Abstract
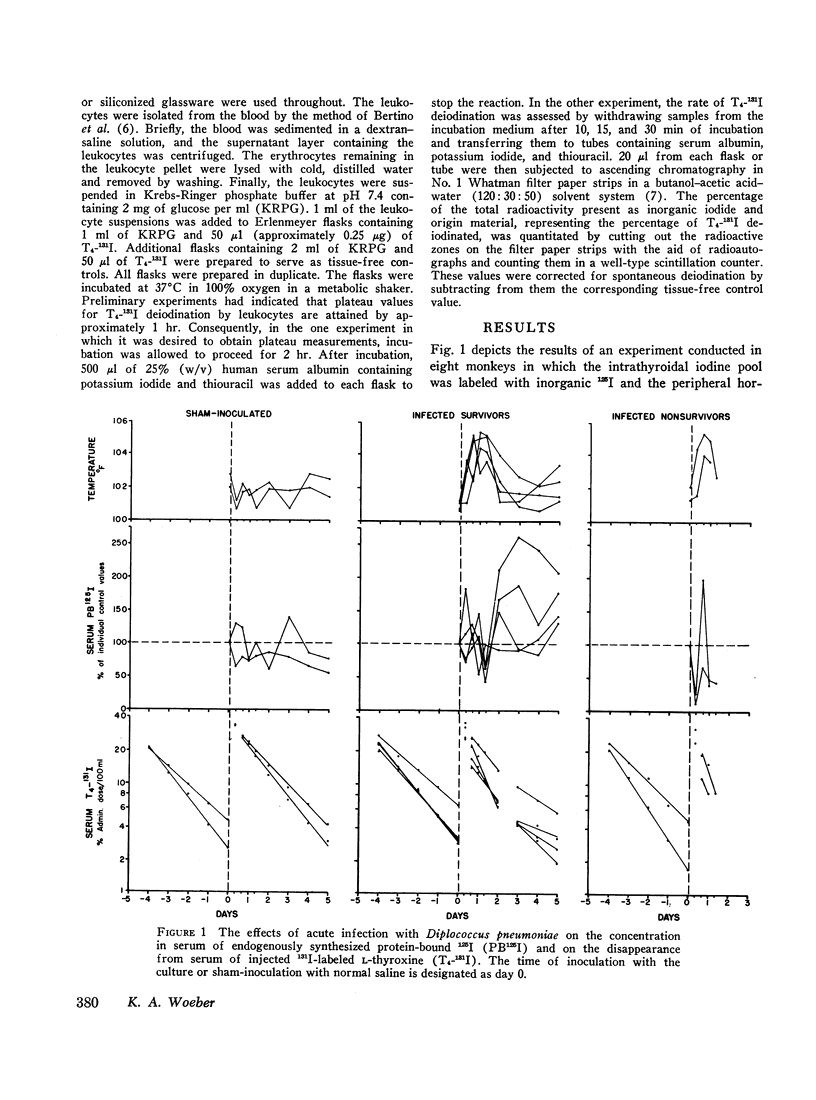
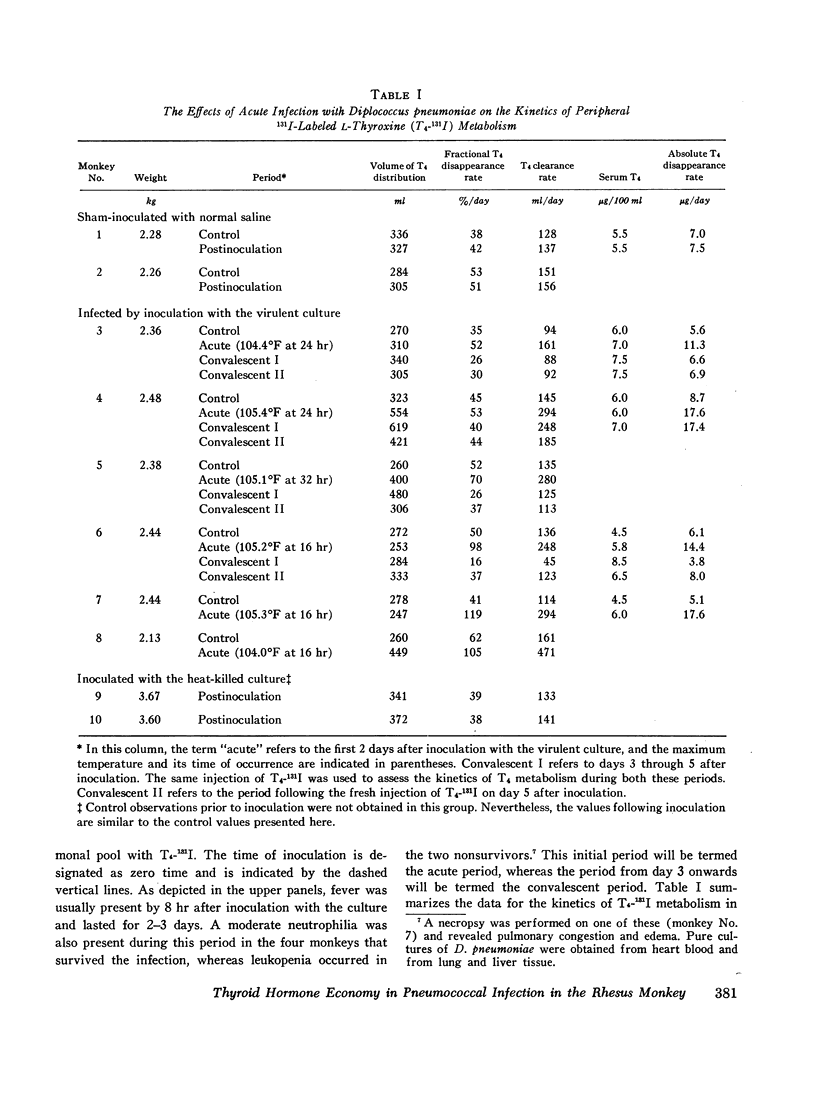
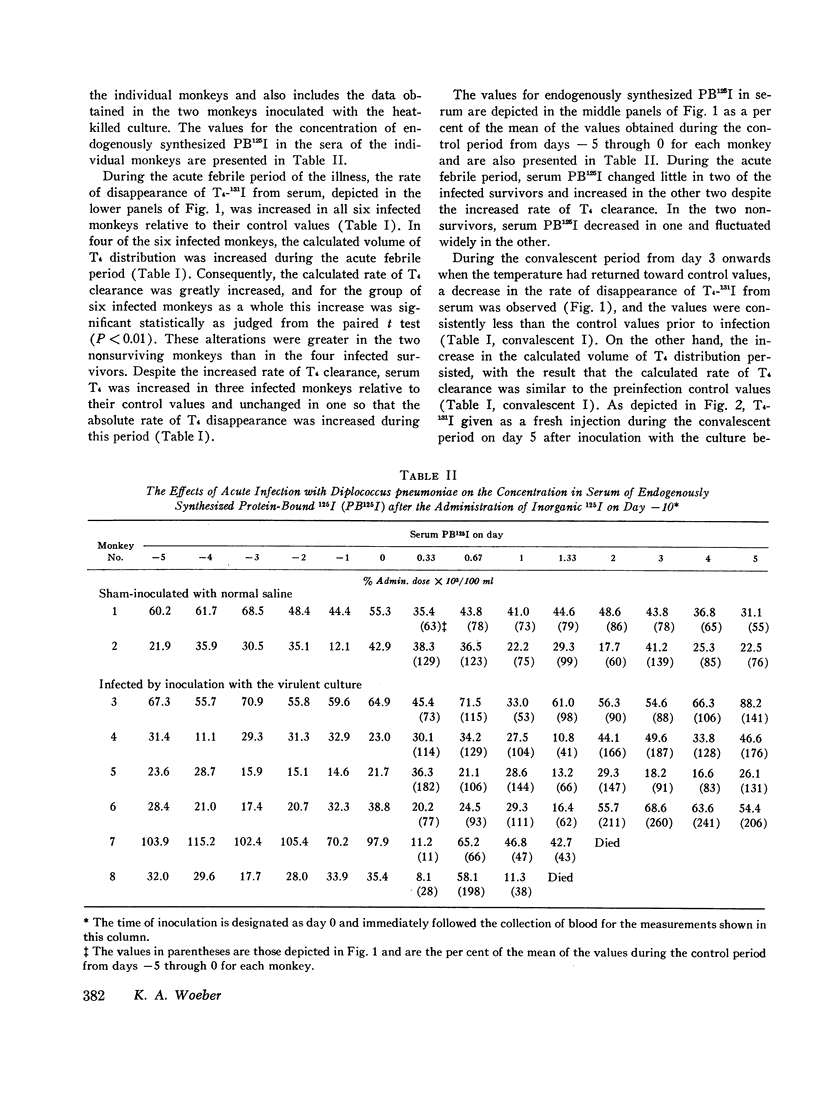
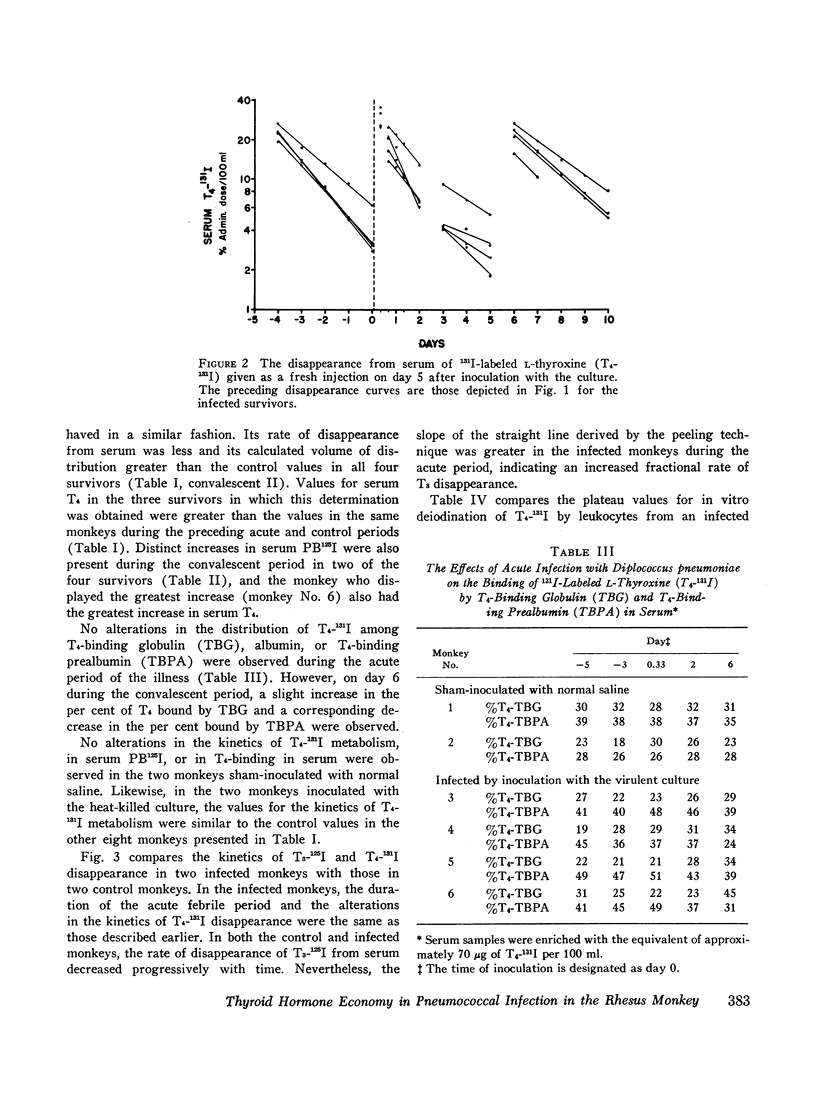
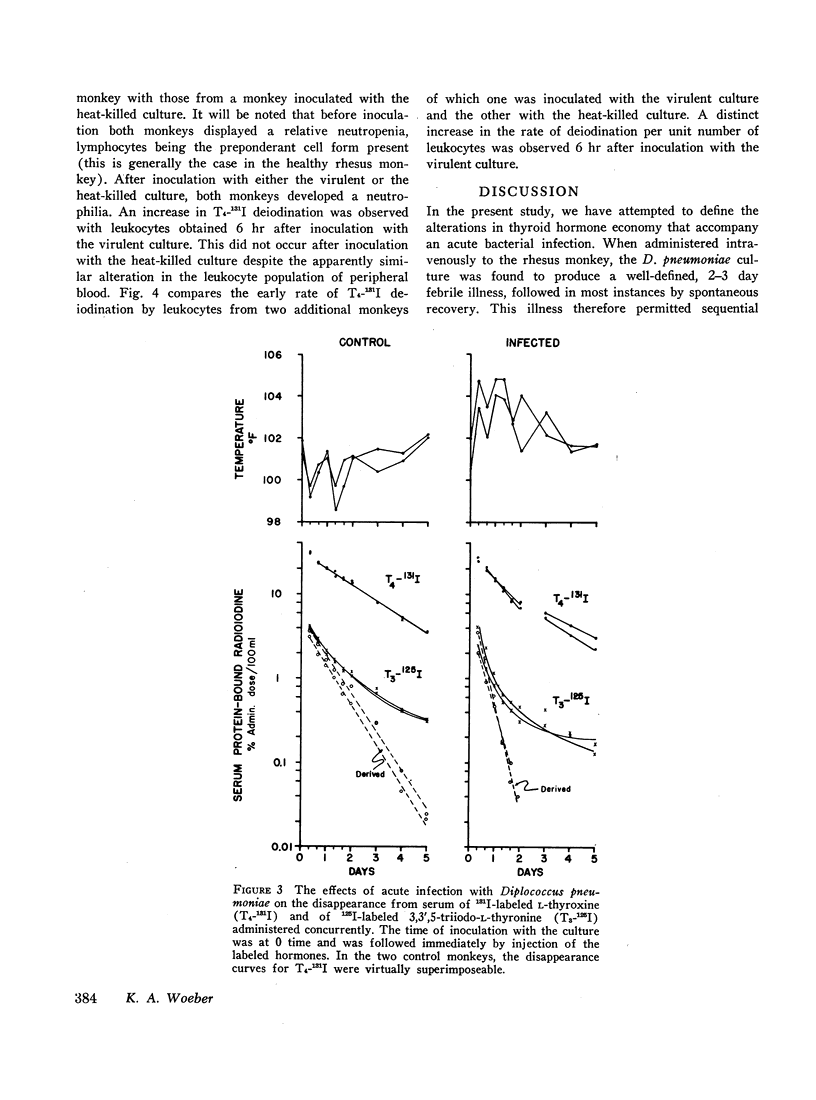
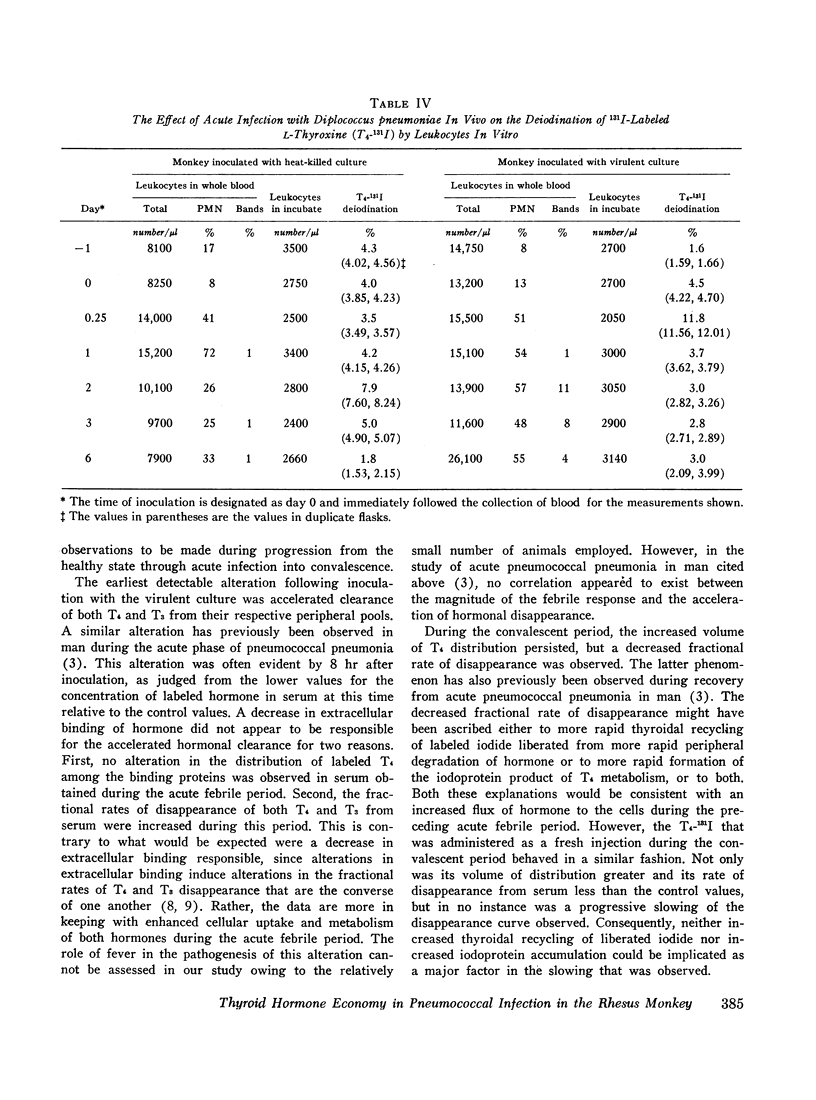
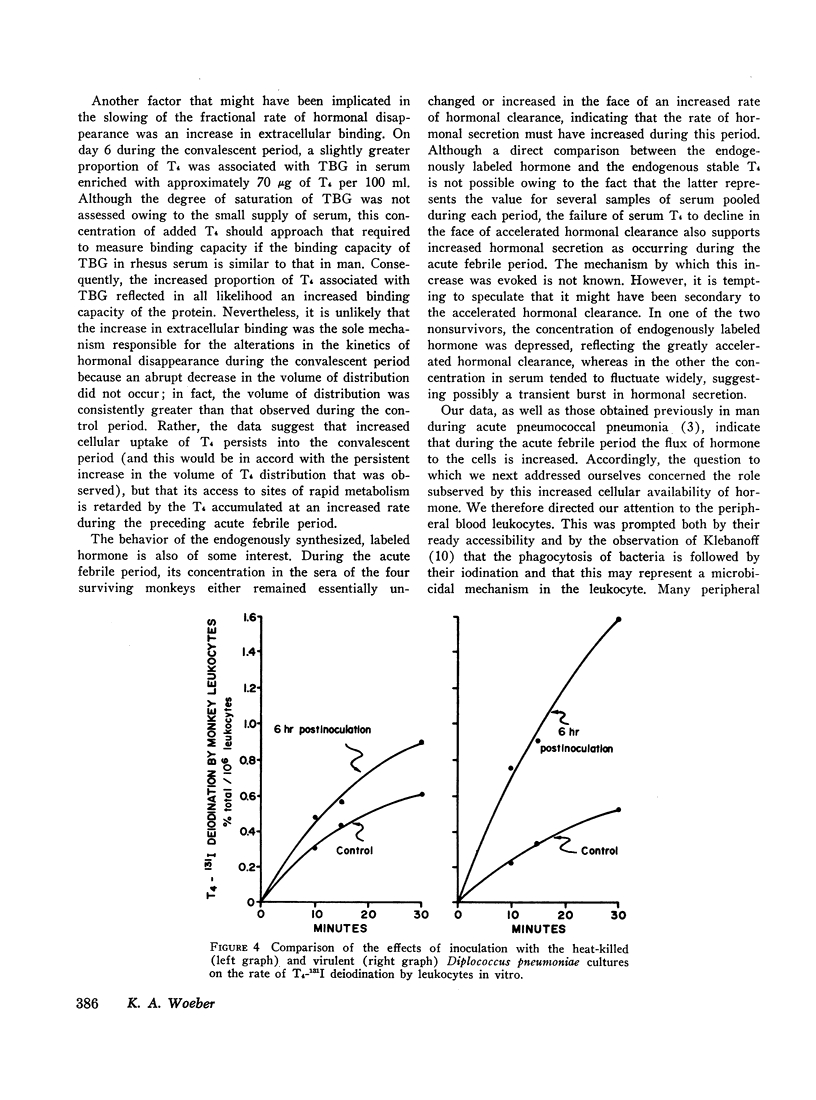
In order to study the alterations in thyroid hormone economy that accompany an acute bacterial infection, rhesus monkeys were inoculated i.v. with a virulent Diplococcus pneumoniae culture containing approximately 108 organisms per dose. This was found to produce a well-defined febrile illness followed in most instances by spontaneous recovery, thereby permitting sequential observations to be made during progression from the healthy state through acute infection into convalescence. During