Abstract
Prostate cancer is one of the most commonly diagnosed cancers and the second leading cause of cancer deaths in Americans. The high mortality rate is mainly attributed to the invasiveness and metastasis of advanced prostate cancer. Targeting the molecules involved in metastasis could be an effective mode of treatment for prostate cancer. In this study, the therapeutic potential of siRNA-mediated targeting of matrix metalloproteinase-9 (MMP-9), urokinase plasminogen activator receptor (uPAR), and cathepsin B (CB) in prostate cancer was carried out using single and bi-cistronic siRNA expressing constructs. Down regulation of MMP-9, uPAR and CB inhibited matrigel invasion, in vitro angiogenesis and wound healing migration ability of PC3 and DU145 prostate cancer cell lines. In addition, the siRNA treatments induced apoptosis in the tumor cells as determined by TUNEL and DNA laddering assays. An attempt to elucidate the apoptotic pathway showed the involvement of FAS-mediated activation of caspases-8 and -7. Further, mice with orthotropic prostate tumors treated with siRNA expressing vectors showed significant inhibition in tumor growth and migration. In conclusion, we report that the siRNA mediated knockdown of MMP-9, uPAR and CB inhibits invasiveness and migration of prostate cancer cells and leads to apoptosis both in vitro and in vivo.
Keywords: RNAi, MMP-9, uPAR, Cathepsin, Prostate cancer, Apoptosis
Introduction
Prostate cancer is the most commonly diagnosed malignancy and the second leading cause of cancer mortality in United States.1 During the year 2008, nearly 1, 86,000 men were newly diagnosed with prostate cancer and ~ 28,000 estimated deaths related to prostate cancer were reported. Localized and early-diagnosed tumors are effectively treated by prostatectomy or radiation therapy. However, a significant number of the patients treated with prostatectomy suffered relapse.2-4 Most of the patients with relapsed disease initially respond to the hormonal ablation therapy, but they eventually progress to hormone refractory prostate cancer and metastases to other organs which no longer responds to hormone ablation therapy.5-7 Metastasis is considered to be an aggressive fatal step in the progression of prostate cancer, which primarily migrate to the bone through lymphatic and hematogeous routes and potentially lead to death of the patients.8
Aggressiveness of tumor is mainly dependent on the extent it can invade and metastasize from the primary tumor site and establish itself at a distant site. Metastasis is a complex process involving adhesion of cell to the extracellular matrix (ECM), degradation of ECM components to release the cells from the primary tumor mass, migration and establishment of tumor cells at the secondary target.9 Dissolution of ECM components, key regulator factors during metastasis, is primarily achieved through the action of several proteolytic enzymes, released either by the tumor or stromal cells present in the tumor microenvironment. Under normal physiological conditions these cellular proteases are under tight regulatory control, however in case of metastatic tumors, these control mechanisms are either lost or imbalanced.10 Moreover, studies have shown a high degree correlation between the enhanced expression and activity of several cellular proteases to the metastatic behavior of the tumor.11 Cellular proteases are generally subdivided as serine, cysteine, aspartic and matrix metalloproteinase (MMP). Among several proteases, MMP's, urokinase plasminogen activator (uPA) system and cathepsins play a major role in ECM degradation either by acting independently or by activating other protease in the breakdown of extracellular proteins contributing to the tumor migration and metastasis.12-14
The uPA system (uPA and its receptor, uPAR) attracted most of the research due to the multifunctional role in cell differentiation, proliferation, cell adhesion, signaling etc. Despite activating uPA, uPAR is associated with various other cell surface molecules, especially the integrins, indicating its role in cell adhesion and migration as well as a signal transducer11,15, which regulate various genes involved in cell proliferation, survival and apoptosis.14,16-18 Even though both uPA and uPAR are expressed in normal and tumor cells, several studies have shown that the activity of uPA and the expression of its receptor are elevated or aberrantly expressed in many malignant tumors including prostate cancer.15 Along with uPA/uPAR system, matrix metalloproteinase (MMP), a zinc dependent proteases, also play a major role in proteolytic degradation of ECM components and aid in tumor invasion and metastasis. MMP, secreted as latent zymogens, are activated by plasmin and their activity is regulated by a family of tissue inhibitors of metalloproteinase (TIMP).19 During tumor metastasis, the balance between the active protease and their inhibitors is disrupted, generally leading to an evaluated MMP expression. Over-expression of MMP is associated with various cancer metastases including those of breast, colon, head and neck, and lung.20-22 In addition to ECM degradation, MMP also induces the release of various growth factors, such as VEGF, which stimulate angiogenesis.23,24 Apart from the extracellular protease, several intracellular proteases are also actively involved in tumor migration and invasion. Cathepsins make up a group of cysteine proteases that are located in lysosomes and function both as an exopeptidase and endopeptidase.25 These cathepsins are actively involved in tumor angiogenesis, apoptosis and inflammatory response, either directly by degrading ECM components such as fibronectin, type I and IV collagen, laminin, or indirectly by activating other proteases such as MMP or the uPA.26,27 Increased expression of cathepsin B (CB) has been reported in various cancer types, including prostate cancer.28 Further, as an intracellular protein, it is also involved in various intracellular proteolysis events and activation of signaling cascades.28
The crucial roles played by MMP-9, uPAR and CB, either individually or in combination with other proteases, in the tumor environment make these molecules highly desirable targets as therapeutic agents. Researchers have successfully blocked or down regulated the expression of these molecules through neutralizing antibodies,29,30 specific inhibitors31,32 and antisense oligonucleotides.33-36 These strategies were shown to successfully inhibit tumor development in various cancer scenarios. Recently, RNA interference (RNAi) has emerged as a highly efficient method for silencing gene expression. RNAi technology comprises a sequence specific post-transcriptional gene silencing using a double-stranded RNA (dsRNA) and shares sequence homology with the targeted gene.37 The present study was aimed to target MMP-9, uPAR and cathepsin-B, using siRNA expressing vectors, in prostate cancer cells in both in vitro and in vivo conditions. In this study, we have shown that plasmid vector expressing siRNA for MMP-9, uPAR and CB effectively abrogated invasion and migration of prostate cancer cells. Further, we have also shown that down regulation of MMP-9, uPAR and CB expression induces apoptotic cell death and inhibits tumor growth and migration under in vivo conditions.
Materials and methods
Cells lines, siRNA vectors and transfection conditions
Human prostate cancer cell lines, PC3 and DU145 cells lines, were obtained from American Type Culture Collection (Manassas, VA) and grown in F-12/K and DMEM/F-12K (1:1) media, respectively containing 10% fetal bovine serum and 1% penicillin/streptomycin. All cell lines were maintained in a 37 °C incubator in a 5% CO2-humidified atmosphere. In the present study, mono-cistronic constructs expressing MMP-9 (pM), uPAR (pU) and CB (pC) siRNAs individually, and two bi-cistronic constructs expressing siRNA in combination of MMP-9 and uPAR (pUM) as well as MMP-9 and CB (pCM) were used. The shRNA used in the present study were expressed under the control of cytomegalovirus promoter in pcDNA3 vector (Invitrogen, CA, USA). Target gene sequences and construction of the above siRNA vectors were described in our earlier work.38,39 . All transfections were performed using Fugene HD Transfection Reagent (Roche Molecular Biochemicals, Indianapolis, IN) as per the manufacturer's instructions. Briefly, a day before transfection 3×105 cells were seeded in a 6 well culture plate and incubated at 37 °C incubator in a 5% CO2-humidified atmosphere. Transfection complex comprising 2 μg of shRNA expressing plasmid and 6 μl of FUGENE HD transfection was added per each well and was further incubated for 48-72 h at 37 °C incubator in a 5% CO2-humidified atmosphere.
