Abstract
Background
Survival varies widely among different neurodegenerative diseases. Data on the role of the Mini Mental State Examination (MMSE) and apolipoprotein E (APOE) in survival are sparse except for Alzheimer's disease (AD).
Methods
We studied mortality of 3,581 patients in an academic clinic from 1993 to 2004. Average follow-up was 4.1 years. We studied patients with amyotrophic lateral sclerosis (ALS) (n = 174), possible AD (n = 206), probable AD (n = 1,175), Parkinson's disease (PD) (n = 661), mild cognitive impairment (MCI) (n = 357), frontotemporal dementia (FTD) (n = 94), Lewy body disease (LBD) (n = 64), and controls (n = 850). We compared patients’ mortality to the US population and to controls.
Results
Mortality ranged from 7% for controls to 58% for ALS patients. The median survival times from initial visit for PD, FTD, probable AD, possible AD, LBD, and ALS were 8.9, 7.0, 5.9, 5.6, 5.3, and 2.7 years, respectively. Mortality rate ratios comparing each disease to controls were 39.43, 7.25, 3.70, 3.51, 2.47, 2.73, and 1.61 for ALS, FTD, LBD, PD, probable AD, possible AD, and MCI, respectively. A lower initial MMSE score was associated with higher mortality for probable AD, PD, and MCI, while APOE4 predicted mortality for PD and LBD. Non-whites had 20% lower mortality rates than whites for all dementias combined, adjusting for education.
Conclusions
All neurologic diseases, including MCI, had increased mortality versus controls. A lower MMSE score and APOE4 presence predicted higher mortality for some patient groups.
Key Words: Neurodegenerative disease, Survival, Apolipoprotein E4, Mini Mental State Examination
Introduction
Survival of patients with neurodegenerative disease is important for projecting societal costs and the burden of disease. The survival of patients with neurodegenerative disease has been investigated in multiple studies of Alzheimer's disease (AD) [1,2,3,4,5,6,7,8,9,10], possible AD [1,2,10,11,12,13], amyotrophic lateral sclerosis (ALS) [14,15,16,17], and Parkinson's disease (PD) [18,19,20,21,22,23,24,25,26]. There are 8 studies (5 in a review article) of survival of patients diagnosed with mild cognitive impairment (MCI) [27,28,29,30]. There are also several studies with data on survival of frontotemporal dementia (FTD) patients [31,32,33,34], and 1 with data on survival of Lewy body disease (LBD) patients [35].
Findings for median survival for these different diseases are not consistent in the literature cited above. Many studies do not report median survival time. Some studies calculate survival from time of referral, others from time of diagnosis, and others from time of symptom onset. Generally, ALS patients show the worst survival, with median survival from any starting point of about 2–3 years. Probable AD patients have a median survival from diagnosis on the order of 3–6 years [34], although adjustment for ‘length bias’ (due to the omission of those with very poor survival from many databases) is likely to shorten this estimate [1]. One study indicated similar survival for possible and probable AD cases [9]. Data for survival for PD patients are sparse, but 1 study reported a median survival from time of diagnosis of 10 years [16]. Studies of MCI patients have not reported median survival, but indicate that the mortality of MCI patients is 50–100% higher than the mortality of controls. Median survival times must also be considered cautiously, as they are taken from Kaplan-Meier curves and are unadjusted for age, which usually plays an important role in survival.
Some studies have investigated the role of cognition [via the Mini Mental State Examination (MMSE), the most common measure] or genetics [most commonly via the presence of the apolipoprotein E4 (APOE4) allele] in survival. Several studies have found that lower initial MMSE scores were associated with higher mortality for AD patients [6,13,35]. Other studies have shown that a higher rate of decline in cognition predicts higher mortality in AD patients [36,37,38,39], although 1 did not [35]. The presence of 1 or 2 APOE4 alleles has been associated with increased mortality among AD patients in some studies (1 in males only) [35,40,41], while other studies have found no association [42,43].
There is less information on the role of MMSE and APOE4 in survival for patients with other types of neurodegenerative diseases, or patients with MCI. There are 2 studies among PD patients indicating that cognitive impairment significantly increases mortality [44,45].
Here, we have investigated survival among a well-characterized population of patients at a referral center with possible AD, probable AD, FTD, LBD, PD, and MCI, as well as survival among controls. This population is larger than that observed in many other studies. We have also considered the role of demographic covariates, comorbidity, MMSE, and APOE4 in predicting survival. Many studies have not been able to evaluate the role of cognitive status and APOE4 in survival.
Methods
Patients with well-defined or suspected diagnoses of possible and probable AD, LBD, FTD, PD, or MCI, as well as controls, were selected from a research registry of subjects seen in the Neurology Department at Emory Wesley Woods Health Center (n = 3,581). Sixty-five percent of the patients’ diagnoses were recently reviewed by Emory's Alzheimer Disease Center to achieve consensus on a research diagnosis. The remaining diagnoses are the clinical diagnosis at the time of last visit.
Patients were required to have been first seen between January 1, 1993 and December 31, 2004, and mortality follow-up was performed through December 31, 2006. The controls were a convenience sample of volunteers who agreed to participate in the research registry, and were either friends or relatives of patients, or volunteers from the community.
