Abstract
The purpose of this study was to ascertain the relative contribution of neural stem/progenitor cells (NSPCs) of the subventricular zone (SVZ) to lineages that repopulate the injured striatum following focal ischemia. We utilized a tamoxifen-inducible Cre/loxP system under control of the nestin promoter, which provides permanent YFP labeling of multipotent nestin+ SVZ-NSPCs prior to ischemic injury and continued YFP expression in all subsequent progeny following stroke. YFP reporter expression was induced in adult male nestin-CreERT2:R26R-YFP mice by tamoxifen administration (180 mg/kg, daily for 5 days). Fourteen days later, mice were subjected to 60 minute transient middle cerebral artery occlusion (MCAO) and sacrificed at 2 days, 2 weeks or 6 weeks post-MCAO for phenotypic fate mapping of YFP+ cells using lineage-specific markers. Migration of YFP+ cells from SVZ into the injured striatal parenchyma was apparent at 2 and 6 weeks, but not 2 days, post-MCAO. At 2 weeks post-MCAO, the average percent distribution of YFP+ cells within the injured striatal parenchyma was as follows: 10% Dcx+ neuroblasts, 15–20% oligodendrocyte progenitors, 59% GFAP+ astrocytes, and only rare NeuN+ postmitotic neurons. A similar phenotypic distribution was observed at 6 weeks, except for an increased average percentage of YFP+ cells that expressed Dcx+ (20%) or NeuN (5%). YFP+ cells did not express endothelial markers, but displayed unique anatomical relationships with striatal vasculature. These results indicate that nestin+ NSPCs within the SVZ mount a multilineage response to stroke that includes a gliogenic component more predominant than previously appreciated.
INTRODUCTION
A promising discovery in stroke research within the past decade is the SVZ response to cerebral ischemia, which may contribute importantly to recovery and repair processes. Focal cerebral ischemia stimulates a cytogenic response from SVZ of both adult rodent (Arvidsson et al. 2002)and human brain (Jin et al. 2006; Macas et al. 2006; Marti-Fabregas et al.). This response is characterized by proliferation of neural stem/progenitor cells (NSPCs) within the SVZ and heterotypic migration of neuroblasts from SVZ into the ischemic brain parenchyma. Although most ectopic neuroblasts undergo cell death within the injured parenchyma, a small number survive and give rise to postmitotic neurons that replace ~0.2% of neurons that are lost to injury within the peri-infarct region (Arvidsson et al. 2002; Thored et al. 2006). In addition to neurogenesis, gliogenesis and angiogenesis contribute to brain repair following stroke, but the relative contribution of the SVZ to new glial or endothelial cell phenotypes following stroke has not been well characterized.
To ascertain the relative contribution of the SVZ-NSPCs to lineages that repopulate striatum following focal cerebral ischemia, we utilized a tamoxifen-inducible Cre/loxP system under control of the nestin promoter to genetically label a large cohort of nestin+ NSPCs within the SVZ prior to the onset of ischemic injury. Administration of tamoxifen to nestin-CreERT2:R26R-YFP bitransgenic mice transiently activates Cre-mediated recombination, resulting in permanent excision of a floxed transcriptional stop sequence upstream of a YFP reporter gene sequence within nestin+ cells (Lagace et al. 2007). This leads to permanent YFP reporter gene expression by NSPCs and all subsequent progeny, thereby providing a genetic marker for tracking the migration of nestin derivatives and for phenotypic fate mapping. Here, we describe the use of nestin-CreERT2:R26R-YFP mice to fate map both neuronal and non-neuronal SVZ derivatives following focal cerebral ischemia induced by transient middle cerebral artery occlusion (MCAO). Our results indicate that the cytogenic response of nestin+ NSPCs within the SVZ is primarily gliogenic following stroke.
MATERIALS and METHODS
Mice
Animal experiments were approved by the University of New Mexico Animal Care and Use Committee in accordance with the NIH Guide for the Care and use of Laboratory Animals. The generation and characterization of the nestin-CreERT2:R26R-YFP strain used in this study were previously described (Lagace et al. 2007). All mice were housed under a 12 h light: 12 h dark cycle with food and water available ad libitum.
Tamoxifen administration
Male nestin-CreERT2:R26R-YFP mice (6–8 weeks old) were administered tamoxifen dissolved in 10% EtOH/90% sunflower oil intraperitoneally (i.p) at the dose of 180 mg/kg daily for 5 consecutive days. Control mice received vehicle injections. This dosing regimen was previously demonstrated to provide maximal recombination with minimal mortality (Lagace et al. 2007)
Middle Cerebral Artery Occlusion (MCAO)
Two weeks following the final injection of tamoxifen or vehicle, nestin-CreERT2:R26R-YFP mice received 60 minute transient MCAO or sham MCAO by intraluminal filament as published previously (Kokovay et al. 2006).
Histology
Mice were overdosed with sodium pentobarbital (150 mg/kg, i.p.; Fort Dodge Animal Health, Fort Dodge, IA), and transcardially perfused with phosphate-buffered saline (PBS) containing 0.1% procaine and 2 U/ml heparin, followed by 4% paraformaldehyde (w/v) in 0.1 M PBS. The brains were post-fixed overnight, cryoprotected with 30% sucrose (w/v) in 0.1 M PBS for 48 hours at 4°C and sectioned at 30 µm thickness in the coronal plane using a freezing sliding knife mictrotome. Floating sections were processed for immunofluorescence as previously published (Roitbak et al. 2008) using the following primary antibodies: goat anti-doublecortin (Dcx; 1:200; Santa Cruz Biotechnology, Santa Cruz CA), mouse anti-glial fibrillary acidic protein (GFAP; 1:500, Sigma, St. Louis, MO), rabbit anti-GLUT-1 (1:200, Abcam, Cambridge, MA), rabbit anti-NG2 (1:200; Millipore, Billerica, MA), rabbit anti-Olig2 (1:1000; Millipore, Billerica, MA), rabbit anti-laminin (1:500; Sigma, St. Louis, MO), chicken anti-GFP (1:1000; Invitrogen, Carlsbad, CA), rabbit anti-Iba-1 (1:500; Wako Chemicals USA, Richmond, VA). Immunofluorescence was visualized using FITC-, CY3- or Cy5-conjugated secondary antibodies (1:250; Jackson ImmunoResearch Laboratories, West Grove, PA). YFP immunofluorescence was visualized using biotinylated donkey anti-chicken secondary antibody and Tyramide-Plus amplification (PerkinElmer Life Sciences, Boston, MA) as previously described (Lagace et al. 2007). For Fluoro-Jade staining, histological sections were incubated with 0.05% potassium permanganate for 15 mins, followed by 30 mins in 0.001% Fluoro-Jade (Histo-Chem Inc., Jefferson, AR).