RT-PCR analysis
After 48 h of transfection, total RNA was extracted from the transfected cells using TRIZOL reagent (Invitrogen, CA) as per standard protocol. DNase-treated RNA was used as a template for reverse transcription (RT) reaction (Invitrogen, CA, USA) followed by PCR analysis using specific primers for MMP-9, uPAR, CB and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The amplified products were analyzed on agarose gel.
Gelatin zymography
The enzymatic activity and molecular weight of electrophoretically separated forms of MMP-9 were determined from the conditioned media by gelatin zymography. Briefly, 48 h after transfection, the cells were washed with phosphate buffered saline (PBS) and incubated in serum-free medium for another 16 h. The serum-free (conditioned) media were assayed for gelatinase activity using 10% SDS gels containing gelatin (0.1 mg/mL). Gels were incubated overnight in Tris-CaCl2 buffer, pH 7.6 and stained with amido black (Sigma Aldrich, St. Louis, MO).
Western blot analysis
After transfection, the cells were washed with PBS and lysed in cell lysis buffer (Tris-buffered saline, 20 mM EDTA and 0.1% Triton X-100 containing 1 mM PMSF and proteinase inhibitors). Equal amounts of protein were fractionated on SDS-PAGE and immunoblotted with primary antibodies, followed by incubation with species specific horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, CA, USA). Detection of signals was carried out using ECL enhanced western blotting detection system (Amersham Pharmacia, Piscataway, NJ) and autoradiography using X-ray films. The following antibodies were used: anti-uPAR (American Diagnostics Inc., Greenwich, CT), anti-Cathepsin-B (Athens Research and Technology, GA), anti-GAPDH (Abcam, Cambridge, MA), Fas-Ligand (Abcam, Cambridge, MA), XIAP, caspase-7, caspase-8 and cleaved caspase-8 (Cell Signaling Technology Inc., Beverly, MA). Antibodies against FAS, FADD, Bcl-2, Bax, total and phospho forms of ERK, Akt were obtained from Santa Cruz Biotechnology, (Santa Cruz, CA).
Cell proliferation assay
Viability of DU145 and PC3 transfected cells was assessed by MTT assay. Seventy-two hours after transfection, ~10,000 cells were seeded in a 96-well plates and incubated for another 24 h. MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] (Sigma Aldrich, St. Louis, MO) was added to the culture medium in each well at a concentration of 500 μg/ml, and plates were incubated for 4 h at 37 °C. DMSO was added to each well and mixed vigorously to completely dissolve the dark blue crystals. Absorbance was measured at 555 nm (Benchmark, Bio-Rad, Hercules, CA).
Matrigel invasion assay
After 72 h of transfection, cells were detached, washed twice in PBS and approximately 1×105 cells suspended in serum-free medium were seeded in the upper chamber of a Transwell insert (8-μm pores) coated with matrigel (1 mg/mL) (Collaborative Research Inc., Boston, MA). The lower chamber was filled with 700 μL of complete medium. After 18 h of incubation period, the non-migrated cells in the upper chamber were gently scraped away, and invaded cells present on the lower surface of the insert were stained with Hema-3 (Fisher Diagnostics, VA). Photographs of the invaded cells were taken with a light microscope (Olympus IX-71).
In vitro angiogenesis assay
Conditioned media after incubating the transfected cells in serum-free media for 16 h was collected and centrifuged to clear the cell debris. Approximately, 8×103 human microvascular endothelial cells (HMEC) were cultured in the conditioned medium in 96-well plates for 48 h. After the incubation period, medium was removed and cells were stained with Hema-3 stain and observed under light microscope.
Wound healing migration assay
The scratch motility assay was used to measure two-dimensional cancer cell movement as described previously.40 Transfected cells were grown to full confluence in 6-well plates. A scratch was made on the cell monolayer using a sterile 200 μL pipette tip. The monolayer was washed twice and incubated for another 18 h in complete medium. Cells were stained with Hema-3 stain and assayed by visualization under a light microscope (Olympus IX-71).
Apoptosis assays
In situ terminal deoxytransferase-mediated dUTP nick end labeling (TUNEL) assay (Roche Molecular Biochemicals, IN, USA) was used to detect apoptosis of the transfected cells. Transfections were carried out in Lab-Tek II 8-well chamber slides (Nalge Nunc International, Naperville, IL) and processed as per the manufacturer's instructions. Positively-stained, fluorescein-labeled cells were visualized and photographed using fluorescence microscopy. Apoptosis was also confirmed by DNA laddering assay by isolated DNA from the transfected and control cells as per the method described.41
Animal experiments
All animal experiments were performed in compliance with institutional guidelines set by the Institutional Animal Care and Use Committee at the University of Illinois College of Medicine at Peoria. Athymic male nude mice (nu/nu; 5-6 weeks of age) were obtained from Harlan Sprague-Dawley (Indianapolis, IN, USA). Orthotopic implantation was carried out as described previously.42 Briefly, after total body anesthesia with ketamine (50 mg/kg) and xylazine (10 mg/kg), a low midline incision was made in the lower abdomen. A suspension of 1 × 106 PC3 cells, with luciferase reporter gene, in 20 μL PBS was injected into a lateral lobe of the mouse prostate. After 7 days of implantation, the tumors were injected with ~150 μg of the respective plasmid expressing siRNA. The primary tumor growth and sites of metastasis were determined by injecting luciferin (intra-peritoneal) at weekly intervals and examining the mice in Xenogen in vivo Imaging System (Xenogen, Alameda, CA, USA). The primary prostate tumor was excised, fixed in formalin and embedded in paraffin for hemtoxylin staining and immunohistology experiments as described previously.
Statistical analysis
Results were analyzed using two-tailed Student's t-test to assess statistical significance. The difference were considered as statistically significant when p<0.05. Densitometry analysis was performed using ImageJ software (National Institutes of Health) to quantify the signal (band) intensities.
