google Scholar]
- Levy D. A., Osler A. G. Studies on the mechanisms of hypersensitivity phenomena. XVI. In vitro assays of reaginic activity in human sera: effect of therapeutic immunization on seasonal titer changes. J Immunol. 1967 Dec;99(6):1068–1077. [PubMed] [Google Scholar]
- Lichtenstein L. M., Levy D. A., Ishizaka K. In vitro reversed anaphylaxis: characteristics of anti-IgE mediated histamine release. Immunology. 1970 Nov;19(5):831–842. [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Norman P. S., Winkenwerder W. L., Osler A. G. In vitro studies of human ragweed allergy: changes in cellular and humoral activity associated with specific desensitization. J Clin Invest. 1966 Jul;45(7):1126–1136. doi: 10.1172/JCI105419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Osler A. G. Studies on the mechanisms of hypersensitivity phenomena. XII. An in vitro study of the reaction between ragweed pollen antigen, allergic human serum and ragweed-sensitive human leukocytes. J Immunol. 1966 Jan;96(1):169–179. [PubMed] [Google Scholar]
- Lowell F. C., Franklin W. A double-blind study of the effectiveness and specificity of injecton therapy in ragweed hay fever. N Engl J Med. 1965 Sep 23;273(13):675–679. doi: 10.1056/NEJM196509232731302. [DOI] [PubMed] [Google Scholar]
- Norman P. S. A rational approach to desensitization. J Allergy. 1969 Sep;44(3):129–145. doi: 10.1016/0021-8707(69)90137-3. [DOI] [PubMed] [Google Scholar]
- Norman P. S., Winkenwerder W. L., Lichtenstein L. M. Immunotherapy of hay fever with ragweed antigen E: comparisons with whole pollen extract and placebos. J Allergy. 1968 Aug;42(2):93–108. doi: 10.1016/0021-8707(68)90139-1. [DOI] [PubMed] [Google Scholar]
- OSLER A. G., LICHTENSTEIN L. M., LEVY D. A. IMMUNOLOGIC ASPECTS OF HUMAN REAGINIC ALLERGY: AN IN VITRO METHOD AND SOME APPLICATIONS. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1965 Feb 16;250:111–124. doi: 10.1007/BF00258551. [DOI] [PubMed] [Google Scholar]
- Ogden E. C., Raynor G. S. A new sampler for airborne pollen: the rotoslide. J Allergy. 1967 Jul;40(1):1–11. doi: 10.1016/0021-8707(67)90053-6. [DOI] [PubMed] [Google Scholar]
- Pruzansky J. J., Patterson R. Immunologic changes during hyposensitization therapy. JAMA. 1968 Feb 26;203(9):805–806. [PubMed] [Google Scholar]
- Sadan N., Rhyne M. B., Mellits E. D., Goldstein E. O., Levy D. A., Lichtenstein L. M. Immunotherapy of pollinosis in children: investigation of the immunologic basis of clinical improvement. N Engl J Med. 1969 Mar 20;280(12):623–627. doi: 10.1056/NEJM196903202801201. [DOI] [PubMed] [Google Scholar]
- Starr M. S., Weinstock M. Studies in pollen allergy. 3. The relationship between blocking antibody levels and symptomatic relief following hyposensitisation with allpyral in hay fever subjects. Int Arch Allergy Appl Immunol. 1970;38(5):514–521. [PubMed] [Google Scholar]
- Tada T., Ishizaka K. Distribution of gamma E-forming cells in lymphoid tissues of the human and monkey. J Immunol. 1970 Feb;104(2):377–387. [PubMed] [Google Scholar]
- VANARSDEL P. P., Jr, MIDDLETON E., Jr The effect of hyposensitization on the in vitro histamine release by specific antigen. J Allergy. 1961 Jul-Aug;32:348–356. doi: 10.1016/0021-8707(61)90103-4. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A. Disorders of immunoglobulin metabolism. N Engl J Med. 1969 Nov 20;281(21):1170–1177. doi: 10.1056/NEJM196911202812107. [DOI] [PubMed] [Google Scholar]