Coexpression of YFP with lineage-specific markers was quantified by random sampling of at least 100 YFP+ cells per mouse for each marker using a 40× oil objective on a Zeiss LSM510 confocal microscope, coupled with rapid z-axis analysis at 1 µm optical section thickness. YFP+ cells were scored as positive or negative for coexpression of each marker. Data are presented as the mean percentage of YFP+ cells coexpressing Dcx, NeuN, NG2, Olig2, or GFAP ± SEM. Statistical analysis was performed using Students t-test, comparing data acquired from 2 vs. 6 week post-MCAO with p<0.05 considered significant.
RESULTS
Stroke induces delayed migration of YFP reporter+ cells from SVZ into adjacent striatal parenchyma
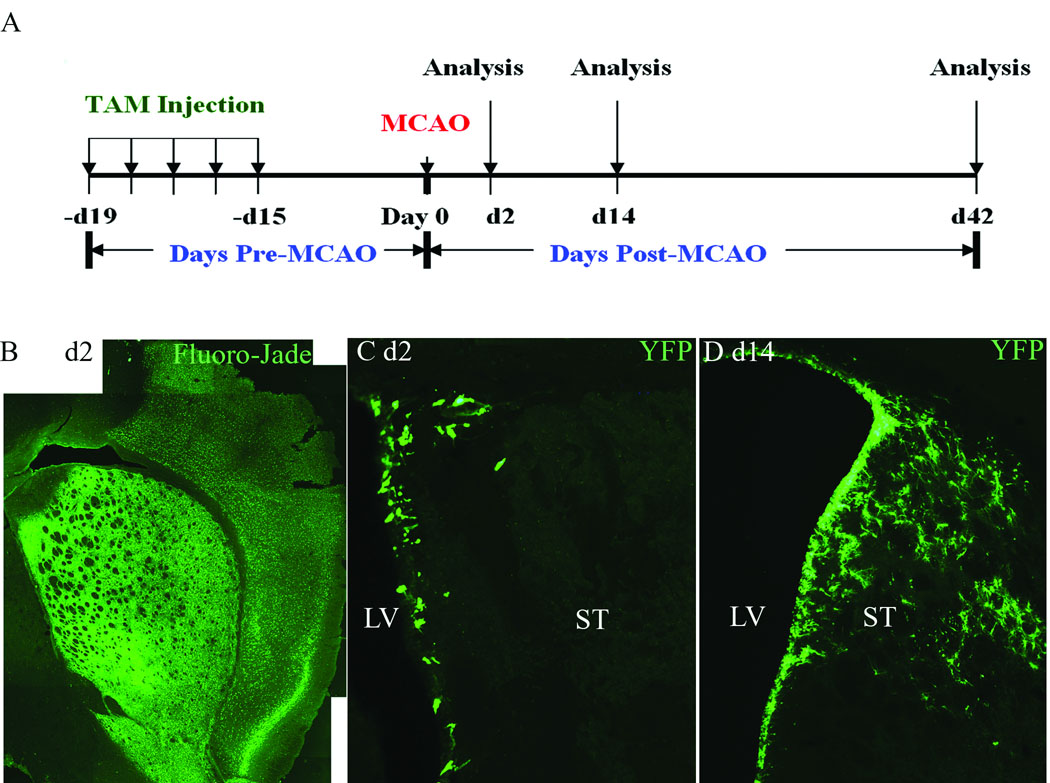
We utilized a previously published protocol for achieving maximum Cre-mediated recombination in the nestin-CreERT2:R26R-YFP mice by administration of tamoxifen (180 mg/kg, i.p.) daily for five consecutive days (Lagace et al. 2007). Two weeks were allowed following the final injection for tamoxifen clearance, and the mice were then subjected to 60 minute transient MCAO (n=12) or sham MCAO (n=2). Nestin-CreERT2:R26R-YFP mice that received vehicle i.p. injections served as additional controls (see below). Mice were sacrificed after 2 days (n=4), 2 weeks (n=4) or 6 weeks (n=4) post-MCAO, and brain tissue was processed for histological evaluation of stroke-induced neurodegeneration, distribution of YFP+ cells and phenotypic fate of YFP+ cells using lineage-specific markers (see Figure 1 A for experimental design).
Figure 1.
A) Experimental design. B) Fluoro-Jade at day 2 post-MCAO. C) YFP immunofluorescence 2 days post-MCAO. D) YFP immunofluorescence at 2 weeks post-MCAO. LV (lateral ventricle), ST (Striatum). Scale Bars = 100 µm.
Neurodegeneration throughout the striatum was apparent as early as 2 days post-MCAO, as evidenced by positive Fluoro-Jade staining throughout the striatum and parietal cortex on the ischemic side (Figure 1 B), however, YFP+ cells remained localized to the SVZ and were not observed within the adjacent striatal parenchyma at this early post-injury time point (Figure 1 C). By two weeks post-MCAO, the density of YFP reporter+ cells was increased throughout the SVZ and adjacent ischemic striatum, heavily populating the striatal parenchyma on the ischemic side (Figure 1 D). A similar distribution and density of YFP+ cells within the injured striatum was apparent at 6 weeks (Figure S1 A, online). YFP reporter+ cells were not present within the contralateral striatum of tamoxifen-induced nestin-CreERT2:R26R-YFP MCAO mice (Figure S1 B, online), or within the ipsilateral striatum of tamoxifen-induced mice that received sham MCAO surgery (Figure S1 C, online).