Results
Down regulation of MMP-9, uPAR and cathepsin B at the RNA and protein levels
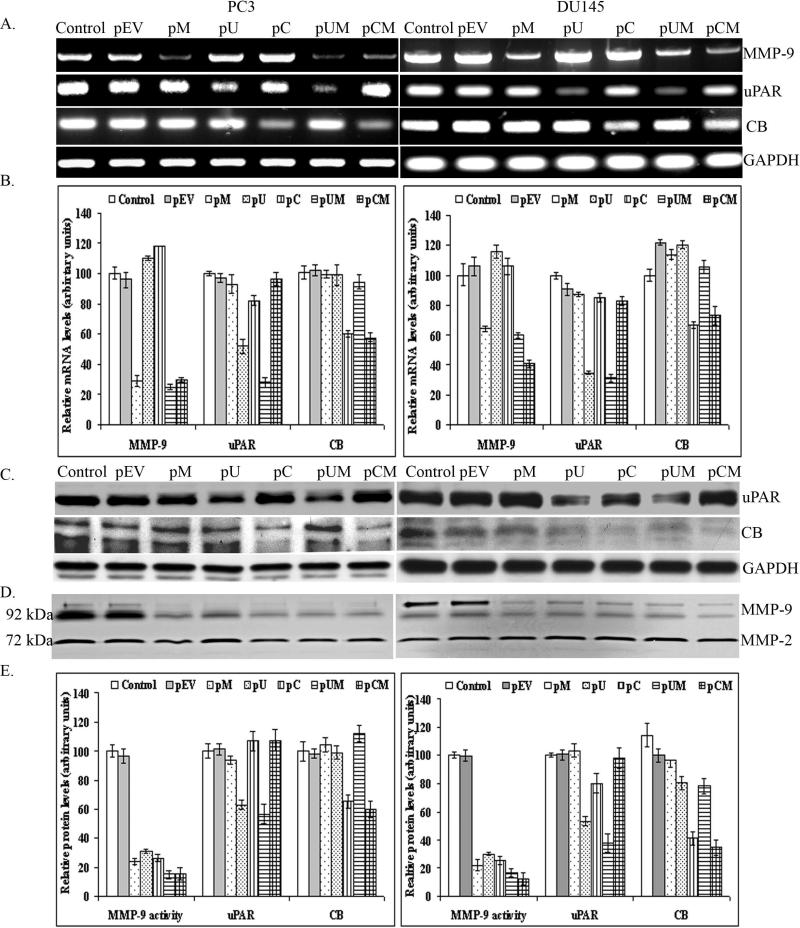
The expression and activation of MMP-9, uPAR and CB is known to play an important role in invasion, survival and progression of human tumors. Moreover, our earlier reports also supported the association of these proteases in breast, lung, glioma and others cancer metastatic models.38,39 Therefore, in the present study we investigated the role of MMP-9, uPAR and CB in two highly aggressive human prostate cancer cell lines, PC3 and DU145. We have used plasmid vectors expressing siRNA for MMP-9 (pM), uPAR (pU) and CB (pC), both individually and in combination of MMP-9 / uPAR (pUM) and MMP-9 / CB (pCM) to inhibit expression of the respective protein(s). RT-PCR analysis of the total RNA extracted from transfected cells showed a significant reduction in the mRNA levels of MMP-9, uPAR and CB in the cells transfected with pM, pU and pC, respectively. Further, transfection with bi-cistronic vectors, pUM and pCM inhibited both of the target genes simultaneously and specifically (Fig. 1A and 1B). The specificity of the plasmids used was demonstrated by comparing the GAPDH mRNA levels as internal control. We further determined the effect of these plasmids on expression of uPAR and CB proteins as well as enzymatic activity of MMP-9. Western blot analysis of the total cell lysate collected from cells transfected with pU and pC showed a significant reduction of uPAR and CB proteins, respectively when compared to the control and cells transfected with empty vector (pEV). Targeting two molecules using a bi-cistronic constructs proved to be effective in simultaneous down regulation of both the target genes as well as inhibiting the target molecules more efficiently than mono-cistronic constructs (Fig 1C). Further, MMP-9 activity in the conditioned media collected from the transfected cells were examined by gelatin zymography. Cells that were transfected pM, pU, pC, pUM and pCM showed a significant reduction in MMP-9 enzymatic activity when compared to the control or pEV transfected cells. However, we observed low or no significant changes in the levels of MMP-2 enzymatic activity in the transfected cells (Fig 1D). Quantitative analysis of uPAR and CB protein signals by densitometry revealed significant decreases in uPAR and CB levels by 63% and 60% in PC3 cells transfected pUM and pCM, respectively (Fig. 1E). While in the case of DU145 cells transfected with pUM and pCM, the uPAR and CB protein levels were reduced by 59% and 64%, respectively. GAPDH protein levels showed equal quantities of protein were loaded.
Figure 1. siRNA mediated downregulation of MMP-9, uPAR and cathepsin B at the RNA and protein levels.
PC3 and DU145 (prostate cancer cell lines) were transfected with either empty vector (pEV) or plasmids encoding siRNA for MMP-9 (pM), uPAR (pU) and Cathepsin-B (pC) (mono-cistronic) or in combination, pUM and pCM (bi-cistronic). (a) After 48 h of transfection, total RNA extracted from controls and transfected cells were used as a template for RT-PCR analysis using MMP-9, uPAR and CB specific primers. GAPDH primers were used to verify the usage of equal amount of total RNA used during cDNA synthesis. (b) The amplified (RT-PCR) fragments were quantified by scanning with densitometry. (c) For western blot analysis, total cells lysates were immunobloted with uPAR and CB specific antibodies. GAPDH was used as protein loading control. (d) Enzymatic activity of MMP-9 was determined by fractionating equal amount of conditioned media (CM) collected from control and transfected cells on 10% SDS-PAGE containing 0.1 mg/ml gelatin. (e) Bands or signals detected by zymography and autoradiography were quantified using densitometry analysis. Data represented is the mean of three different experiments and bar are mean S.D (p < 0.01).
Effect on cell proliferation, ERK and Akt activation
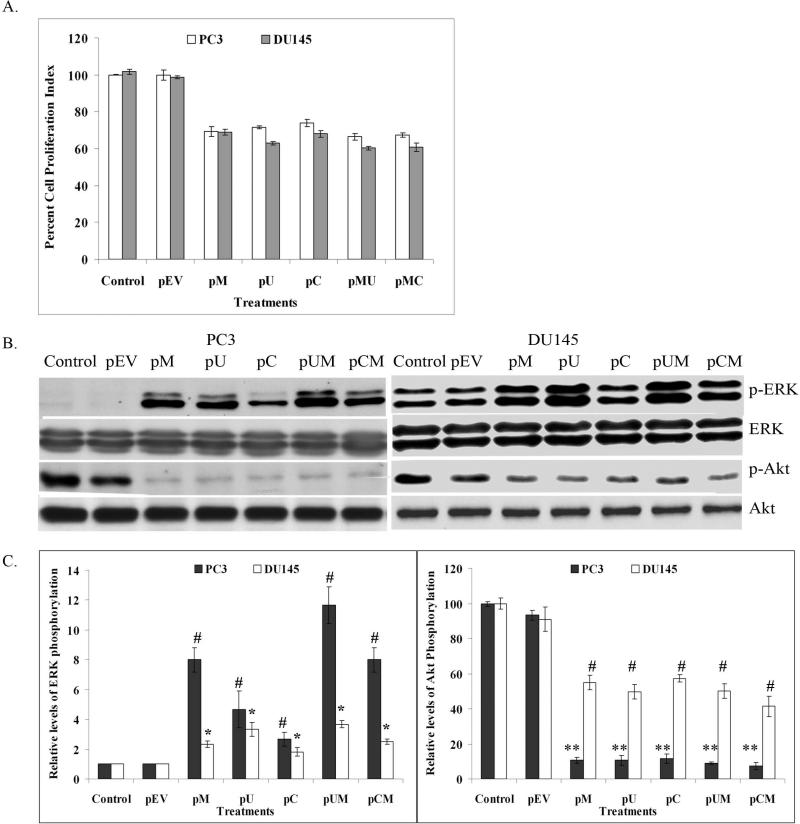
MTT assay was carried out to determine the cell proliferation index in the transfected cells. Cell proliferation was significantly reduced in both PC3 and DU145 cells transfected with pM, pU, pC, pUM and pCM compared to control and pEV transfected cells (Fig 2 A). We further analyzed the effect of the down regulation of MMP-9, uPAR and CB on ERK and Akt signaling molecules, which are considered as major survival molecules. Western blotting analysis was performed to detect the total and phosphorylated levels of ERK and Akt in the transfected cells using specific antibodies. No significant difference was observed in the total ERK and Akt levels in the transfected cells, however, phosphorylation of Akt decreased significantly in the cells treated with pM, pU, pC, pUM and pCM when compared to the parental cells or cells treated with pEV (Fig. 2B and 2C). In contrast to this, we observed significant increases in the phosphorylation of ERK1 (p44) and ERK2 (p42) in cells transfected with pM, pU, pC, pUM and pCM when compared to control and pEV transfected cells (Fig. 2B and 2C).
Figure 2. MTT assay and activation of signaling molecules.
Knockdown of MMP-9, uPAR and CB in PC3 and DU145 cells reduced cell proliferation and effect the activation on ERK and Akt. (a) Cell proliferation index of PC3 and DU145 cells transfected siRNA plasmids and controls was assessed by MTT assay. Each bar represents the mean of triplicate analysis of mean ± S.D (p < 0.05). (b) Western blot analysis showing the effect of siRNA expressing plasmids on total and phosphorylated forms of ERK and Akt in PC3 and DU145 cell lines. (c) Phsophorylation of ERK and Akt were quantified using densitometry analysis. Data represented is the mean of three different experiments and bar are mean S.D (* p < 0.01, ** p < 0.05 and # p< 0.01).