Mortality follow-up was conducted by matching patient IDs with the National Death Index (NDI). Matching was done via Social Security Numbers when available or a combination of name, sex, and date of birth when the Social Security Number was not available. The NDI returns all possible matches and classifies them as either ‘true’ or ‘false’, with ‘true’ matching on most or all submitted identifiers, and further assigns subjects probability-of-matching scores. We generally accepted true match or nonmatch according to NDI's classification of ‘true’ or ‘false’, unless the data on probability of matching indicated a close call in which cases there was individual review. The NDI is the principal source of cause-of-death information in the US for deaths after 1978 and has been shown in studies submitting data on known decedents to provide complete and accurate death information [46].
Cognition was assessed by the MMSE, and was available for 66% of subjects (69% with the exception of ALS patients, who were rarely administered the MMSE). The MMSE was generally given upon initial visit and annually thereafter. Data were available regarding APOE4 for 68% of the cohort. The main determinant of having APOE data was having volunteered to participate in research studies. Over 97% of subjects who had volunteered for research (65% of subjects) had APOE data, while only 16% of the remaining subjects had APOE data. A similar but not so extreme pattern was seen for MMSE, where 74% of research subjects had MMSE data, compared to 51% of nonresearch subjects. Those missing MMSE among nonresearch subjects were predominantly ALS patients and controls (both missing in over 90% of subjects), among whom the MMSE is not usually administered. Among the remainder of nonresearch subjects, the percent missing MMSE was similar to research subjects, i.e., 25%.
Comorbidity data were also available for 77% of the cohort. These data were available as ‘disease present’ or ‘disease absent’ for heart disease, stroke, diabetes, and hypertension; 4 variables for these 4 diseases were entered simultaneously in all models controlling for comorbidity. These data came largely from self-report at the time a subject was enrolled as a research volunteer in the Neurology Clinic, although some subjects had further exams which either led to confirmation or changing the original self-report.
Multivariate Cox regression analyses, using SAS PHREG, were run for all disease groups versus controls. Covariates for race, sex, and education were included in all models, age was either controlled via matching or was entered in the model, and some models also examined comorbidity (available for 77% of the cohort), MMSE, and APOE4. The time variable for Cox regression was usually age, which had the effect of matching on age. Risk sets were formed for each case based on surviving past the age at which the case died. Time at risk began at the time of first visit. We also conducted one set of analyses in which age was one of the variables of interest, and in this analysis the time variable was the follow-up time from initial visits.
In analyzing the effect of MMSE or APOE4 by disease group, when an adequate distribution of MMSE scores was available, we used cut points 0–19, 20–24, and 25–30 (the referent). For those disease groups without an adequate distribution of scores we used 0–24 and 25–30 (referent). Tests for trend with MMSE were evaluated by the p value for the coefficient for MMSE as a continuous variable.
Kaplan-Meier survival curves were generated to evaluate the survival of each disease group; survival time began at the time of first visit to the clinics. Median survival times were calculated using the midpoint of the 2 time points around the 50% intersection of the survival curve. Median survival could not be calculated for MCI patients and controls because the survival function did not reach 50% (too few deaths). In addition, life table analyses were run for each group using the National Institute for Occupational Safety and Health Life Table Analysis System. Standardized mortality ratios (SMRs) were calculated by comparing the study subjects to the national population stratified by age (5-year categories), race, sex, and calendar period. SMRs are essentially the mortality rate of the disease group (deaths/person-time at risk, which begins at the time of first visit) divided by the mortality rate of the US population of similar age, race, sex, and calendar year.
Results
The NDI returned death matches on 30% of the cohort. After review of questionable matches, we reclassified 69 records incorrectly classified as nonmatches, for a false negative rate of 6.9% (false negatives divided by true positives). We found no false positives.
Table 1 provides descriptive statistics for the cohort. The largest group in the cohort was made up of individuals diagnosed with probable AD, followed by the control and PD groups. The cohort was followed for an average of 4.1 years, and slightly more than half of the cohort was female, with larger percentages in the AD and control groups.
Table 1.
Descriptive statistics on population in mortality study of Emory neurology subjects (first seen before December 31, 2004, followed for mortality through December 31, 2006)
| Number | Percent | Mean age at first visit | Mean years of follow-up | Dead n | Dead % | Male % | Mean first MMSE score | MMSE available % | APOE available % | APOE4 (if APOE available), % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALS | 174 | 4.9 | 54.0 | 2.3 | 101 | 58.1 | 63.2 | 24.1 | 4.6 | 36.8 | 25.0 |
| Possible AD | 206 | 5.8 | 75.5 | 3.6 | 79 | 38.4 | 35.9 | 18.6 | 77.2 | 87.9 | 52.5 |
| Probable AD | 1,175 | 32.8 | 76.3 | 4.3 | 535 | 45.5 | 32.1 | 17.3 | 76.9 | 59.2 | 64.9 |
| FTD | 94 | 2.6 | 65.7 | 3.8 | 33 | 35.1 | 54.3 | 20.4 | 66.0 | 76.6 | 38.9 |
| LBD | 64 | 1.8 | 74.4 | 3.7 | 29 | 45.3 | 56.3 | 19.5 | 76.6 | 71.9 | 52.2 |
| MCI | 357 | 10.0 | 72.1 | 4.8 | 74 | 20.7 | 44.1 | 25.7 | 73.4 | 56.6 | 49.0 |
| PD | 661 | 18.5 | 67.0 | 3.8 | 161 | 24.4 | 63.6 | 26.6 | 70.1 | 91.8 | 26.2 |
| Controls | 850 | 23.7 | 60.6 | 4.3 | 59 | 6.9 | 37.7 | 28.7 | 53.9 | 67.2 | 35.6 |
| Total | 3,581 | 100 | 69.0 | 4.1 | 1,071 | 29.9 | 43.2 | 22.5 | 66.0 | 68.1 | 44.1 |
As noted, 66% of the cohort had at least 1 MMSE, generally taken at the time of initial visit, while 68% were genotyped for APOE. We explored reasons for missing any data on MMSE and found no correlation with age, sex, or race. As expected, ALS patients were far less likely than other patient groups to have an MMSE; controls were also less likely to have an MMSE than most patient groups since the cognitive assessments were often obtained for clinical purposes. Controls, MCI, probable AD, and ALS were less likely to have data on APOE than other disease categories.
