Abstract
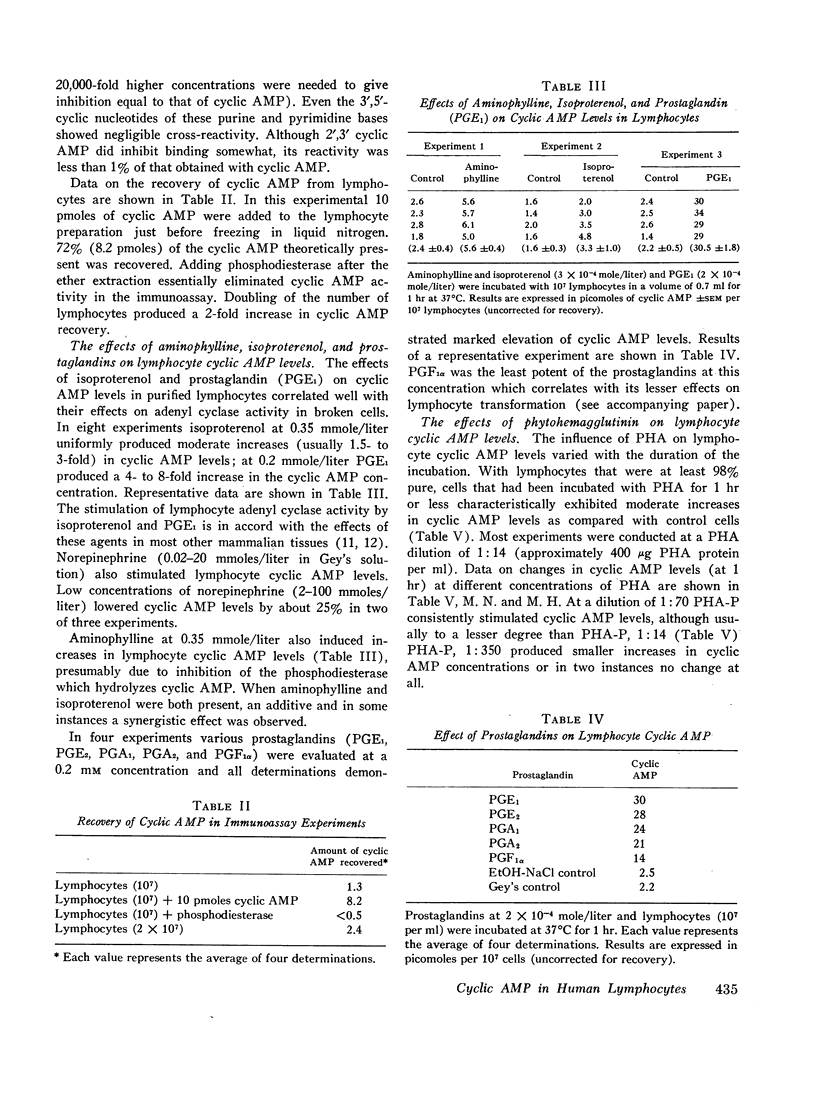
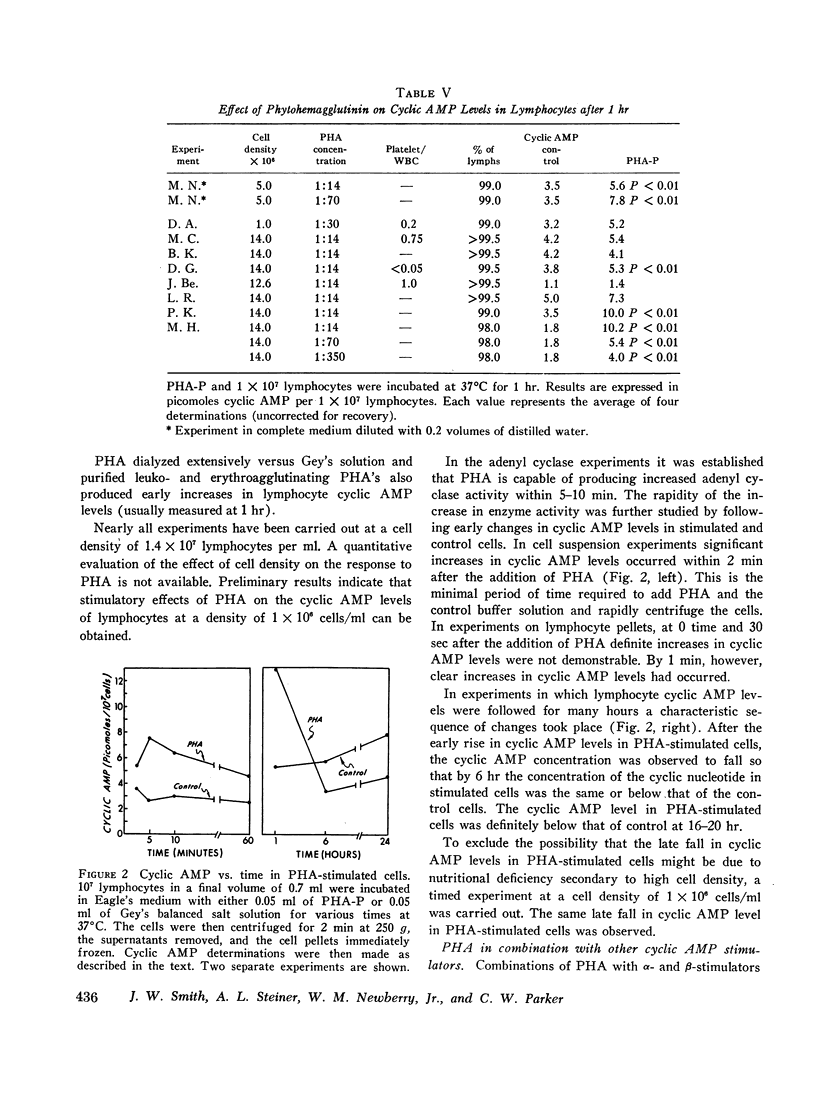
We have studied cyclic adenosine 3′,5′-monophosphate (cyclic AMP) concentrations in human peripheral blood lymphocytes after stimulation with phytohemagglutinin (PHA), isoproterenol, prostaglandins, and aminophylline. Purified lymphocytes were obtained by nylon fiber chromatography, and low speed centrifugation to remove platelets. Cyclic AMP levels were determined by a highly sensitive radioimmunoassay. At concentrations of 0.1-1.0 mmoles/liter isoproterenol and aminophylline produced moderate increases in cyclic AMP concentrations, whereas prostaglandins produced marked elevations. High concentrations of PHA produced 25-300% increases in cyclic AMP levels, alterations being demonstrated within 1-2 min. The early changes in cyclic AMP concentration appear to precede previously reported metabolic changes in PHA-stimulated cells. After 6 hr cyclic AMP levels in PHA-stimulated cells had usually fallen to the levels of control cells. After 24 hr the level in PHA-stimulated cells was characteristically below that of the control cells.
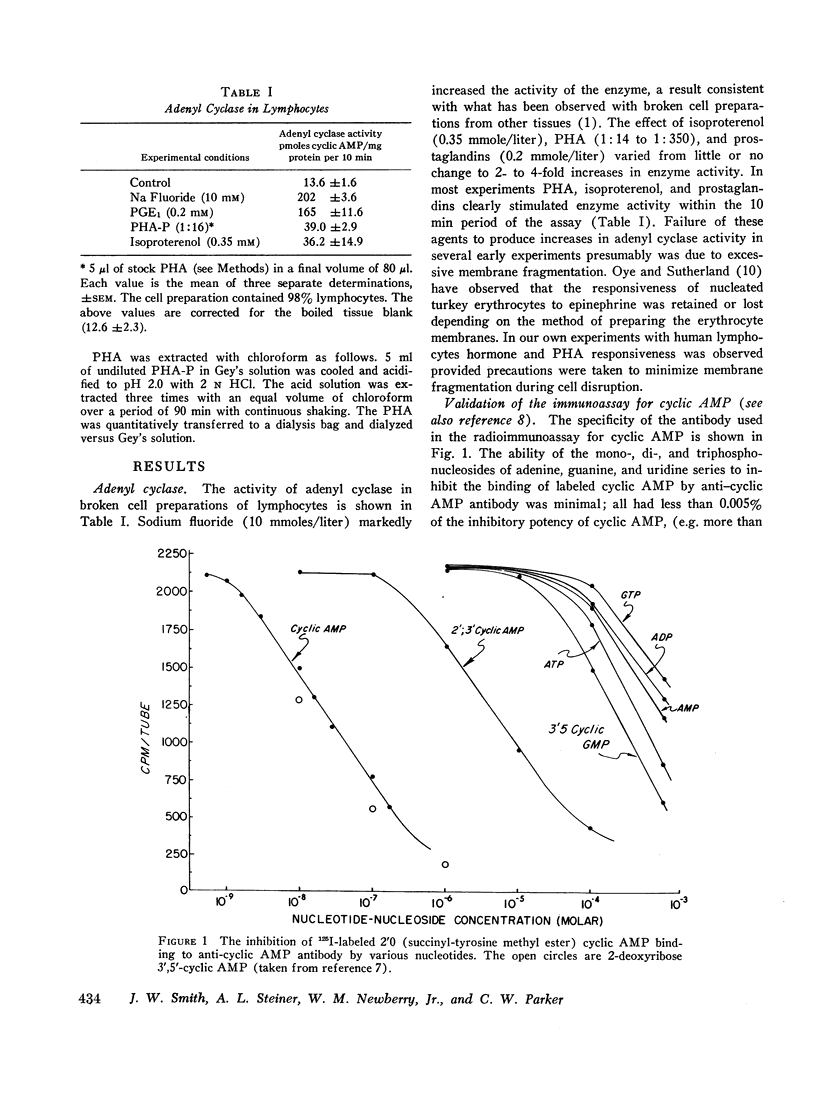
Adenyl cyclase, the enzyme which converts ATP to cyclic AMP, was measured in lymphocyte homogenates. Adenyl cyclase activity was rapidly stimulated by fluoride, isoproterenol, prostaglandins, and PHA. Since adenyl cyclase is characteristically localized in external cell membranes, our results are consistent with an initial action of PHA at this level.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fisher D. B., Mueller G. C. An early alteration in the phospholipid metabolism of lymphocytes by phytohemagglutinin. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1396–1402. doi: 10.1073/pnas.60.