Down regulation of MMP-9, uPAR and cathepsin B reduced tumor invasion, in vitro angiogenesis and migration
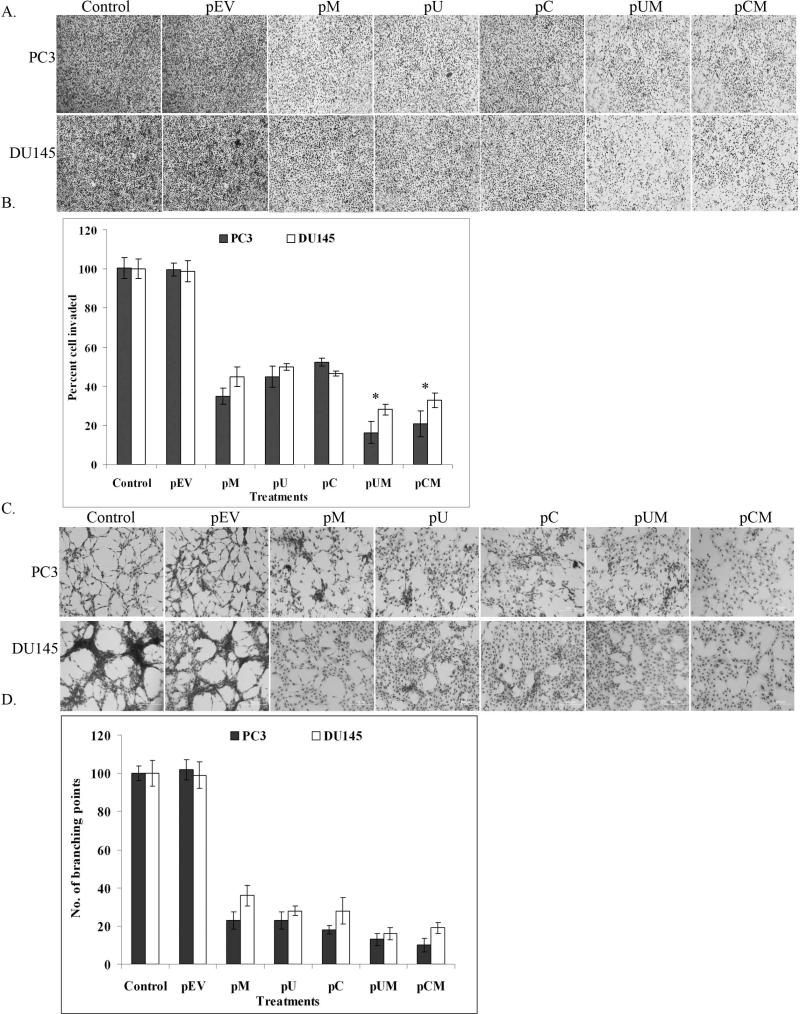
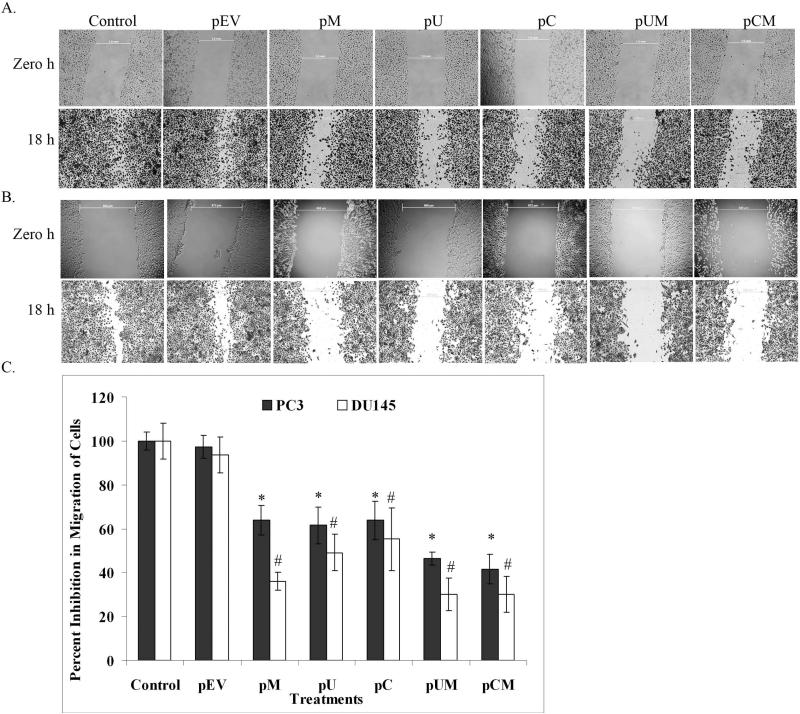
To determine the functional significance of MMP-9, uPAR and CB in prostate cancer cell lines, we compared the matrigel invasion, angiogenesis and wound healing migration ability of the transfected cells to the control cells. The ability of the transfected cells to invade the matrigel-coated filters was significantly decreased when compared to controls. The most significant decrease in invasive ability was observed in cells transfected with the bi-cistronic vectors (Fig. 3A). Quantitative analysis showed that only 19% and 25% of PC3 cells transfected with pUM and pCM, respectively, invaded through matrigel coated transwell insert as compared to controls (Fig. 3B). Similarly observations were even made in DU145 cells where only 28% and 32% of pUM and pCM transfected cells, respectively invaded the matrigel coated transwell insert as compared to controls. Moreover, nearly 40% of the PC3 and DU145 cells transfected with pM, pU, and pC invaded through matrigel coated transwell compared to control and pEV transfected cells. Well-defined capillary network formation was observed when HMEC cells were cultured in conditioned media collected from control or pEV transfected cells. In contrast, the conditioned media collected from transfected cells failed to induce the capillary network formation of HMEC cells (Fig. 3C). The induction of capillary like network by HMEC cells grown in cancer cell conditioned media was quantified by counting the number of branching points. Conditioned media from pUM and pCM transfected PC3 cells showed 87% and 90 % reduction in branching points respectively compared to controls (Fig. 3D). Whereas the conditioned media of pUM and pCM transfected DU145 cells showed a decrease by 84% and 81%, respectively in the induction of branching points compared to controls (Fig. 3D). Apart from significant inhibition in matrigel invasion, the transfected cells also showed reduced cell mobility as determined by scratch wound-healing migration assay. Microscopic examination showed that the control and pEV transfected cells migrated prominently towards each other after 18 h of scratch. In contrast, the migration of the cells transfected with plasmid expressing siRNA were significantly reduced (Fig 4A). The difference in distance migrated by controls between zero hours and 18 h was measured and compared with that of the transfected cells. Quantification of the wound healing migration assay illustrated that migration of PC3 cells transfected with pUM and pCM was decreased by nearly 54% and 59%, respectively, when compared with control and pEV-transfected cells (Fig. 4B). Whereas the migration of DU145 cells transfected with pUM and pCM was reduced by ~70% compared to the respective controls (Fig. 4B).
Figure 3. Down regulation of MMP-9, uPAR and cathepsin B reduced tumor invasion and angiogenesis.
(a) Matrigel invasion assay was performed after transfecting PC3 and DU145 cells with pM, pU, pC, pUM, pCM and pEV. After 48 h of transfection, the cells (1 × 105) were allowed to invade through the transwell inserts coated with matrigel (1 mg/ml). After 18 h of incubation, the invaded cells were stained with Hema-3, photographed under light microscope and counted. (b) Invasion assay was quantified by counting the number of cells invaded through the matrigel in at least 5 representative fields. Bars represent the mean S.D from three different experiments, * (p < 0.01) represents difference between controls and pUM and pCM transfected cells. (c) In vitro angiogenesis assay was performed by incubating human microvascular endothelial cells in conditioned media collected from transfected and control cells. After 48 h incubation period the cells were stained with Hema-3 stain and photographed. (d) Angiogenesis was quantified by counting the number of branching points formed by HMEC grown in the cancer cell conditioned media with and without treatments. Values are mean S.D (* p<0.05) from three different experiments.