Table 2 shows the distribution of education and comorbidities in this population; these were covariates included in some regression models. The two AD groups had lower education than the other disease groups, as might be expected from the literature suggesting that lower education is a risk factor. ALS patients had an elevated prevalence of diabetes, heart disease, and hypertension relative to other groups.
Table 2.
Mean years of education and prevalence of comorbidities in individuals included in Cox regression model
| Number | Mean years of education | Diabetes, % | Heart disease % | Stroke % | Hypertension % | |
|---|---|---|---|---|---|---|
| ALS | 141 | 14.2 | 16.3 | 12.1 | 0.7 | 53.2 |
| FTD | 75 | 13.9 | 6.7 | 2.7 | 0.0 | 44.0 |
| LBD | 45 | 14.0 | 2.2 | 8.9 | 0.0 | 42.2 |
| PD | 512 | 14.5 | 12.7 | 5.3 | 2.7 | 38.7 |
| Probable AD | 789 | 12.8 | 10.9 | 5.7 | 1.4 | 34.7 |
| Possible AD | 154 | 12.6 | 13.0 | 5.2 | 1.3 | 30.5 |
| MCI | 273 | 14.3 | 12.8 | 4.8 | 1.5 | 39.2 |
| Controls | 635 | 14.4 | 11.8 | 4.1 | 1.4 | 38.3 |
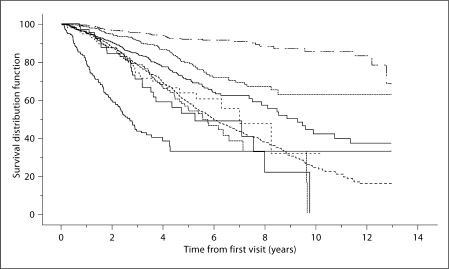
Figure 1 shows the Kaplan-Meier survival curves for this population, with survival time beginning at the time of first visit. The median estimated survival times from initial visit for PD, FTD, probable AD, possible AD, LBD, and ALS were 8.9, 7.0, 5.9, 5.6, 5.3, and 2.7 years, respectively. Median survival time for MCI and controls could not be reliably calculated due to the relatively small number of deaths. However, neither the curves nor the median survival times are corrected for age (the most important covariate), race, sex, or education.
Fig. 1.
Kaplan-Meier survival curves, in order, top to bottom (at 5 years): controls, MCI, PD, FTD, probable AD, possible AD, LBD, and ALS.
Table 3 shows the ratio of the mortality rate of each group to the mortality rates of the US population (the SMR for all causes combined), adjusted for age, race, sex, and calendar time. It also shows the mortality rate of each group compared directly to controls via the RR. Compared to the US population, ALS patients have the highest mortality, FTD the next, while LDB, PD, possible AD, and probable AD follow in that order. MCI patients and controls have borderline significant or significantly lower mortality than the general population, likely reflecting a relatively healthy base population which reaches an academic medical center, as well as possibly a ‘healthy volunteer’ effect for the controls. The pattern for RRs, comparing each group to the controls, is the same as for the SMRs. ALS has the highest mortality, and MCI the lowest, relative to controls. Adjustment for comorbidity did not change these results appreciably, and was omitted in final models.
Table 3.
Mortality rate ratios of different patient groups compared to the US population (SMR) and directly to controls (RR)
| Dead | Person-years | Crude ratea | SMRa | RRb | |
|---|---|---|---|---|---|
| ALS | 101 | 408.8 | 0.247 | 23.35 (19.0–28.4) | 39.43 (28.23–55.08) |
| FTD | 33 | 356.4 | 0.093 | 3.47 (2.37–4.89) | 7.25 (4.69–11.21) |
| LBD | 29 | 238 | 0.122 | 2.42 (1.62–3.48) | 3.70 (2.34–5.85) |
| PD | 161 | 2,492 | 0.065 | 1.99 (1.69–2.32) | 3.51 (2.58–4.76) |
| Possible AD | 79 | 751.9 | 0.105 | 1.84 (1.45–2.29) | 2.47 (1.75–3.48) |
| Probable AD | 535 | 5,053.5 | 0.106 | 1.70 (1.56–1.85) | 2.73 (2.07–3.60) |
| MCI | 74 | 1,701.6 | 0.043 | 0.84 (0.66–1.05) | 1.61 (1.14–2.28) |
| Controls | 59 | 3,630.4 | 0.016 | 0.57 (0.43–0.73) | 1 |
SMR, adjusted for age, race, sex, and calendar time.
Mortality rate ratio of each group compared to controls. Adjusted for race, sex, and education; analysis via Cox regression with age as the time variable.