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. L., Rosenau W., Burke G. C. Fractionation of phytohemagglutinin. I. Purification of the RNA and DNA synthesis-stimulating substances and evidence that they are not proteins. Proc Natl Acad Sci U S A. 1969 Sep;64(1):283–289. doi: 10.1073/pnas.64.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Cooper H. L. Rapidly labeled cytoplasmic RNA in normal and phytohaemagglutinin-stimulated human lymphocytes. Biochim Biophys Acta. 1969 Jul 22;186(1):62–84. doi: 10.1016/0005-2787(69)90490-0. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphorylation of nuclear protein early in the course of gene activation in lymphocytes. Science. 1966 Nov 11;154(3750):780–781. doi: 10.1126/science.154.3750.780. [DOI] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan T. A. Phosphorylation of liver histone following the administration of glucagon and insulin. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1276–1283. doi: 10.1073/pnas.64.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey G. S., Epstein S. E. Myocardial adenyl cyclase: activation by thyroid hormones and evidence for two adenyl cyclase systems. J Clin Invest. 1969 Sep;48(9):1663–1669. doi: 10.1172/JCI106131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis N., Majerus P. W. Lipid metabolism in human platelets. II. De novo phospholipid synthesis and the effect of thrombin on the pattern of synthesis. J Clin Invest. 1969 Nov;48(11):2114–2123. doi: 10.1172/JCI106178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Margolis S. Histamine release in vitro: inhibition by catecholamines and methylxanthines. Science. 1968 Aug 30;161(3844):902–903. doi: 10.1126/science.161.3844.902. [DOI] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Oye I., Sutherland E. W. The effect of epinephrine and other agents on adenyl cyclase in the cell membrane of avian erythrocytes. Biochim Biophys Acta. 1966 Oct 31;127(2):347–354. doi: 10.1016/0304-4165(66)90389-8. [DOI] [PubMed] [Google Scholar]