Figure 4. Down regulation of MMP-9, uPAR and CB in reduced prostate cancer cell migration.
Wound healing migration assay was performed to determine the motility of transfected (a) PC3 and (b) DU145 cell lines. After 72 of transfection, cell mono-layers were wounded with a sterile 200 μL pipette tip and washed with culture medium. Cells were photographed at zero h and incubated in complete medium for 18 h. The cell motility was then assayed by staining with Hema-3 and observing under light microscope. (c) Quantification was carried out by measuring the distance migrated compared to the controls. Bars indicated the mean S.D. from three different experiment (*p<0.01 and # p<0.05).
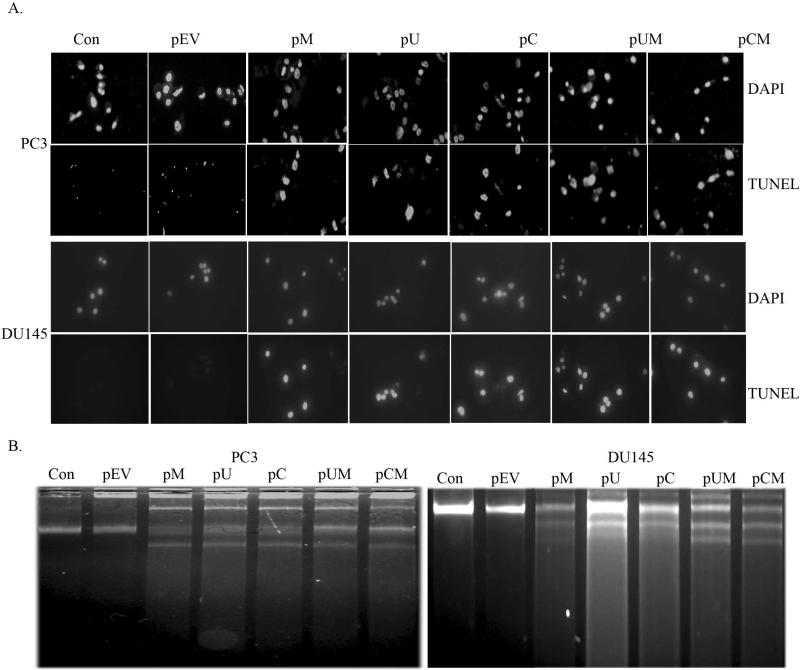
Down regulation of MMP-9, uPAR and cathepsin B induces apoptosis
With the MMT assay showing a significant inhibition in cell proliferation, we made an attempt to determine the possible induction of apoptosis in MMP-9, uPAR and CB down-regulated prostate cancer cells by carrying out TUNEL and DNA laddering assays. In TUNEL assay, the cells were visualized under a fluorescent microscope using appropriate filter sets. The characteristic green fluorescence emission was prominently observed in cells transfected with pM, pU, pC, pUM and pCM, which indicates the apoptotic nature of the cells. No such emission was observed in the non-apoptotic cells, which were primarily the control and pEV transfected cells (Fig 5A). Further evidence was provided by carrying out a DNA laddering assay to determine the fragmentation of cellular DNA, a characteristic feature of apoptotic cells. Agarose gel electrophoreses of the total DNA isolated from cells transfected with pM, pU, pC, pUM and pCM showed the characteristic pattern of DNA laddering, while the same was not observed in control and pEV transfected cells (Fig. 5B). Both high as well as low molecular weight DNA fragmentation was noticed in the transfected cells.
Figure 5. Down regulation of MMP-9, uPAR and cathepsin B induces apoptosis.
Induction of apoptosis by siRNA mediated down regulation of MMP-9, uPAR and CB was determined by TUNEL assay and DNA laddering assay. (a) After 72 h of transfection, prostate cancer cell lines PC3 and DU145 were stained for apoptosis using TdT-mediated dUTP nick end labeling (TUNEL) assay. Data shown form representative fields. (b) Induction of apoptosis in the transfected cells was confirmed by DNA fragmentation assay. Approximately 2×106 were re-suspended in 200 μl of PBS, fixed in 70% ethanol and stored at -20°C overnight. Cell pellet was re-suspended in 40 μL of phosphate-citrate buffer and incubated at room temperature for 30 min. The supernatant (DNA extract) was collected in new tubes following centrifugation. Three microliter aliquots each of 0.25% Nonidet P-40 and RNase A (1 mg/mL) were added to the extracts and incubated at 37°C for 1 h. Proteinase K was added to 100 μg/mL and incubation was continued for another hour. An aliquot of each DNA extract was analyzed on 1.5% agarose gel containing ethidium bromide by standard gel electrophoresis.
Apoptotic pathway
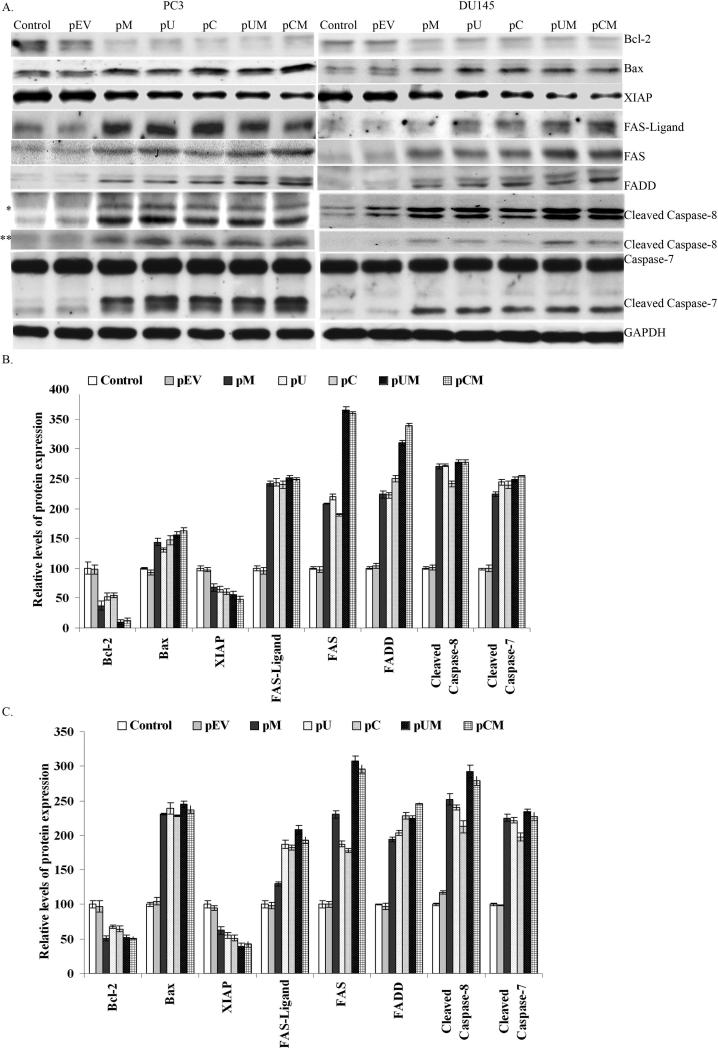
We then made an attempt to determine the possible molecules or the pathway involved in inducing apoptosis in the cancer cells by down regulating MMP-9, uPAR and CB. Western blot analysis of the cell lysates revealed that the down regulation of MMP-9, uPAR and CB activated several pro-apoptotic molecules (including Bax and caspases) and inhibited the expression of anti-apoptotic molecules, mainly XIAP and Bcl-2 (Fig. 6). With the activation pro-apoptotic molecules, we further attempted to determine the involvement of caspases in the induction of apoptosis in PC3 and DU145 cells. Figure 6 indicated that down regulation of MMP-9, uPAR and CB prostate cancer cells induced cleavage of caspase-8 and caspase-7. In contrast, the respective cleaved products were comparatively low in the control and pEV transfected cells. We next determined the involvement of various upstream molecules involved in activation of caspase-8 and extrinsic apoptotic pathway. The levels of FAS and FAS-ligand were observed to be significantly increased (up to 2 folds) in cells transfected with pM, pU, pC, pUM and pCM as compared to control and pEV transfected cells (Fig 6 B and C). The downstream process involved in the Fas/Fas-L system is its association with death domain receptors. Western blot analysis showed an increase in the expression levels of FAS associated death domain (FADD) levels in the cells transfected with siRNA expressing plasmids as compared to controls (Fig. 6). Quantification of the signals by densitometry analysis showed more than 3 and 2 folds increase in the FADD expression levels in PC3 (Fig 6 B)and DU145 (Fig 6 C) cells respectively, transfected with bi-cistronic shRNA plasmids (pUM and pCM). FADD serves as a single transducer of Fas induced apoptosis where the cytosolic domain binds to capsase-8, in order to transduce death signal.
