Abstract
The two enzymes required for de novo glutathione synthesis, glutamyl cysteine synthetase and glutathione synthetase, have been demonstrated in hemolysates of human erythrocytes. Glutamyl cysteine synthetase requires glutamic acid, cysteine, adenosine triphosphate (ATP), and magnesium ions to form γ-glutamyl cysteine. The activity of this enzyme in hemolysates from 25 normal subjects was 0.43±0.04 μmole glutamyl cysteine formed per g hemoglobin per min. Glutathione synthetase requires γ-glutamyl cysteine, glycine, ATP, and magnesium ions to form glutathione. The activity of this enzyme in hemolysates from 25 normal subjects was 0.19±0.03 μmole glutathione formed per g hemoglobin per min. Glutathione synthetase also catalyzes an exchange reaction between glycine and glutathione, but this reaction is not significant under the conditions used for assay of hemolysates. The capacity for erythrocytes to synthesize glutathione exceeds the rate of glutathione turnover by 150-fold, indicating that there is considerable reserve capacity for glutathione synthesis. A patient with erythrocyte glutathione synthetase deficiency has been described. The inability of patients' extracts to synthesize glutathione is corrected by the addition of pure glutathione synthetase, indicating that there is no inhibitor in the patients' erythrocytes.
Full text
PDF