the acute febrile period of the infection, the clearance of both exogenously labeled L-thyroxine (T4) and 3,3′,5-triiodo-L-thyronine (T3) from their peripheral pools was accelerated. This alteration was often evident by 8 hr after inoculation with the virulent culture and could not be ascribed to a decrease in extracellular binding. Despite the accelerated hormonal clearance, the concentrations of both endogenously labeled thyroid hormone and stable T4 in the sera of the surviving monkeys remained essentially unchanged or increased, indicating that hormonal secretion must have increased during this period. During the convalescent period, hormonal clearance was similar to preinfection control values. Leukocytes isolated from blood obtained 6 hr after inoculation with the virulent culture displayed enhanced T4-deiodinative activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTINO J. R., SILBER R., FREEMAN M., ALENTY A., ALBRECHT M., GABRIO B. W., HUENNEKENS F. M. STUDIES ON NORMAL AND LEUKEMIC LEUKOCYTES. IV. TETRAHYDROFOLATE-DEPENDENT ENZYME SYSTEMS AND DIHYDROFOLIC REDUCTASE. J Clin Invest. 1963 Dec;42:1899–1907. doi: 10.1172/JCI104875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELZINGA K. E., CARR E. A., Jr, BEIERWALTES W. H. Adaptation of the standard Durrum-type cell for reverse-flow paper electrophoresis. Am J Clin Pathol. 1961 Aug;36:125–131. doi: 10.1093/ajcp/36.2.125. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Rechcigl M., Jr Factors influencing myeloperoxidase and catalase activities in