Figure 6. Elucidation of apoptotic pathway.
siRNA mediated down regulation of MMP-9, uPAR and CB induces apoptosis by activating FAS, FADD, cleavage of caspase-8 and caspase-7. (a) After 72 h, total cell lysates collected from control and transfected cells were subjected to SDS-PAGE analysis and transferred onto nitrocellulose membrane. The membranes were immunoblotted overnight by incubating with Bcl-2, Bax, FAS-ligand, FAS, FADD, XIAP, caspase-8 and caspase-7 specific antibodies. * and ** represents two cleaved forms of capsase-8 (44/42 kDa and 18 kDa forms, respectively). Signal detection was carried out by ECL reagent and autoradiography with X-ray film. GAPDH antibodies were used to confirm equal loading of proteins in each lane. (b) Band intensity of respective proteins were quantified by densitometry analysis and normalized with GAPDH levels as shown in the bar graph (mean S.D; p<0.05).
Animal experiments
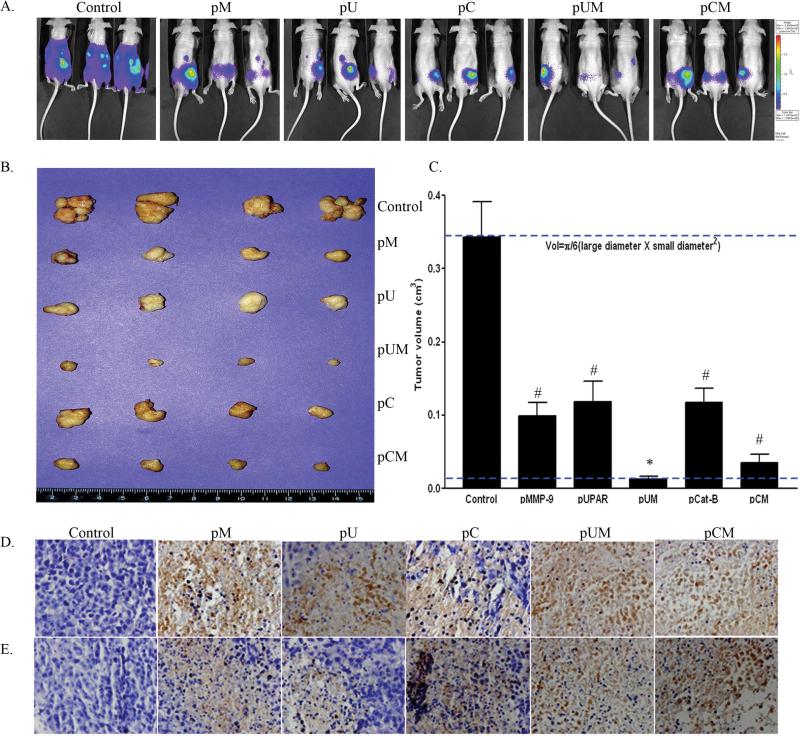
Given our in vitro results, we made an attempt to determine the effect of these plasmid based siRNA constructs in vivo. PC3-M cells with lucfierase reporter gene were inoculated in the lateral lobe of mouse prostate. Seven days post-inoculating PC3 cells into the lateral lobe mouse prostate, the animals were injected with pM, pU, pC, pUM, pCM and pEV. The control mice (pEV treated) were sacrificed three weeks after implantation as was necessitated by the morbidity resulting from the tumors that had formed in control groups. Prostate tumor growth and progression was monitored at weekly intervals by measuring the luciferase activity using the Xenogene in vivo imaging system. Photon counts from the tumor region were measured on days 7, 15 and 22. Control mice developed larger tumor at 21 days post implantation compared to mice treated with siRNA expressing constructs (Fig. 7A). Based on the tumor volume and luciferase activity, the control mice were sacrificed at 22 days (3rd week) after tumor implantation, whereas the siRNA treated mice were scarified later (after 4 weeks of tumor implantation). No significant secondary tumors were observed in mice treated with the siRNA expressing plasmids, whereas control mice developed secondary tumors mostly at the diaphragm. Tumors were dissected, weighed and the volume was measured. We noted a significant variation in the size and volume of the tumors in mice treated with siRNA plasmid as compared to control mice (Fig. 7B). Tumor volumes were measured using mathematical formula (Volume= π/6 X (larger diameter X smaller diameter2) (Fig. 7C). Although tumors treated with mono-cistronic plasmids (pM, pU and PC) did inhibit tumor growth to a large extent, a remarkable reduction of ~93% and 87% in tumor weight was observed in mice treated with bi-cistronic constructs, pUM and pCM, respectively. Immunohistochemical analysis of prostate tumor sections revealed an increased expression of FAS-L and cleaved caspase-8 in tumors treated with siRNA constructs as compared to controls (Fig. 7D and 7E).
Figure 7. siRNA expressing constructs (pM, pU, pC, pUM and pCM) regresses orthotropic tumor development in nude mice.
PC3 cells with luciferase reporter gene were inoculated to the lateral lobe of prostate gland of nude mice. After 7 days of implantation, mice were treated by injecting pM, pU, pC, pUM, pCM and pEV constructs. (a) Tumor progression in the control and transfected mice was assessed by measuring luciferase activity at regular intervals using Xenogen in vivo imaging system. (b) The mice were sacrificed and tumors were dissected and photographed. The volume of the primary tumors was measured, as described in Materials and Methods and (c) quantified. Bars indicated the mean S.D. by measuring the tumor volume at three different tumor areas (# p<0.05 and * p<0.01). Immuohistochemical analysis of (d) Fas-Ligand and (e) cleaved caspase-8 was performed in paraffin-embedded prostate tumor section of mice treated with pEV and siRNA expressing plasmids.
Discussion
Several studies aimed to control cancer either by direct inhibition or blocking the activity of molecules associated with ECM degradation and angiogenesis were well documented.9 Earlier work from our laboratory has shown the importance of the uPA system in prostate cancer metastasis both under in vitro and in vivo conditions.42 Apart from the uPA system, therapeutic strategies targeting other proteases, especially MMP and cathepsins in prostate cancer cells were limited. Therefore, the present study was aimed to knockdown MMP-9, uPAR and CB in prostate cancer cells and to demonstrate the therapeutic potential of plasmid-based siRNA constructs. As a preliminary data, a comparison between the expression pattern of MMP-9, uPAR and CB in three prostate cancer cell lines (PC3, DU145 and LNCaP) and their invasive ability showed a direct correlation between the MMP-9 enzymatic activity and uPAR expression with the invasive ability. PC3 and DU145 (androgen independent) cell lines, well known for their high metastatic behavior, expressed significantly high levels of uPAR and MMP-9 compared to LNCaP (androgen dependent), a poor metastatic prostate cancer cell line.42,43 However, the CB levels were observed to be high in DU145 followed by LNCaP and PC3 cells.44 Significance of higher CB expression in LNCaP than PC3 cells is yet to be understood. With the preliminary information, we further used PC3 and DU145 (metastatic prostate cancer cell lines) which expressed high levels of MMP-9 and uPAR compared to LNCaP cells.