YFP reporter fidelity is maintained in adult nestin-CreERT2:R26R-YFP mice following stroke
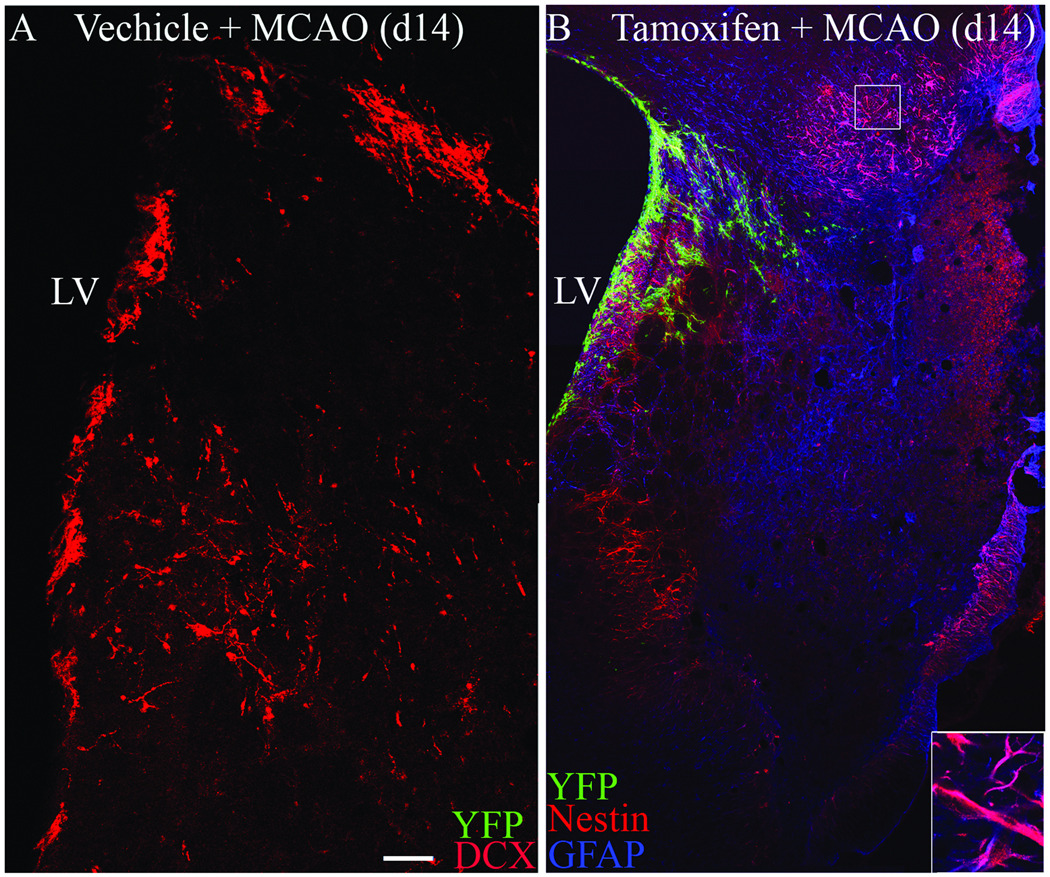
Nestin-CreERT2:R26R-YFP mice that received vehicle i.p. injections were used to verify the stringency of Cre-mediated recombination under conditions of cerebral ischemia. In contrast to tamoxifen-treated mice, YFP+ cells were not observed within the SVZ or within the injured striatal parenchyma of vehicle-injected nestin-CreERT2:R26R-YFP mice as assessed at 2 weeks post-MCAO. However, Dcx+ migrating neuroblasts were readily detected in vehicle-treated mice at this time point, confirming a normal neurogenic response to stroke in this control group (Figure 2 A). Thus, Cre-mediated recombination does not occur in the absence of tamoxifen and does not occur spontaneously in response to MCAO injury in vehicle-treated nestin-CreERT2:R26R-YFP mice. In mice treated with tamoxifen 2 weeks prior to MCAO, YFP expression was also not observed within reactive astrocytes that upregulate nestin following MCAO (Figure 2 B), indicating that the fourteen day washout interval between tamoxifen administration and MCAO was sufficient time to ensure that all recombination occurred prior to the onset of ischemic injury. Taken together, these results verify the fidelity of the nestin-CreERT2 system under conditions of focal cerebral ischemia induced by transient 60 minute MCAO.
Figure 2.
A) Dcx (red) and YFP (green) immunofluorescence in vehicle-injected control Nestin-CreERT2:R26R-YFP mice. B) Nestin (red), GFAP (blue) and YFP (green) immunofluorescence in tamoxifen-treated Nestin-CreERT2:R26R-YFP mice at d14 post-MCAO. LV (lateral ventricle). Scale bar = 100 µm.