polymorphonuclear leukocytes. Biochim Biophys Acta. 1967 Oct 9;148(1):243–250. doi: 10.1016/0304-4165(67)90299-1. [DOI] [PubMed] [Google Scholar]
- GALTON V. A., INGBAR S. H. ROLE OF PEROXIDASE AND CATALASE IN THE PHYSIOLOGICAL DEIODINATION OF THYROXINE. Endocrinology. 1963 Nov;73:596–605. doi: 10.1210/endo-73-5-596. [DOI] [PubMed] [Google Scholar]
- GALTON V. A., INGBAR S. H. The mechanism of protein iodination during the metabolism of thyroid hormones by peripheral tissues. Endocrinology. 1961 Jul;69:30–38. doi: 10.1210/endo-69-1-30. [DOI] [PubMed] [Google Scholar]
- Gregerman R. I., Solomon N. Acceleration of thyroxine and triiodothyronine turnover during bacterial pulmonary infections and fever: implications for the functional state of the thyroid during stress and in senescence. J Clin Endocrinol Metab. 1967 Jan;27(1):93–105. doi: 10.1210/jcem-27-1-93. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY B. E., PATTEE C. J. DETERMINATION OF THYROXINE UTILIZING THE PROPERTY OF PROTEIN-BINDING. J Clin Endocrinol Metab. 1964 Feb;24:187–196. doi: 10.1210/jcem-24-2-187. [DOI] [PubMed] [Google Scholar]
- Paul B., Sbarra A. J. The role of the phagocyte in host-parasite interactions. 13. The direct quantitative estimation of H2O2 in phagocytizing cells. Biochim Biophys Acta. 1968 Feb 1;156(1):168–178. doi: 10.1016/0304-4165(68)90116-5. [DOI] [PubMed] [Google Scholar]
- REICHLIN S., GLASER R. J. Thyroid function in experimental streptococcal pneumonia in the rat. J Exp Med. 1958 Feb 1;107(2):219–236. doi: 10.1084/jem.107.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shambaugh G. E., 3rd, Beisel W. R. Alterations in thyroid physiology during pneumococcal septicemia in the rat. Endocrinology. 1966 Sep;79(3):511–523. doi: 10.1210/endo-79-3-511. [DOI] [PubMed] [Google Scholar]
- Woeber K. A., Hecker E., Ingbar S. H. The effects of an acute load of thyroxine on the transport and peripheral metabolism of triiodothyronine in man. J Clin Invest. 1970 Apr;49(4):650–654. doi: 10.1172/JCI106276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninovich A. A., Volpe R., Ezrin C. Effects of variations of thyroxine-binding globulin capacity on the disappearance of triiodothyronine from the plasma. J Clin Endocrinol Metab. 1969 Dec;29(12):1601–1607. doi: 10.1210/jcem-29-12-1601. [DOI] [PubMed] [Google Scholar]