Table 4 shows the effect of age, race, sex, education, and comorbid conditions on mortality for each of the disease groups and controls. A higher age increases risk significantly for a majority of diseases, while female gender was significantly protective for FTD, probable AD, and controls. Non-white race and increased education were significantly associated with lower mortality in probable AD, while education was also associated in controls. The presence of stroke was associated with increased mortality in both ALS and probable AD. No comorbid conditions were important in either LBD or possible AD.
Table 4.
Demographic and comorbidity significant predictors of mortality by disease group1
| Increased age | Non-white versus white | Female versus male | Increased years of education | Diabetes | Heart disease | Stroke | Hypertension | |
|---|---|---|---|---|---|---|---|---|
| ALS | ∗∗ (+) | ∗(+) | ||||||
| FTD | ∗∗(−) | |||||||
| LBD | ||||||||
| PD | ∗∗(+) | ∗∗(+) | ||||||
| Probable AD | ∗∗(+) | ∗∗(−) | ∗∗(−) | ∗∗(−) | ∗∗(+) | ∗(−) | ∗∗(+) | |
| Possible AD | ||||||||
| MCI | ∗∗(+) | |||||||
| Controls | ∗∗(+) | ∗(−) | ∗∗(−) |
p < 0.10
p < 0.05. (+) or (−) indicates direction of association, history of disease via self-report.
Multivariate models, Cox regression, with time variable as time since initial visit, all covariates in the model, same model for each group.
There is a significantly increased mortality for those with lower MMSE scores at the time of initial visit for probable AD, PD, and LBD (table 5). For the AD patients, there is a significant trend of increased mortality when MMSE is entered in the model as a continuous variable, despite the apparent lack of trend in the categorical analysis, presumably reflecting the large number of AD patients. The decreased risk of death of controls with lower MMSE is probably due to chance, as the confidence interval is very wide.
Table 5.
Mortality RRs within each disease group by MMSE category
| MMSE category | Number | Dead % | Rate ratio | 95% CI | p value test for trend (MMSE continuous) | |
|---|---|---|---|---|---|---|
| Possible | 0–19 | 78 | 46.2 | 1.75 | 0.80–3.81 | 0.137 |
| AD | 20–24 | 45 | 37.8 | 1.61 | 0.70–3.69 | |
| 25–30 | 36 | 25.0 | 1.00 | |||
| Probable | 0–19 | 517 | 52.2 | 1.07 | 0.75–1.52 | 0.038 |
| AD | 20–24 | 244 | 39.8 | 1.07 | 0.74–1.55 | |
| 25–30 | 142 | 28.9 | 1.00 | |||
| FTD | 0–24 | 39 | 53.9 | 4.02 | 1.31–12.31 | 0.190 |
| 25–30 | 23 | 17.4 | 1.00 | |||
| LBD | 0–24 | 34 | 41.2 | 1.26 | 0.38–4.11 | 0.149 |
| 25–30 | 15 | 26.7 | 1.00 | |||
| MCI | 0–24 | 73 | 34.3 | 1.46 | 0.82–2.61 | 0.005 |
| 25–30 | 189 | 19.6 | 1.00 | |||
| PD | 0–19 | 30 | 56.7 | 2.05 | 1.16–3.63 | 0.002 |
| 20–24 | 64 | 39.1 | 0.90 | 0.53–1.52 | ||
| 25–30 | 369 | 15.5 | 1.00 | |||
| Controls | 0–24 | 17 | 17.7 | 0.59 | 0.17–2.13 | 0.967 |
| 25–30 | 441 | 9.5 | 1.00 | |||
First available MMSE after initial visit. Data adjusted for age, race, sex, and education. Adjustment for comorbidity did not change results. MMSE score ≥25 served as the referent group. MMSE categories collapsed for controls, LBD, and MCI due to small numbers. ALS not included due to small numbers with MMSE.
We explored further the effect of race on mortality by patient group, while controlling for initial MMSE, age, and sex. For possible and probable AD combined, non-whites (largely African Americans) had a borderline significantly decreased risk of mortality (RR = 0.83, 0.67–1.03). Combining all the dementias (possible AD, probable AD, LBD, and FTD), non-whites also had a significantly decreased risk of dying (RR = 0.80, 0.64–0.92). In contrast, among controls, non-whites had a borderline increased risk of dying (RR = 2.29, 0.98–5.36). These findings persisted when education, which is correlated with race, was entered into the model.
Table 6 shows that the presence of the APOE4 allele is associated with a significantly increased mortality risk for PD and LBD patients after adjusting for race, sex, education and age via matching in Cox regression. It is also close to significance (at the 0.05 level) for ALS patients.
Table 6.
Mortality RR by presence of APOE41 for each disease group
| Number | Dead % | Rate ratio | 95% CI | ||
|---|---|---|---|---|---|
| ALS | APOE4 present | 16 | 62.5 | 1.96 | 0.89–4.31 |
| APOE4 absent (ref) | 48 | 70.8 | 1.00 | ||
| Possible | APOE4 present | 95 | 33.7 | 1.21 | 0.74–1.97 |
| AD | APOE4 absent (ref) | 86 | 46.5 | 1.00 | |
| Probable | APOE4 present | 452 | 39.6 | 1.11 | 0.86–1.41 |
| AD | APOE4 absent (ref) | 244 | 45.5 | 1.00 | |
| FTD | APOE4 present | 28 | 28.6 | 1.15 | 0.48–2.76 |
| APOE4 absent (ref) | 44 | 34.1 | 1.00 | ||
| LBD | APOE4 present | 24 | 58.3 | 3.51 | 1.16–10.58 |
| APOE4 absent (ref) | 22 | 27.3 | 1.00 | ||
| MCI | APOE4 present | 99 | 14.1 | 1.02 | 0.49–2.13 |
| APOE4 absent (ref) | 103 | 17.5 | 1.00 | ||
| PD | APOE4 present | 159 | 28.3 | 1.73 | 1.19–2.52 |
| APOE4 absent (ref) | 448 | 21.0 | 1.00 | ||
| Controls | APOE4 present | 203 | 8.4 | 1.28 | 0.69–2.40 |
| APOE4 absent (ref) | 368 | 9.5 | 1.00 | ||
Data adjusted for race, sex, and education via Cox regression with age as the time variable. Adjustment for comorbidity did not change results. APOE4 considered present if subject either heterozygous or homozygous.