Multilineage analysis of YFP reporter+ cells
Neuronal lineage
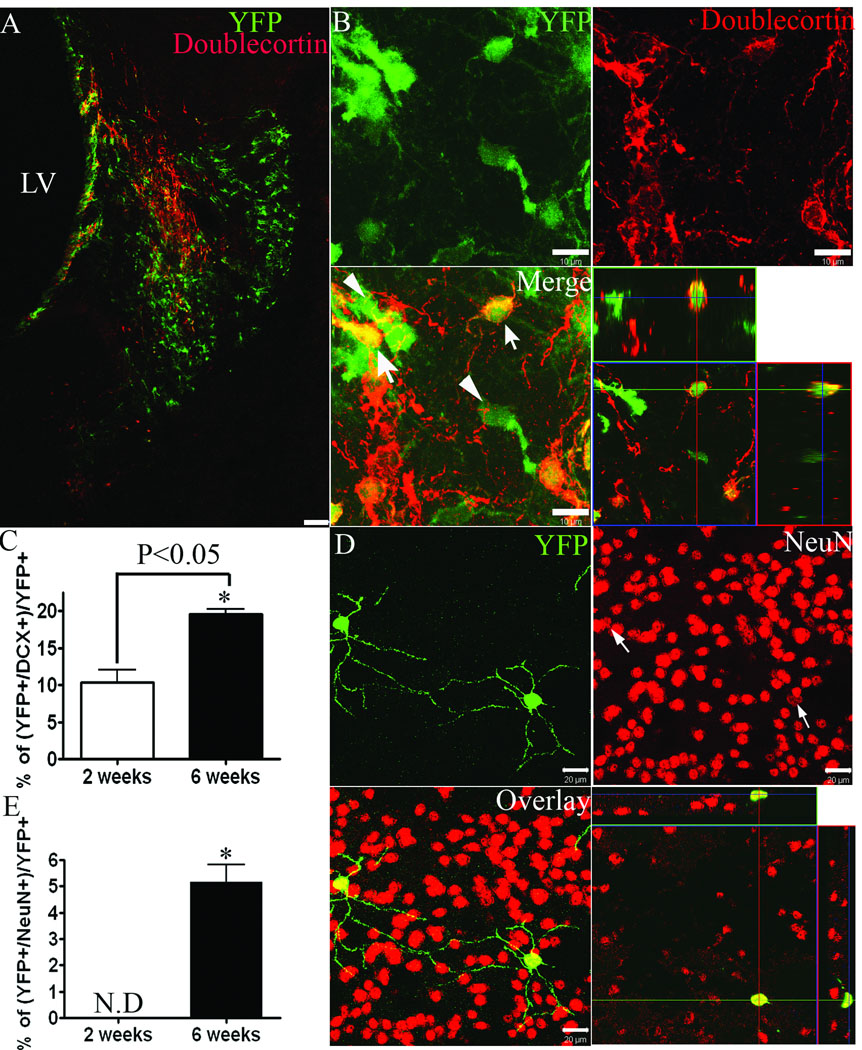
To determine the relative proportion of YFP reporter+ cells within the ischemic striatum that represent migratory neuroblasts and/or postmitotic neurons, histological sections from tamoxifen-treated mice were assessed for co-labeling of YFP with Dcx or NeuN immunofluorescence, respectively. Dcx+ neuroblasts were observed throughout the ischemic striatum (Figure 3 A), but were not present within the contralateral striatum or within the striatum of sham-operated controls (data not shown). Dcx+ cells comprised 10 ± 3 % and 20 ± 1% of all YFP+ cells within the injured striatum at 2 and 6 weeks post-MCAO, respectively (Figure 3 A–C). The majority of Dcx+ cells within the injured straitum were negative for YFP reporter expression at both 2 and 6 weeks post-MCAO (80 ± 3% and 67 ± 17%, respectively). YFP+ cells within the injured striatum that co-expressed the mature neuronal marker, NeuN, were only rarely observed at 2 weeks (<1 NeuN+/YFP+ colabeled cell per histological section, data not shown), but represented 5 ± 1% of all YFP+ cells by 6 weeks post-MCAO (Figure 3 D,E).
Figure 3.
A) Doublecortin (red) and YFP (green) immunofluorescence at 2 weeks post-MCAO. B) Higher power images with orthogonal view of confocal z-stack. DCX+/YFP+ cells (arrows) and DCX−/YFP+ cells (arrowheads). C, E) Percentage of YFP reporter+ cells that co-express Dcx (C) or NeuN (E). D) NeuN+ (red) and YFP (green) immunofluorescence at 6 weeks post-MCAO. ND = <1 cell per section. *p<0.05. Scale bars = 100 µm (A) or 10 µm (B,D).
Oligodendrocyte lineage
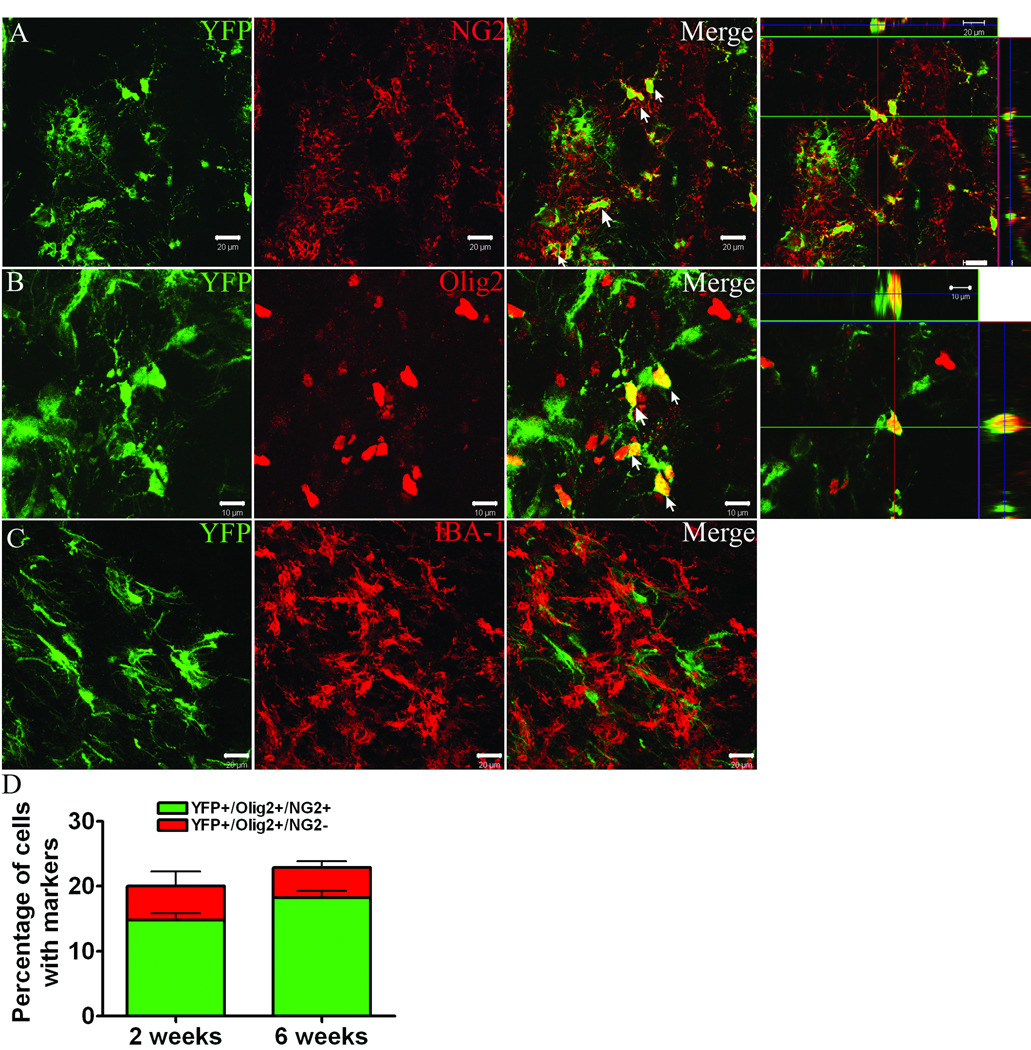
To determine the relative contribution of YFP+ cells to the oligodendrocyte lineage we utilized NG2 proteoglycan and the transcription factor, Olig2, as markers of oligodendrocyte progenitors (Figure 4 A,B). Triple labeling revealed that 15 ± 2% and 19 ± 4% of all YFP+ cells within injured striatum co-expressed both NG2 and Olig2 markers at 2 and 6 weeks post-MCAO, respectively (Figure 4 D). Only a small percentage of YFP+ cells were Olig2+/NG2− (5 ± 2% and 5 ± 1% at 2 and 6 weeks, respectively). YFP+ oligodendrocyte progenitors were abundant throughout the penumbral region that spanned from lateral ventricle to infarct boundary, but were sparse within the SVZ in both lesioned and unlesioned hemispheres (Figure S2 A, online). YFP+ oligodendrocyte progenitors displayed a multi-processed morphology and were negative for the microglial marker, Iba1 (Figure 4 C). YFP+ cells immunofluorescent for the mature oligodendrocyte markers, CC1 or RIP, were not observed at 2 or 6 weeks post-MCAO, although YFP+ cells with morphology reminiscent of mature oligodendrocytes were observed within the corpus callosum (Figure S2 B, online).