The aggressiveness in invasion and migration of the tumor cells determine the metastatic potential of the cancer. Several studies have demonstrated the crucial role of the cellular proteases in tumor growth and metastasis.14 In the present study we examined the functional importance of these cellular proteases in prostate cancer cell invasion and migration. Our studies have shown that the down regulation of MMP-9, uPAR and CB using plasmids expressing siRNA significantly inhibited tumor invasion, migration as well as angiogenic ability of both PC3 and DU145 cells, indicating the active involvement of these molecules in tumor metastasis. Previous studies have shown that down regulation of MMP-9 either using specific inhibitors,45 antisense oligonucleotides46 or siRNA in breast tumor cells,47 glioma48 and lung cancer cells inhibited tumor cell invasion and migration. Similarly, targeting uPA/uPAR system, either by inhibiting uPA activity or knockdown of uPA, uPAR expression and blocking their interactions reduced tumor growth, invasion and angiogenesis in various cancers.15, 42 Our findings also confirm that down regulation of uPAR inhibited matrigel invasion, migration and angiogenic ability of PC3 and DU145 cell lines. Inhibition of invasion, migration and angiogenesis of uPAR knockout prostate cancer cells might be either due to decreased activation of uPA or due to the inhibition of downstream signaling caused by lateral interaction of uPAR with integrins. uPA, a serine proteases produced as an inactive enzyme (pro-uPA), which upon binding to uPAR becomes activated and cleaves plasminogen into plasmin. Further, the plasmin induces ECM degradation either by directly cleaving various extracellular proteins or by activating other proteases involved in the process.15 Interestingly we even noticed a preferentially inhibition in the enzymatic activity of MMP-9 (a 92 kDa collagenase IV) but not MMP-2 (72 kDa collagenase IV) (see Fig. 1 C) in uPAR and CB knockdown prostate cancer cell lines along with MMP-9 down regulated cells, indicating the specific role played by uPAR and CB in activation of MMP-9. Among the several MMPs, MMP-9 is associated with cancer metastasis by degrading type IV collagen of basal lamina of basement membrane and thus aids in tumor invasion and angiogenesis in the tumor microenvironment.22 Along with MMPs and the uPA/uPAR system, reports have even showed that both intra- and extracellular CB contribute to tumor invasiveness and its down regulation have inhibited the invasiveness of meningioma,49 lung cancer cells and glioma.38 Similarly, we have also shown that down regulation of CB significantly inhibited invasive ability and migration of prostate cancer cell lines. Further, by comparing our results, it was clear that the simultaneous down regulation of two molecules using bi-cistronic vectors proved to be more effective in inhibiting cell invasion and migration than specifically targeting a single gene/molecule. The synergistic effect of bi-cistronic vectors indicates that each of these molecules might have specific and independent role in cell invasion, migration and angiogenesis. Studies have also shown that the cellular protease can either act independently as well as coordinates with other proteases in the process of tumor invasion and angiogenesis.9,50 Collectively, our present studies established the functional significance of MMP-9, uPAR and CB in the prostate cancer cell lines. However, further studies are needed to understand the coordinated role played by each protease in tumor invasion.
Given our results showing the inhibition of tumor invasion and migration of the transfected prostate cancer cells, we studied the effect of MMP-9, uPAR and CB down regulation on cell proliferation and the activation of intracellular signaling molecules. Among several signaling pathways, ERK and PI3-K/Akt pathways were well correlated with cancer cell growth, proliferation, differentiation, survival and mobility.16 Our results show that plasmid based siRNA constructs inhibited the phosphorylation of Akt, a vital survival molecule that plays an important role in cell proliferation and differentiation. In contrast to this, the ERK system was activated (i.e., phosphorylation of ERK), which was proposed to provide a protective effect against apoptosis.51,52 Even though majority of the reports indicate that the activation of ERK signaling pathway increases the apoptotic threshold of cancer cells, some studies have also shown the pro-apoptotic influence of ERK activation.51,53,54 Recently, our results have demonstrated that MMP-9 down regulation induces apoptosis following the activation of the ERK. Evidences were also provided to demonstrate that ERK activation plays a vital role in MMP-9 mediated apoptosis.55
The present study also demonstrated the apoptosis-inducing ability of the siRNA mediated targeting of MMP-9, uPAR and CB in prostate cancer cells. This was in accordance with several earlier reports on siRNA mediated knockdown of these molecules in breast cancer, lung cancer, glioma etc. We have carried out DNA fragmentation and TUNEL assays to confirm apoptosis in the transfected cells. Apoptosis is majorly activated by either extrinsic or intrinsic pathways, which converge via activation caspases and ultimately initiate cell death. Further evidence was provided by observing a decreased expression of anti-apoptotic molecules of Bcl-2 and XIAP, enhanced expression of pro-apoptotic molecule such as Bax and activation of caspase-8 (initiator caspases) and caspase-7 (effector caspases). However, activation of caspase-3 was not seen. Similar to our observation, caspase-8 mediated apoptosis without caspase-3 activation was reported in uPAR and CB down regulated SNB19 glioma cells.56 Activation of apoptosis might be due the induction of intracellular death signaling process which in turn might have stimulated by the extra cellular death signals released in MMP-9, uPAR and CB down regulated prostate cancer cells. However, the death signal initiating mechanism is yet to be elucidated. With the activation of caspase-8, we made an attempt to elucidate the activation of Fas/Fas-ligand system in inducing apoptosis in siRNA transfected prostate cancer cell lines. Several reports have shown that Fas/Fasligand system is enhanced in the cancer cells treated with anti-cancer chemotherapeutic agents.57 Apart from chemotherapeutic agents, down regulation of MMPs was reported to induce Fas-mediated apoptosis and inhibit tumor progression in lung cancer58 and breast cancer.59 Similarly, in the present study the expressions of Fas-ligand as well as its receptor were enhanced in the siRNA plasmid transfected prostate cancer cells, providing further insight to the earlier reports. The increased expression of Fas-ligand / Fas in MMP-9, uPAR and CB down regulated cells is yet to be understood. However, certain earlier reports also suggest that the presence of uPAR/ uPA system (extra cellular protease) might have a shielding effect on Fas receptor which inhibits the activation of caspases-8. Fas-ligand functions both as autocrine as well as paracrine apoptotic mediator by binding to its receptor, Fas. Upon binding, the Fas/Fas-ligand system associates in activation of the death domains, Fas associated death domains (FADD), by its ligation (oligomerisation).60,61 This multiprotein death-inducing signaling complex could stimulate the activation of the initiator caspases which in turn activates other effectors caspases and progresses to apoptosis.62,63 The increased expression of Fas-ligand, Fas, FADD, cleavage of procaspase-8 (initiator caspases) and procaspase-7 (effectors caspases) supports the activation of extrinsic apoptotic pathway in MMP-9, uPAR and CB down-regulated prostate cancer cells. However, due to the enhancement of Bax and decrement of Bcl-2 proteins in the cell lysate of transfected cells, the involvement of intrinsic apoptotic pathway cannot be ruled out. Extended studies on both the apoptotic pathways might lead to the identification of new downstream targets useful for cancer treatment.
The induction of apoptosis and reduction in the invasive ability of prostate cancer lines in vitro provided us a platform to determine the efficiency of using siRNA plasmids in tumor regression under in vivo conditions. Earlier studies using antisense technology and the siRNA-mediated approach have been successfully used as targeting tools to suppress tumor development in animal models. Similarly in the present study, we show that plasmids expressing siRNA for MMP-9, uPAR and CB can effectively regresses the in vivo established tumor growth and its migration to secondary sites (Fig. 7A). Furthermore, the bi-cistronic plasmids proved to be more effective in inhibiting tumor growth compared to the mono-cistronic plasmids.