We also explored interactions between APOE4 and initial MMSE, but did not see any convincing patterns. We also adjusted the APOE4 findings for MMSE instead of education, but this caused a significant loss of data and did not change results remarkably.
Discussion
Strengths of our study include use of a well-characterized patient population with relatively large numbers of subjects, including those with MMSE and APOE data. Limitations include a lack of data on disease onset, and small numbers for some disease groups (possible AD, LBD, dementia with Lewy bodies). The lack of data on disease onset is a limitation shared by many studies that analyze mortality of patients with neurodegenerative disease, in particular those that are based in referral clinics such as our own. The length of follow-up in our study (mean 4.1 years), although shorter than desired, nonetheless was sufficiently long such that 30% of subjects died, providing enough deaths to allow reasonable statistical power for most analyses.
Our findings of very high mortality for ALS patients, and markedly high mortality for FTD patients, generally conform to the literature. Our observation of higher mortality among FTD patients compared to AD patients corresponds to 2 earlier findings [31,34], but differs from another [33], which reported similar mortality in these two groups. The small sample size of the latter study may account for this difference. Other authors have found faster decline in FTD compared to AD for measures other than survival [47,48].
Our findings parallel those of Claus et al. [11] in indicating that possible AD and probable AD cases have similar mortality. Our observation of a significant 59% excess of mortality for MCI patients compared to controls also parallels other such findings in the literature [27].
It should be noted that our population was better educated than the US general population, reflecting the fact that the Emory is a referral center for patients with neurodegenerative disease and its patients have relatively more resources than other patients. Restricting our population to those alive and aged 75 years and older in 2005, the percentages with less than high school education, high school or some college, and college degree or more were 20, 30, and 50%, respectively. The corresponding numbers for the US population in 2005 were 29, 48, and 23% (www.census.gov/population/www/socdemo/education/cps2005.html). Among our population, the controls were the best educated (9, 28, and 63%, respectively), reflecting the well-known ‘healthy volunteer effect’ [49]. This probably accounts for the fact that controls in our population had a mortality rate which was only 57% of the corresponding US mortality rate matched for age, race, sex, and calendar time, since it is well known that education is a strong determinant of mortality [50].
Our findings that worse initial MMSE shortens survival for probable AD patients is supported by the prior literature. Our observations that a lower initial MMSE shortens survival for PD patients (significant at the 0.05 level), FTD patients (borderline significant), and MCI patients (borderline significant) represent new findings, although there are 2 studies among PD patients indicating that cognitive impairment significantly increases mortality [44,45]. Roberson et al. [34] did not find an effect of MMSE on survival in FTD patients.
Our finding of a negative effect of the APOE4 allele on survival in PD patients has been seen in 1 prior study [51]. Another study also found that APOE4 increased the risk of psychosis in PD patients [52]. On the other hand, our finding of an increased risk of mortality for LBD patients, and to some extent for ALS patients, does not appear to have been reported earlier.
Our lack of findings of many effects of comorbidity on survival may reflect the limited data available on comorbidity, which came largely from self-reports. As self-reports may be inaccurate, this may have contributed to some misclassification of comorbidity, which would tend to obscure comorbidity effects (bias to the null). Furthermore, the lack of data on severity of comorbidity may have limited our ability to detect comorbidity effects.
Our finding that non-whites (predominantly African Americans) with dementia had lower mortality rates than whites, even after controlling for initial cognitive status, parallels 2 recent studies [9,10]. It also indirectly parallels recent US mortality data which show that non-whites in the US have half the death rates from AD compared to whites, after controlling for education [49]. The mortality data contrast with AD incidence data, where African Americans have the same or higher incidence rates than whites [53,54,55,56]. This difference suggests the possibility of better survival of African Americans, although differential coding of AD by race on death certificates is another explanation. This finding contrasts with the effect of race on mortality in controls, where non-whites have much higher mortality, as is true in the general population.
The reasons for the improved survival in non-whites versus whites among patients with neurodegenerative disease are not known. Authors of the 2 other survival studies [9,10] with this finding have speculated that possible reasons include (1) better comorbidity profile among non-whites, or receiving more aggressive therapy for their comorbidities, (2) underlying unknown genetic variants which affect survival, (3) differences by race in decisions about prolonging life after AD onset, or (4) earlier detection of disease (and hence longer apparent survival) among non-whites. Our data have been adjusted for comorbidity, so it seems that explanation 1 above is unlikely. The other 3 hypotheses are not testable in our data. Hence, more investigation is needed to address the cause of racial differences in survival among individuals with neurodegenerative disease.
Acknowledgements
This work was supported by a grant for the Emory Alzheimer's Disease Research Center (NIH-NIA 5 P50 AG025688).