Figure 4.
Dual immunofluorescence for YFP (green) with A) NG2 (red) B) Olig2 (red) and C) Iba-1 (red) at 2 weeks post-MCAO. D) Percentage of YFP+ cells that express Olig2 and NG2 at 2 and 6 weeks post-MCAO. Scale bars = 20 µm (A,C) or 10 µm (B).
Astrocyte lineage
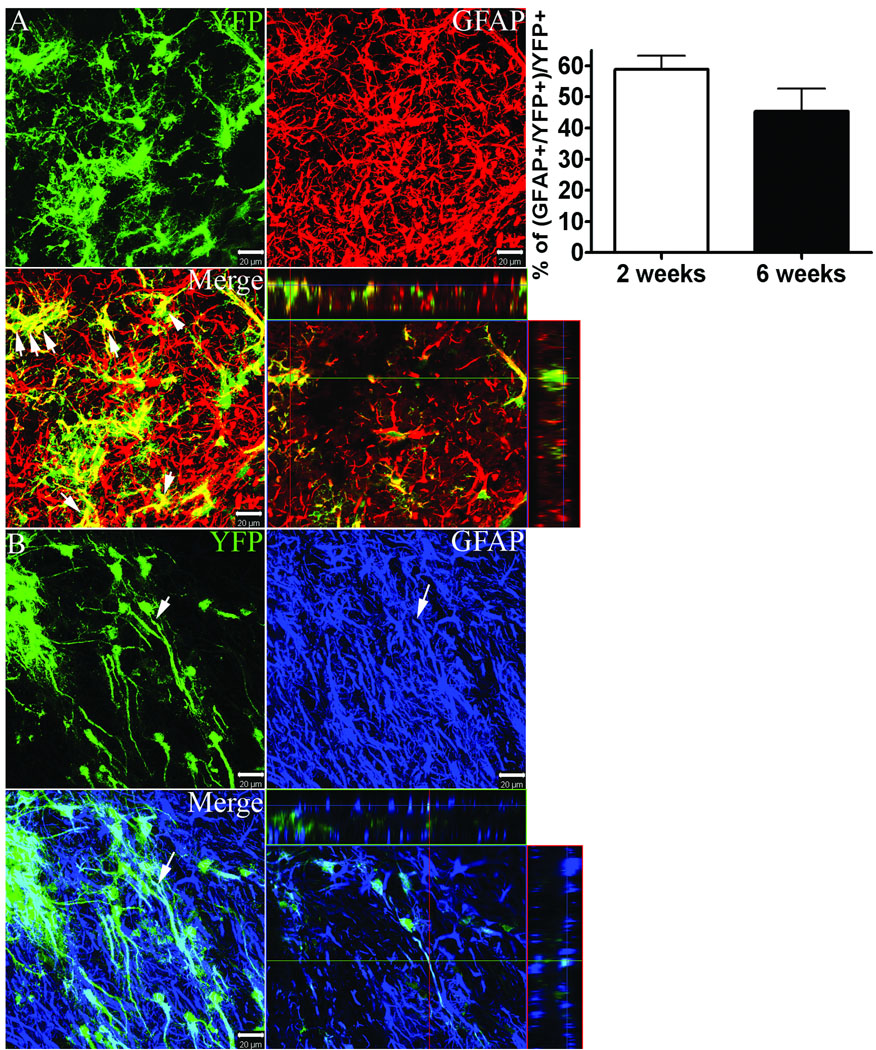
YFP reporter+ cells that co-expressed the astrocytic marker, GFAP, were abundant within the penumbral regions between lateral ventricle and ischemic core; 58 ± 4% and 45 ± 7% of all YFP+ cells were co-labeled with GFAP at 2 and 6 weeks, respectively (Figure 5). GFAP+/YFP+ co-labeled cells displayed diverse morphologies ranging from multipolar highly branched hypertrophied astrocytes (Figure 5 A) to radial glia-like morphologies characterized by long slender sparsely branched processes (Figure 5 B).
Figure 5.
Dual immunofluorescence for YFP (green) with GFAP (red or blue) at 2 weeks post-MCAO. Graph depicts the percentage of YFP+ cells that express GFAP at 2 and 6 weeks post-MCAO. Scale bars = 20 µm.