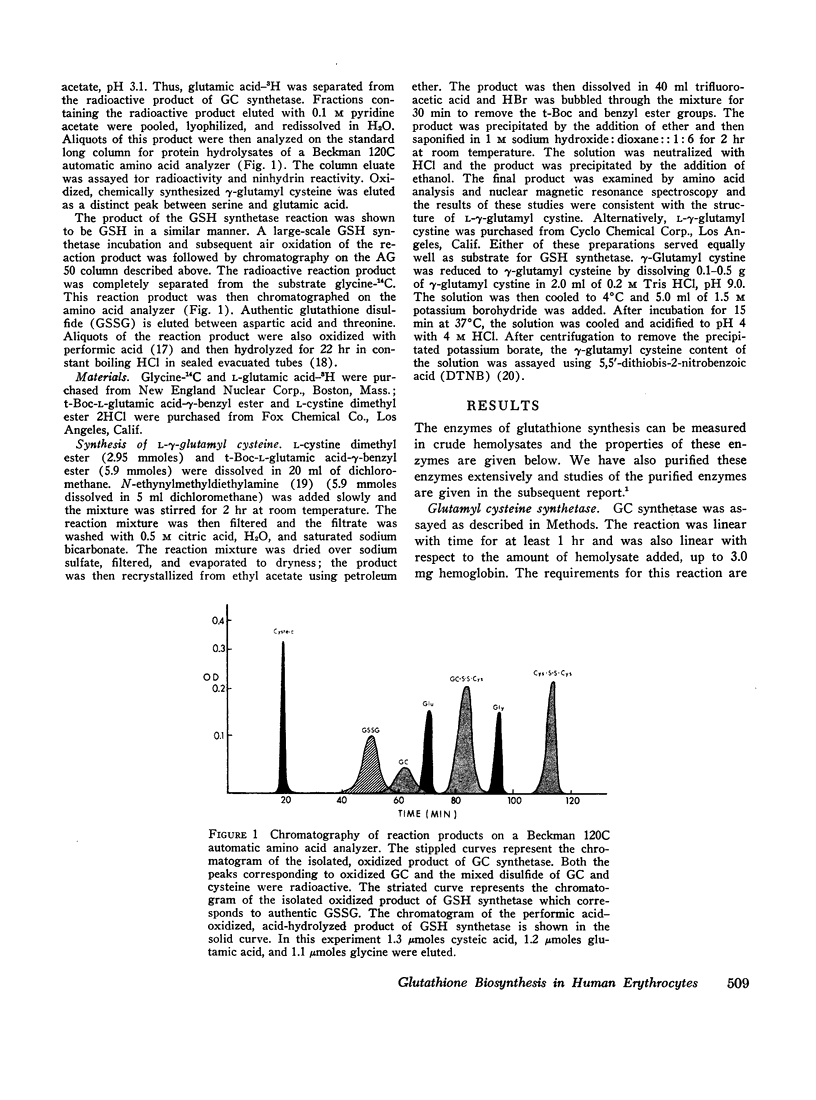

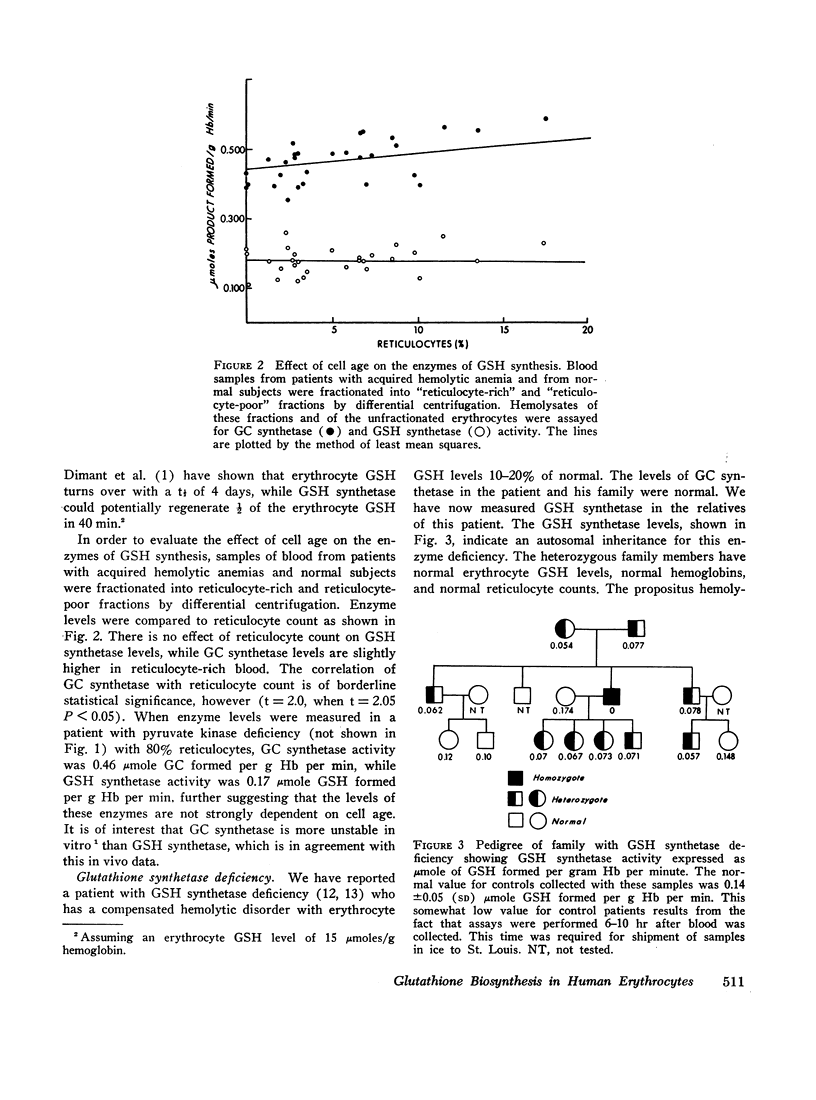


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boivin P., Galand C. La synthèse du glutathion au cours de l'anémie hémolytique congénitale avec déficit en glutathion réduit. Déficit congénital en glutathion-synthérase érythrocytaire? Nouv Rev Fr Hematol. 1965 Sep-Oct;5(5):707–720. [PubMed] [Google Scholar]
- DIMANT E., LANDSBERG E., LONDON I. M. The metabolic behavior of reduced glutathione in human and avian erythrocytes. J Biol Chem. 1955 Apr;213(2):769–776. [PubMed] [Google Scholar]
- ELDER H. A., MORTENSEN R. A. The incorporation of labeled glycine into erythrocyte glutathione. J Biol Chem. 1956 Jan;218(1):261–267. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- HOCHBERG A., RIGBI M., DIMANT E. THE INCORPORATION IN VITRO OF GLYCINE AND L-GLUTAMIC ACID INTO GLUTATHIONE OF HUMAN ERYTHROCYTES. Biochim Biophys Acta. 1964 Sep 4;90:464–471. doi: 10.1016/0304-4165(64)90225-9. [DOI] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. II. Studies in vivo. J Clin Invest. 1962 Jul;41:1514–1523. doi: 10.1172/JCI104607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. C. Studies in the enzymology of glutathione metabolism in human erythrocytes. Biochem J. 1969 Feb;111(3):309–315. doi: 10.1042/bj1110309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASBEKAR D. K., SREENIVASAN A. Biosynthesis of glutathione by rat erythrocytes. Biochem J. 1959 Jul;72:389–395. doi: 10.1042/bj0720389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELES S., BLOCK K. Enzymatic synthesis of gamma-glutamylcysteine. J Biol Chem. 1955 Jun;214(2):639–646. [PubMed] [Google Scholar]
- MORTENSEN R. A., HALEY M. I., ELDER H. A. The turnover of erythrocyte glutathione in the rat. J Biol Chem. 1956 Jan;218(1):269–273. [PubMed] [Google Scholar]
- Majerus P. W., Lastra R. Fatty acid biosynthesis in human leukocytes. J Clin Invest. 1967 Oct;46(10):1596–1602. doi: 10.1172/JCI105651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Smith M. B., Clamon G. H. Lipid metabolism in human platelets. I. Evidence for a complete fatty acid synthesizing system. J Clin Invest. 1969 Jan;48(1):156–164. doi: 10.1172/JCI105964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler D. N., Majerus P. W., Minnich V., Hess C. E., Garrick M. D. Glutathione synthetase deficiency as a cause of hereditary hemolytic disease. N Engl J Med. 1970 Dec 3;283(23):1253–1257. doi: 10.1056/NEJM197012032832304. [DOI] [PubMed] [Google Scholar]
- Prins H. K., Oort M., Zürcher C., Beckers T. Congenital nonspherocytic hemolytic anemia, associated with glutathione deficiency of the erythrocytes. Hematologic, biochemical and genetic studies. Blood. 1966 Feb;27(2):145–166. [PubMed] [Google Scholar]
- SNOKE J. E., BLOCH K. Studies on the mechanism of action of glutathione synthetase. J Biol Chem. 1955 Apr;213(2):825–835. [PubMed] [Google Scholar]
- SNOKE J. E., YANARI S., BLOCH K. Synthesis of glutathione from gamma-glutamylcysteine. J Biol Chem. 1953 Apr;201(2):573–586. [PubMed] [Google Scholar]
- Sass M. D. Glutathione synthesis in cell-free preparations from erythrocytes of different ages. Clin Chim Acta. 1968 Oct;22(2):207–210. doi: 10.1016/0009-8981(68)90359-8. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. The transport of oxidized glutathione from human erythrocytes. J Biol Chem. 1969 Jan 10;244(1):9–16. [PubMed] [Google Scholar]