References
- 1.Wolfson C, Wolfson DB, Asgharian M, M'Lan CE, Ostbye T, Rockwood K, Hogan DB, Clinical Progression of Dementia Study Group A reevaluation of the duration of survival after the onset of dementia. N Engl J Med. 2001;344:1111–1116. doi: 10.1056/NEJM200104123441501. [DOI] [PubMed] [Google Scholar]
- 2.Villareal DT, Grant E, Miller JP, Storandt M, McKeel DW, Morris JC. Clinical outcomes of possible versus probable Alzheimer's disease. Neurology. 2003;61:661–667. doi: 10.1212/wnl.61.5.661. [DOI] [PubMed] [Google Scholar]
- 3.Singer RB. Mortality derived from 5-year survival in patients with Alzheimer disease. J Insur Med. 2005;37:264–271. [PubMed] [Google Scholar]
- 4.Waring SC, Doody RS, Pavlik VN, Massman PJ, Chan W. Survival among patients with dementia from a large multi-ethnic population. Alzheimer Dis Assoc Disord. 2005;19:178–183. doi: 10.1097/01.wad.0000189033.35579.2d. [DOI] [PubMed] [Google Scholar]
- 5.Barclay LL, Zemcov A, Blass JP, Sansone J. Survival in Alzheimer's disease and vascular dementias. Neurology. 1985;35:834–840. doi: 10.1212/wnl.35.6.834. [DOI] [PubMed] [Google Scholar]
- 6.Walsh JS, Welch HG, Larson EB. Survival of outpatients with Alzheimer-type dementia. Ann Intern Med. 1990;113:429–434. doi: 10.7326/0003-4819-113-6-429. [DOI] [PubMed] [Google Scholar]
- 7.Brookmeyer R, Corrada MM, Curriero FC, Kawas C. Survival following a diagnosis of Alzheimer disease. Arch Neurol. 2002;59:1764–1767. doi: 10.1001/archneur.59.11.1764. [DOI] [PubMed] [Google Scholar]
- 8.Larson EB, Shadlen MF, Wang L, McCormick WC, Bowen JD, Teri L, Kukull WA. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140:501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 9.Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N, Stern Y. Survival in Alzheimer disease: a multiethnic, population-based study of incident cases. Neurology. 2008;71:1489–1495. doi: 10.1212/01.wnl.0000334278.11022.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta KM, Yaffe K, Pérez-Stable EJ, Stewart A, Barnes D, Kurland BF, Miller BL. Race/ethnic differences in AD survival in US Alzheimer's Disease Centers. Neurology. 2008;70:1163–1170. doi: 10.1212/01.wnl.0000285287.99923.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claus JJ, van Gool WA, Teunisse S, Walstra GJ, Kwa VI, Hijdra A, Verbeeten B, Jr, Koelman JH, Bour LJ, Ongerboer De Visser BW. Predicting survival in patients with early Alzheimer's disease. Dement Geriatr Cogn Disord. 1998;9:284–293. doi: 10.1159/000017073. [DOI] [PubMed] [Google Scholar]
- 12.Lopez OL, Becker JT, Klunk W, Saxton J, Hamilton RL, Kaufer DI, Sweet RA, Cidis Meltzer C, Wisniewski S, Kamboh MI, DeKosky ST. Research evaluation and diagnosis of possible Alzheimer's disease over the last two decades. Part 2. Neurology. 2000;55:1863–1869. doi: 10.1212/wnl.55.12.1863. [DOI] [PubMed] [Google Scholar]
- 13.Moritz DJ, Fox PJ, Luscombe FA, Kraemer HC. Neurological and psychiatric predictors of mortality in patients with Alzheimer disease in California. Arch Neurol. 1997;54:878–885. doi: 10.1001/archneur.1997.00550190066016. [DOI] [PubMed] [Google Scholar]
- 14.Mandrioli J, Faglioni P, Nichelli P, Sola P. Amyotrophic lateral sclerosis: prognostic indicators of survival. Amyotroph Lateral Scler. 2006;7:211–220. doi: 10.1080/17482960600947648. [DOI] [PubMed] [Google Scholar]
- 15.Czaplinski A, Yen AA, Appel SH. Amyotrophic lateral sclerosis: early predictors of prolonged survival. J Neurol. 2006;253:1428–1436. doi: 10.1007/s00415-006-0226-8. [DOI] [PubMed] [Google Scholar]
- 16.Forbes RB, Colville S, Swingler RJ, Scottish ALS/MND Register The epidemiology of amyotrophic lateral sclerosis (ALS/MND) in people aged 80 or over. Age Ageing. 2004;33:131–134. doi: 10.1093/ageing/afh013. [DOI] [PubMed] [Google Scholar]
- 17.Testa D, Lovati R, Ferrarini M, Salmoiraghi F, Filippini G. Survival of 793 patients with amyotrophic lateral sclerosis diagnosed over a 28-year period. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:208–212. [PubMed] [Google Scholar]
- 18.Elbaz A, Bower JH, Peterson BJ, Maraganore DM, McDonnell SK, Ahlskog JE, Schaid DJ, Rocca WA. Survival study of Parkinson disease in Olmsted County, Minnesota. Arch Neurol. 2003;60:91–96. doi: 10.1001/archneur.60.1.91. [DOI] [PubMed] [Google Scholar]
- 19.Fall PA, Saleh A, Fredrickson M, Olsson JE, Granérus AK. Survival time, mortality, and cause of death in elderly patients with Parkinson's disease: a 9-year follow-up. Mov Disord. 2003;18:1312–1316. doi: 10.1002/mds.10537. [DOI] [PubMed] [Google Scholar]