YFP+ cells display unique anatomical relationships with striatal vasculature
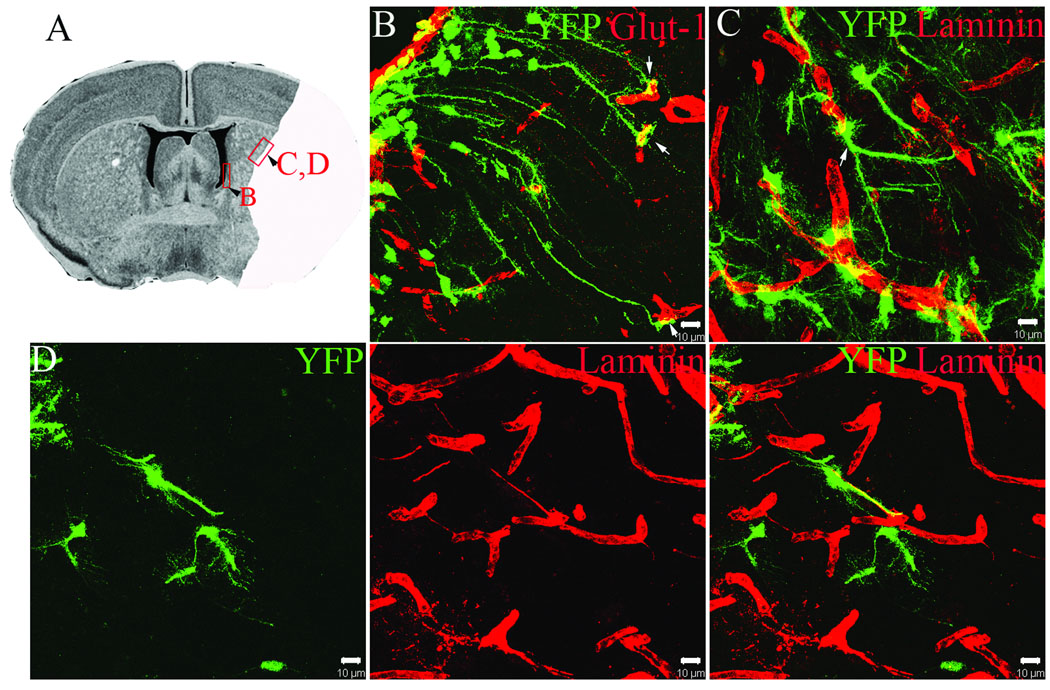
Previous studies have demonstrated that exposure of isolated neural stem cells to endothelial cells in co-culture can induce neural stem cell expression of endothelial lineage markers (Roitbak et al. 2008). Since stroke-induced neurogenesis is thought to occur within an angiogenic niche, we assessed vascular phenotype in YFP+ cells in the MCAO paradigm using laminin and the high affinity Glut-1 glucose transporter. Although we did not observe YFP+ cells that adopted an endothelial cell fate, co-labeling with endothelial markers revealed a striking relationship of YFP+ cells with microvasculature, both within the SVZ and within the ischemic striatum. As shown in Figure 6, YFP reporter+ cells within the SVZ were found to extend long radial processes from the SVZ into the surrounding brain parenchyma to terminate an endfoot-like terminal on capillaries (Figure 6 B). These cells likely represent B1 type astrocytic stem cells (Doetsch 2003). These cells were observed within the base of the SVZ at the level of medial septum, near the nucleus accumbens on both injured and uninjured hemispheres, and extended processes up to 180 µm in length to terminate on blood vessels. Most SVZ-derived YFP reporter+ cells that had migrated into ischemic striatum were found in close physical contact with vasculature, with soma either directly juxtaposed to blood vessels or contacting blood vessels via endfoot-like processes (Figure 6 C, D). In some cases, YFP+ cells were closely associated with fine endothelial extensions that connected adjacent capillaries reminiscent of angiogenic microvasculature (Figure 6 D).
Figure 6.
A) Boxed images on histological section are shown in higher power in B–D. Solid area represents region of ischemic damage. B) Dual immunofluorescence for YFP (green) and GLUT-1 (red) demonstrating YFP+ processes from radial glial like cells in the SVZ with endothelial end-feet. C) YFP reporter+ cells (green) that have migrated into the ischemic border zone and are associated with laminin+ (red) cerebral blood vessels. D) YFP reporter+ cells (green) within the ischemic striatum associated with processes of laminin+ (red) angiogenic blood vessels. Scale bars = 10 µm (B–D).
DISCUSSION
In the present study, we utilized tamoxifen inducible nestin-CreERT2:R26R-YFP mice to label a large cohort of NSPCs within the adult subventricular zone prior to injury and followed migration and lineage fate of the NSPCs out to 6 weeks post-MCAO. This genetic fate-mapping approach permits long-term evaluation of a large cohort of SVZ NSPCs using an irreversible YFP reporter system, thereby enabling a comprehensive examination of lineage fate following stroke.
We found that Dcx+ neuroblasts accounted for up to 20% of all YFP+ cells within the ischemic striatal parenchyma by 6 weeks post-MCAO. In mouse, transgenic ablation of Dcx-expressing cells worsens stroke outcome (Jin et al., 2010) indicating that these cells may be an important therapeutic target. Although NeuN+/YFP+ co-labeled cells were only sparsely represented at 2 weeks, their numbers increased to 5% of all YFP+ cells by 6 weeks post-MCAO. Since approximately 80% of migrating neuroblasts were YFP− in our study, it is likely that up to 20% of all neuroblasts survive to become NeuN+ postmitotic neurons by 6 weeks post-MCAO, which is consistent with previous estimates (Arvidsson et al. 2002). The presence of Dcx+/YFP− cells may reflect suboptimal labeling efficiency within SVZ, since previous studies have demonstrated that Dcx+ neuroblasts within ischemic striatum arise primarily from SVZ astrocytic stem cells of the nestin lineage (Yamashita et al. 2006). A previous study by Burns et al. (Burns et al. 2007) using a different strain of tamoxifen-inducible nestin-CreER:GFP bitransgenic mice reported only very few GFP-positive cells outside the SVZ following MCAO. Ectopic migration of Dcx+ neuroblasts into the ipsilateral striatum was negligible in that study, possibly due to the early time point of analysis at 1 week post-MCAO or due to less effective MCAO-induced injury.