- Parker C. W., Vavra J. D. Immunosuppression. Prog Hematol. 1969;6:1–81. [PubMed] [Google Scholar]
- Pogo B. G., Allfrey V. G., Mirsky A. E. RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc Natl Acad Sci U S A. 1966 Apr;55(4):805–812. doi: 10.1073/pnas.55.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas D. A., Tisdale V. V. Bio-assay and dose-response of the mitogenic activity of the phytohemagglutinin of Phaseolus vulgaris. Experientia. 1969 Apr 15;25(4):399–400. doi: 10.1007/BF01899946. [DOI] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Adenyl cyclase as an adrenergic receptor. Ann N Y Acad Sci. 1967 Feb 10;139(3):703–723. doi: 10.1111/j.1749-6632.1967.tb41239.x. [DOI] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle J. R., Kipnis D. M. An adrenergic receptor mechanism for the control of cyclic 3'5' adenosine monophosphate synthesis in tissues. Biochem Biophys Res Commun. 1967 Sep 7;28(5):797–802. doi: 10.1016/0006-291x(67)90388-9. [DOI] [PubMed] [Google Scholar]
- Vaughan M., Murad F. Adenyl cyclase activity in particles from fat cells. Biochemistry. 1969 Jul;8(7):3092–3099. doi: 10.1021/bi00835a060. [DOI] [PubMed] [Google Scholar]
- Weiss B. Similarities and differences in the norepinephrine-and sodium fluoride-sensitive adenyl cyclase system. J Pharmacol Exp Ther. 1969 Apr;166(2):330–338. [PubMed] [Google Scholar]
- Wolfe S. M., Shulman N. R. Adenyl cyclase activity in human platelets. Biochem Biophys Res Commun. 1969 Apr 29;35(2):265–272. doi: 10.1016/0006-291x(69)90277-0. [DOI] [PubMed] [Google Scholar]