- 20.Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Rudolph A, Lang AE, Parkinson Study Group Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort. Neurology. 2005;64:87–93. doi: 10.1212/01.WNL.0000148603.44618.19. [DOI] [PubMed] [Google Scholar]
- 21.D'Amelio M, Ragonese P, Morgante L, Reggio A, Callari G, Salemi G, Savettieri G. Long-term survival of Parkinson's disease: a population-based study. J Neurol. 2006;253:33–37. doi: 10.1007/s00415-005-0916-7. [DOI] [PubMed] [Google Scholar]
- 22.Jellinger KA, Wenning GK, Seppi K. Predictors of survival in dementia with lewy bodies and Parkinson dementia. Neurodegener Dis. 2007;4:428–430. doi: 10.1159/000107703. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Zhang SM, Schwarzschild MA, Hernán MA, Ascherio A. Survival of Parkinson's disease patients in a large prospective cohort of male health professionals. Mov Disord. 2006;21:1002–1007. doi: 10.1002/mds.20881. [DOI] [PubMed] [Google Scholar]
- 24.Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Parkinson disease and risk of mortality: a prospective comorbidity-matched cohort study. Neurology. 2008;70:1423–1430. doi: 10.1212/01.wnl.0000310414.85144.ee. [DOI] [PubMed] [Google Scholar]
- 25.Herlofson K, Lie SA, Arsland D, Larsen JP. Mortality and Parkinson disease: a community-based study. Neurology. 2004;62:937–942. doi: 10.1212/01.wnl.0000115116.56955.50. [DOI] [PubMed] [Google Scholar]
- 26.Hughes TA, Ross HF, Mindham RH, Spokes EG. Mortality in Parkinson's disease and its association with dementia and depression. Acta Neurol Scand. 2004;110:118–123. doi: 10.1111/j.1600-0404.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 27.Guehne U, Angermeyer MC, Riedel-Heller S. Is mortality increased in mildly cognitively impaired individuals? A systematic literature review. Dement Geriatr Cogn Disord. 2006;21:403–410. doi: 10.1159/000092846. [DOI] [PubMed] [Google Scholar]
- 28.Hunderfund AL, Roberts RO, Slusser TC, Leibson CL, Geda YE, Ivnik RJ, Tangalos EG, Petersen RC. Mortality in amnestic mild cognitive impairment: a prospective community study. Neurology. 2006;67:1764–1768. doi: 10.1212/01.wnl.0000244430.39969.5f. [DOI] [PubMed] [Google Scholar]
- 29.Guehne U, Luck T, Busse A, Angermeyer MC, Riedel-Heller SG. Mortality in individuals with mild cognitive impairment. Results of the Leipzig Longitudinal Study of the Aged. Neuroepidemiology. 2007;29:226–234. doi: 10.1159/000112479. [DOI] [PubMed] [Google Scholar]
- 30.Lee HB, Kasper JD, Shore AD, Yokley JL, Black BS, Rabins PV. Level of cognitive impairment predicts mortality in high-risk community samples: the memory and medical care study. J Neuropsychiatry Clin Neurosci. 2006;18:543–546. doi: 10.1176/jnp.2006.18.4.543. [DOI] [PubMed] [Google Scholar]
- 31.Rascovsky K, Salmon DP, Lipton AM, Leverenz JB, DeCarli C, Jagust WJ, Clark CM, Mendez MF, Tang-Wai DF, Graff-Radford NR, Galasko D. Rate of progression differs in frontotemporal dementia and Alzheimer disease. Neurology. 2005;65:397–403. doi: 10.1212/01.wnl.0000171343.43314.6e. [DOI] [PubMed] [Google Scholar]
- 32.Hodges JR, Davies R, Xuereb J, Kril J, Halliday G. Survival in frontotemporal dementia. Neurology. 2003;61:349–354. doi: 10.1212/01.wnl.0000078928.20107.52. [DOI] [PubMed] [Google Scholar]
- 33.Pasquier F, Richard F, Lebert F. Natural history of frontotemporal dementia: comparison with Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;17:253–257. doi: 10.1159/000077148. [DOI] [PubMed] [Google Scholar]
- 34.Roberson ED, Hesse JH, Rose KD, Slama H, Johnson JK, Yaffe K, Forman MS, Miller CA, Trojanowski JQ, Kramer JH, Miller BL. Frontotemporal dementia progresses to death faster than Alzheimer disease. Neurology. 2005;65:719–725. doi: 10.1212/01.wnl.0000173837.82820.9f. [DOI] [PubMed] [Google Scholar]
- 35.Bonsignore M, Heun R. Mortality in Alzheimer's disease. Dement Geriatr Cogn Disord. 2003;15:231–236. doi: 10.1159/000068779. [DOI] [PubMed] [Google Scholar]
- 36.Carcaillon L, Pérès K, Péré JJ, Helmer C, Orgogozo JM, Dartigues JF. Fast cognitive decline at the time of dementia diagnosis: a major prognostic factor for survival in the community. Dement Geriatr Cogn Disord. 2007;23:439–445. doi: 10.1159/000102017. [DOI] [PubMed] [Google Scholar]