We found that 18–21% of all YFP reporter+ cells within the injured striatal parenchyma at 6 weeks post-MCAO represent NG2+/Olig2+ oligodendrocyte progenitor cells. NG2 is a chondroitin sulfate proteoglycan expressed on the surface of oligodendrocyte progenitors (Nishiyama et al. 2009), and Olig2 is a transcription factor necessary for the development of the oligodendrocyte lineage (Takebayashi et al. 2002). In adult brain, the majority of Olig2+ progenitor cells within the white matter transiently co-express NG2 and eventually give rise to NG2− oligodendrocytes, whereas in grey matter most Olig2+ cells remain as non-proliferating Olig2+/NG2+ progenitors (Dimou et al. 2008). Our study suggests that the latter population of oligodendrocyte progenitors is partially replaced by SVZ-NSPCs following stroke, since we did not observe co-labeling for mature oligodendrocyte markers within the ischemic parenchyma, even by 6 weeks.
GFAP+ astrocytes comprise the largest fraction of all YFP reporter+ cells within the ischemic striatum at both 2 weeks (59%) and 6 weeks (45%) post-MCAO. Astrocytes are a heterogeneous population of cells within the CNS that play both beneficial and detrimental roles following injury (Sofroniew 2009). Glial scarring within the CNS is recognized as a major impediment to neurite outgrowth and regeneration (Silver and Miller 2004). On the other hand, ablation of reactive astrocytes in adult mice (Bush et al. 1999) or disruption of cytokine signaling in reactive astrocytes (Herrmann et al. 2008; Okada et al. 2006) leads to spread of inflammation and increased injury responses. Furthermore, GFAP-null mice demonstrate increased kainate excitoxicity (Otani et al. 2006). Pharmacological inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia (Hayakawa et al., 2010). Recent studies have shown that neural stem cells within the spinal cord give rise to a functionally distinct subset of astrocytes following injury, which do not produce axonal growth inhibitory chondroitin sulfate proteoglycans (CSPGs) and appear to promote axonal sprouting (Meletis et al. 2008). Buffo et al. recently reported that mature astrocytes have the capacity to re-enter the cell cycle, dedifferentiate and resume multipotency in response to brain injury (Buffo et al. 2008). Future studies will be required to determine the extent to which SVZ-derived astrocytes within the ischemic parenchyma promote regenerative processes vs. contribute to glial scarring and inhibition of neurite outgrowth following focal ischemia.
YFP reporter+ cells displayed unique anatomical association with microvasculature within the SVZ and ischemic striatal parenchyma. Many YFP+ cells within the SVZ were found to extend a long basal process from the SVZ into the brain parenchyma to make contact with blood vessels through an endfoot-like structure. Mirzadeh et al. recently demonstrated that >95% of type B1 astrocytes within the SVZ have an apical ciliated process that is in contact with the ventricle, and a long basal process that extends several hundred microns into the brain parenchyma to contact a blood vessel via an endfoot (Mirzadeh et al. 2008). The functional significance of vascular contact of neural stem cells may reflect the need for vascular-derived factors important in maintaining the stem-cell phenotype (Shen et al. 2004), or allow signals from CSF and bloodstream to be integrated (Tavazoie et al. 2008). Neural stem cells within the SVZ are also associated with extravascular basal lamina unique to the subependymal layer, termed fractones, purported to provide a specialized extracellular matrix within the neural stem cell niche (Mercier et al. 2002).
YFP+ cells were also found in close association with the microvasculature throughout the injured striatum. This anatomical relationship likely reflects the use of vascular basal lamina as a migratory substrate (Ohab et al. 2006), and/or the importance of reciprocal signaling between SVZ derivatives and nascent vasculature during the repair process (Louissaint et al. 2002; Roitbak et al. 2008). The migration of neuroblasts along nonstereotypical routes toward the injured area occurs in response to chemotactic growth factors and chemokines that also signal angiogenesis, including stromal cell-derived growth factor (SDF-1) (Imitola et al. 2004), vascular endothelial growth factor (VEGF) (Zhang et al. 2003) and angiopoietin 2 (Liu et al. 2009). SDF-1- and VEGF-induced migration of adult NSPCs in culture is mediated by matrix metalloproteinase (MMP)-3 and MMP-9 expression and activation in NSPCs (Barkho et al. 2008). Pharmacological inhibition of MMP activity also impairs stroke-induced neurogenesis in vivo (Lee et al. 2006). Thus, the angiogenic and neurogenic responses to stroke are tightly linked.
In conclusion, our genetic-lineage tracing study shows that stroke induces a multilineage cytogenic response in which nestin+ neural stem cells of the SVZ produce neuroblasts, postmitotic neurons, oligodendrocyte progenitor cells and astrocytic cells that repopulate the striatal parenchyma out to at least 6 weeks following the ischemic event. These studies are important in light of recent evidence for increased proliferation of GFAP+ cells within the ribbon layer of human ipsilateral SVZ at early times following stroke (Marti-Fabregas et al., 2010) and subsequent appearance of cells expressing markers of newborn neurons (PSA-NCAM and Dcx) within the infarct penumbra of human brain weeks following the onset of stroke symptoms (Jin et al. 2006; Macas et al. 2006). A recent study by Ohira et al., (2010), provides evidence for cortical neurogenesis from non-nestin derivatives within subpial regions of the neocortex in response to global forebrain ischemia in mice (Ohira et al.). In addition, induced repetitive cortical spreading depression, an epiphenomenon of stroke injury in humans and rodents, has recently been reported to stimulate hippocampal neurogenesis and subpial cortical neurogenesis (Urbach et al. 2008; Yanamoto et al. 2005). However, we did not observe contribution of YFP+ cells to superficial gliosis or evidence for cellular migration from subpial regions of cortex following MCAO.
An important finding of the current study is the percent distribution of YFP+ SVZ derivatives; in which glia represent ~67–78% of all YFP reporter+ cells, indicating a significant gliogenic component. It is currently unclear whether YFP+ SVZ cells adopt a glial fate prior to migration or after they arrive within the striatal parenchyma. Previous studies have shown that transplantation of multipotent SVZ cells directly into nonneurogenic brain regions limits neurogenic potential and promotes astrocyte differentiation (Alvarez-Buylla and Lim 2004; Zheng et al. 2006), underscoring the importance of microenvironment in determining NSPC lineage fate. The current studies provide a baseline for the use of nestin-CreERT2 transgenic mice to study the molecular regulation of migration and differentiation of NSPCs following ischemic injury through selective knockout or overexpression of genes within nestin+ NSPCs and their progeny. Understanding the molecular regulation of lineage fate of NSPCs following injury should lead to improved strategies for facilitating structural and functional recovery.