- 37.Helmer C, Andrieu S, Pérès K, Orgogozo JM, Vellas B, Dartigues JF, REAL.fr Group Predictive value of 6-month decline in ADAS-cog for survival without severe Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;23:168–174. doi: 10.1159/000098516. [DOI] [PubMed] [Google Scholar]
- 38.Wilson RS, Li Y, Aggarwal NT, McCann JJ, Gilley DW, Bienias JL, Barnes LL, Evans DA. Cognitive decline and survival in Alzheimer's disease. Int J Geriatr Psychiatry. 2006;21:356–362. doi: 10.1002/gps.1472. [DOI] [PubMed] [Google Scholar]
- 39.Hui JS, Wilson RS, Bennett DA, Bienias JL, Gilley DW, Evans DA. Rate of cognitive decline and mortality in Alzheimer's disease. Neurology. 2003;61:1356–1361. doi: 10.1212/01.wnl.0000094327.68399.59. [DOI] [PubMed] [Google Scholar]
- 40.Dal Forno G, Carson KA, Brookmeyer R, Troncoso J, Kawas CH, Brandt J. APOE genotype and survival in men and women with Alzheimer's disease. Neurology. 2002;58:1045–1050. doi: 10.1212/wnl.58.7.1045. [DOI] [PubMed] [Google Scholar]
- 41.Tilvis RS, Strandberg TE, Juva K. Apolipoprotein E phenotypes, dementia and mortality in a prospective population sample. J Am Geriatr Soc. 1998;46:712–715. doi: 10.1111/j.1532-5415.1998.tb03805.x. [DOI] [PubMed] [Google Scholar]
- 42.Juva K, Verkkoniemi A, Viramo P, Polvikoski T, Kainulainen K, Kontula K, Sulkava R. APOE epsilon4 does not predict mortality, cognitive decline, or dementia in the oldest old. Neurology. 2000;54:412–415. doi: 10.1212/wnl.54.2.412. [DOI] [PubMed] [Google Scholar]
- 43.Slooter AJ, Houwing-Duistermaat JJ, van Harskamp F, Cruts M, Van Broeckhoven C, Breteler MM, Hofman A, Stijnen T, van Duijn CM. Apolipoprotein E genotype and progression of Alzheimer's disease: the Rotterdam Study. J Neurol. 1999;246:304–308. doi: 10.1007/s004150050351. [DOI] [PubMed] [Google Scholar]
- 44.Ebmeier KP, Calder SA, Crawford JR, Stewart L, Besson JA, Mutch WJ. Parkinson's disease in Aberdeen: survival after 3.5 years. Acta Neurol Scand. 1990;81:294–299. doi: 10.1111/j.1600-0404.1990.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez HH, Lapane KL. Predictors of mortality among nursing home residents with a diagnosis of Parkinson's disease. Med Sci Monit. 2002;8:CR241–CR246. [PubMed] [Google Scholar]
- 46.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12:462–468. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 47.Binetti G, Locascio JJ, Corkin S, Vonsattel JP, Growdon JH. Differences between Pick disease and Alzheimer disease in clinical appearance and rate of cognitive decline. Arch Neurol. 2000;57:225–232. doi: 10.1001/archneur.57.2.225. [DOI] [PubMed] [Google Scholar]
- 48.Chan D, Fox NC, Jenkins R, Scahill RI, Crum WR, Rossor MN. Rates of global and regional cerebral atrophy in AD and frontotemporal dementia. Neurology. 2001;57:1756–1763. doi: 10.1212/wnl.57.10.1756. [DOI] [PubMed] [Google Scholar]
- 49.Pinsky PF, Miller A, Kramer BS, Church T, Reding D, Prorok P, Gelmann E, Schoen RE, Buys S, Hayes RB, Berg CD. Evidence of a healthy volunteer effect in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2007;165:8874–8881. doi: 10.1093/aje/kwk075. [DOI] [PubMed] [Google Scholar]
- 50.Steenland K, Henley J, Thun M. All cause and cause-specific mortality by educational status in two million people in two ACS cohorts. Am J Epidemiol. 2002;156:11–21. doi: 10.1093/aje/kwf001. [DOI] [PubMed] [Google Scholar]
- 51.de Lau LM, Schipper CM, Hofman A, Koudstaal PJ, Breteler MM. Prognosis of Parkinson disease: risk of dementia and mortality: the Rotterdam Study. Arch Neurol. 2005;62:1265–1269. doi: 10.1001/archneur.62.8.1265. [DOI] [PubMed] [Google Scholar]
- 52.Feldman B, Chapman J, Korczyn AD. Apolipoprotein epsilon4 advances appearance of psychosis in patients with Parkinson's disease. Acta Neurol Scand. 2006;113:14–17. doi: 10.1111/j.1600-0404.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- 53.Steenland K, Macneil J, Vega I, Levey A. Recent mortality patterns in Alzheimer's Disease. Alzheimer Dis Assoc Disord. 2009;23:165–170. doi: 10.1097/wad.0b013e3181902c3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 55.Evans DA, Bennett DA, Wilson RS, Bienias JL, Morris MC, Scherr PA, Hebert LE, Aggarwal N, Beckett LA, Joglekar R, Berry-Kravis E, Schneider J. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 56.Fillenbaum GG, Heyman A, Huber MS, Woodbury MA, Leiss J, Schmader KE, Bohannon A, Trapp-Moen B. The prevalence and 3-year incidence of dementia in older Black and White community residents. J Clin Epidemiol. 1998;51:587–595. doi: 10.1016/s0895-4356(98)00024-9. [DOI] [PubMed] [Google Scholar]