Supplementary Material
A) YFP+ cells within the ischemic striatum at 6 weeks post-MCAO. B) YFP immunofluorescence on the non-ischemic hemisphere of a tamoxifen-injected mouse at 2 weeks post-MCAO. C) YFP (green) and NeuN (red) immunofluorescence of ipsilateral hemisphere 2 weeks after sham MCAO surgery. CTX (cortex), STM (striatum). Scale bars = 100 µm (A) or 20 µm (B,C).
A) YFP and Olig2 (left) or NG2 (right) immunofluorescence within the subventricular zone. B) YFP+ cells within the corpus callosum (CC); higher power shown on right panel. LV (lateral ventricle). Scale bars = 10 µm.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (R01 NS047373), the American Heart Association (09GRNT2290178 and 0810071Z), and Dedicated Health Research Funds from the University of New Mexico School of Medicine C-2310-RAC/Bridge. Confocal images were generated using the University of New Mexico Cancer Center Fluorescence Microscopy Facility, supported as detailed at http://hsc.unm.edu/crtc/microscopy/facility.html. The authors thank Kelsey Thomas for outstanding technical assistance.
Contributor Information
Lu Li, Department of Neurosciences, University of New Mexico Health Sciences Center, Albuquerque, New Mexico 87131.
Kate M. Harms, Department of Neurosciences, University of New Mexico Health Sciences Center, Albuquerque, New Mexico 87131
P. Britten Ventura, Department of Neurosciences, University of New Mexico Health Sciences Center, Albuquerque, New Mexico 87131
Diane C. Lagace, Department of Cellular and Molecular Medicine, University of Ottawa, Ontario K1H 8M5
Amelia J. Eisch, Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, Texas 75390-9070
Lee Anna Cunningham, Department of Neurosciences, University of New Mexico Health Sciences Center, Albuquerque, New Mexico 87131.
REFERENCES
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41(5):683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Barkho BZ, Munoz AE, Li X, Li L, Cunningham LA, Zhao X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells. 2008;26(12):3139–3149. doi: 10.1634/stemcells.2008-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105(9):3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KA, Ayoub AE, Breunig JJ, Adhami F, Weng WL, Colbert MC, Rakic P, Kuan CY. Nestin-CreER Mice Reveal DNA Synthesis by Nonapoptotic Neurons following Cerebral Ischemia-Hypoxia. Cereb Cortex. 2007 doi: 10.1093/cercor/bhl164. [DOI] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23(2):297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28(41):10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6(11):1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Nakano T, Irie K, Higuchi S, Fujioka M, Orito K, Iwasaki K, Jin G, Lo EH, Mishima K, et al. Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 30(4):871–882. doi: 10.1038/jcbfm.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28(28):7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101(52):18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Greenberg DA. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1000154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103(35):13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokovay E, Li L, Cunningham LA. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab. 2006;26(4):545–555. doi: 10.1038/sj.jcbfm.9600214. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27(46):12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, Lo EH. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26(13):3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Zhang RL, Hozeska-Solgot A, Gregg SC, Buller B, Lu M, Zhang ZG. Angiopoietin 2 mediates the differentiation and migration of neural progenitor cells in the subventricular zone after stroke. J Biol Chem. 2009;284(34):22680–22689. doi: 10.1074/jbc.M109.006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34(6):945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26(50):13114–13119. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Fabregas J, Romaguera-Ros M, Gomez-Pinedo U, Martinez-Ramirez S, Jimenez-Xarrie E, Marin R, Marti-Vilalta JL, Garcia-Verdugo JM. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology. 74(5):357–365. doi: 10.1212/WNL.0b013e3181cbccec. [DOI] [PubMed] [Google Scholar]
- Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6(7):e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451(2):170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10(1):9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, et al. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci. 13(2):173–179. doi: 10.1038/nn.2473. [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12(7):829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- Otani N, Nawashiro H, Fukui S, Ooigawa H, Ohsumi A, Toyooka T, Shima K, Gomi H, Brenner M. Enhanced hippocampal neurodegeneration after traumatic or kainate excitotoxicity in GFAP-null mice. J Clin Neurosci. 2006;13(9):934–938. doi: 10.1016/j.jocn.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Roitbak T, Li L, Cunningham LA. Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1alpha-regulated VEGF signaling. J Cereb Blood Flow Metab. 2008;28(9):1530–1542. doi: 10.1038/jcbfm.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K, Nabeshima Y. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12(13):1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24(3):739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Urbach A, Redecker C, Witte OW. Induction of neurogenesis in the adult dentate gyrus by cortical spreading depression. Stroke. 2008;39(11):3064–3072. doi: 10.1161/STROKEAHA.108.518076. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26(24):6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamoto H, Miyamoto S, Tohnai N, Nagata I, Xue JH, Nakano Y, Nakajo Y, Kikuchi H. Induced spreading depression activates persistent neurogenesis in the subventricular zone, generating cells with markers for divided and early committed neurons in the caudate putamen and cortex. Stroke. 2005;36(7):1544–1550. doi: 10.1161/01.STR.0000169903.09253.c7. [DOI] [PubMed] [Google Scholar]
- Zhang H, Vutskits L, Pepper MS, Kiss JZ. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J Cell Biol. 2003;163(6):1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Rossignol C, Leibovici A, Anderson KJ, Steindler DA, Weiss MD. Transplantation of multipotent astrocytic stem cells into a rat model of neonatal hypoxic-ischemic encephalopathy. Brain Res. 2006;1112(1):99–105. doi: 10.1016/j.brainres.2006.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) YFP+ cells within the ischemic striatum at 6 weeks post-MCAO. B) YFP immunofluorescence on the non-ischemic hemisphere of a tamoxifen-injected mouse at 2 weeks post-MCAO. C) YFP (green) and NeuN (red) immunofluorescence of ipsilateral hemisphere 2 weeks after sham MCAO surgery. CTX (cortex), STM (striatum). Scale bars = 100 µm (A) or 20 µm (B,C).
A) YFP and Olig2 (left) or NG2 (right) immunofluorescence within the subventricular zone. B) YFP+ cells within the corpus callosum (CC); higher power shown on right panel. LV (lateral ventricle). Scale bars = 10 µm.