In conclusion, our studies clearly demonstrate that the down regulation of MMP-9, uPAR and cathepsin B in prostate cancer cell lines reduced tumor invasion and migration in both in vivo and in vitro conditions. Our in vivo results also revealed a significant reduction in tumor volume when treated with bi-cistronic siRNA plasmids, further indicating the potential of targeting two genes simultaneously using a same vector construct.
Acknowledgement
We thank Shellee Abraham for manuscript preparation. We also thank Diana Meister and Sushma Jasti for manuscript review.
This research was supported by National Cancer Institute Grant CA75557, CA116708, CA138409 and Caterpillar, Inc., OSF St. Francis, Inc. Peoria, IL (to J.S.R.). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Abbreviation
- MMP
matrix metalloproteases
- uPAR
urokinase-type plasminogen activator receptor
- CB
cathepsin-B
- ECM
extra cellular matrix
- CMV
cytomegalovirus
- RT-PCR
reverse transcription polymerase chain reaction
- SDS-PAGE
sodium dodecyl sulphate- polyacrlyamide gel electrophoresis
- PBS
phosphate buffered saline
- HMEC
human micro vascular endothelial cells
- FADD
Fas associated death domain
Footnotes
- Arun Kumar Nalla This author declares no conflict of interest.
- Bharathi Gorantla This author declares no conflict of interest.
- Christopher S. Gondi This author declares no conflict of interest.
- Sajani S. Lakka This author declares no conflict of interest.
- Jasti S. Rao Dr. Rao's work has been funded by NIH.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Albertsen PC, Hanley JA, Barrows GH, Penson DF, Kowalczyk PD, Sanders MM, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248–1253. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
- 3.Bianco FJ, Jr., Wood DP, Jr., Gomes de OJ, Nemeth JA, Beaman AA, Cher ML. Proliferation of prostate cancer cells in the bone marrow predicts recurrence in patients with localized prostate cancer. Prostate. 2001;49:235–242. doi: 10.1002/pros.10018. [DOI] [PubMed] [Google Scholar]
- 4.Roth BJ. Prostate cancer chemotherapy: emerging from the shadows. J Clin Oncol. 2005;23:3302–3303. doi: 10.1200/JCO.2005.11.933. [DOI] [PubMed] [Google Scholar]
- 5.Chen AC, Petrylak DP. Complications of androgen-deprivation therapy in men with prostate cancer. Curr Urol Rep. 2005;6:210–216. doi: 10.1007/s11934-005-0009-2. [DOI] [PubMed] [Google Scholar]
- 6.Ferrero JM. [Hormone resistant metastatic prostate cancer: analysis of two phase III clinical studies]. Bull Cancer. 2005;92:425–427. [PubMed] [Google Scholar]
- 7.Petrylak DP. Future directions in the treatment of androgen-independent prostate cancer. Urology. 2005;65:8–12. doi: 10.1016/j.urology.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 9.Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall DM. Molecular interactions in cancer cell metastasis. Acta Histochem. 2009 doi: 10.1016/j.acthis.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 11.Duffy MJ. The urokinase plasminogen activator system: role in malignancy. Curr Pharm Des. 2004;10:39–49. doi: 10.2174/1381612043453559. [DOI] [PubMed] [Google Scholar]
- 12.Dano K, Behrendt N, Hoyer-Hansen G, Johnsen M, Lund LR, Ploug M, et al. Plasminogen activation and cancer. Thromb Haemost. 2005;93:676–681. doi: 10.1160/TH05-01-0054. [DOI] [PubMed] [Google Scholar]
- 13.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 14.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 15.Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev. 2008;34:122–136. doi: 10.1016/j.ctrv.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res. 2003;63:1684–1695. [PubMed] [Google Scholar]
- 17.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 18.Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307:269–273. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- 19.Khasigov PZ, Podobed OV, Gracheva TS, Salbiev KD, Grachev SV, Berezov TT. Role of matrix metalloproteinases and their inhibitors in tumor invasion and metastasis. Biochemistry (Mosc) 2003;68:711–717. doi: 10.1023/a:1025051214001. [DOI] [PubMed] [Google Scholar]
- 20.Freije JM, ez-Itza I, Balbin M, Sanchez LM, Blasco R, Tolivia J, et al. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994;269:16766–16773. [PubMed] [Google Scholar]
- 21.Muller D, Wolf C, Abecassis J, Millon R, Engelmann A, Bronner G, et al. Increased stromelysin 3 gene expression is associated with increased local invasiveness in head and neck squamous cell carcinomas. Cancer Res. 1993;53:165–169. [PubMed] [Google Scholar]
- 22.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 23.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mira E, Lacalle RA, Buesa JM, de Buitrago GG, Jimenez-Baranda S, Gomez-Mouton C, et al. Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface. J Cell Sci. 2004;117:1847–1857. doi: 10.1242/jcs.01035. [DOI] [PubMed] [Google Scholar]
- 25.Tu C, Ortega-Cava CF, Chen G, Fernandes ND, Cavallo-Medved D, Sloane BF, et al. Lysosomal cathepsin B participates in the podosome-mediated extracellular matrix degradation and invasion via secreted lysosomes in v-Src fibroblasts. Cancer Res. 2008;68:9147–9156. doi: 10.1158/0008-5472.CAN-07-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Creemers LB, Hoeben KA, Jansen DC, Buttle DJ, Beertsen W, Everts V. Participation of intracellular cysteine proteinases, in particular cathepsin B, in degradation of collagen in periosteal tissue explants. Matrix Biol. 1998;16:575–584. doi: 10.1016/s0945-053x(98)90068-3. [DOI] [PubMed] [Google Scholar]
- 27.Yin LL, Chung CM, Chen J, Fok KL, Ng CP, Jia RR, et al. A suppressor of multiple extracellular matrix-degrading proteases and cancer metastasis. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang JH, Lee SH, Lee KH, Lee KY, Kim H, Ryu JK, et al. Cathepsin B is a target of Hedgehog signaling in pancreatic cancer. Cancer Lett. 2009;273:266–272. doi: 10.1016/j.canlet.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 29.Rabbani SA, Gladu J. Urokinase receptor antibody can reduce tumor volume and detect the presence of occult tumor metastases in vivo. Cancer Res. 2002;62:2390–2397. [PubMed] [Google Scholar]
- 30.Trauth BC, Klas C, Peters AM, Matzku S, Moller P, Falk W, et al. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 31.Frlan R, Gobec S. Inhibitors of cathepsin B. Curr Med Chem. 2006;13:2309–2327. doi: 10.2174/092986706777935122. [DOI] [PubMed] [Google Scholar]
- 32.Nemeth JA, Yousif R, Herzog M, Che M, Upadhyay J, Shekarriz B, et al. Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis. J Natl Cancer Inst. 2002;94:17–25. doi: 10.1093/jnci/94.1.17. [DOI] [PubMed] [Google Scholar]
- 33.Arens N, Gandhari M, Bleyl U, Hildenbrand R. In vitro suppression of urokinase plasminogen activator in breast cancer cells--a comparison of two antisense strategies. Int J Oncol. 2005;26:113–119. [PubMed] [Google Scholar]
- 34.D'Alessio S, Margheri F, Pucci M, Del Rosso A, Monia BP, Bologna M, et al. Antisense oligodeoxynucleotides for urokinase-plasminogen activator receptor have anti-invasive and anti-proliferative effects in vitro and inhibit spontaneous metastases of human melanoma in mice. Int J Cancer. 2004;110:125–133. doi: 10.1002/ijc.20077. [DOI] [PubMed] [Google Scholar]
- 35.Gondi CS, Lakka SS, Yanamandra N, Siddique K, Dinh DH, Olivero WC, et al. Expression of antisense uPAR and antisense uPA from a bicistronic adenoviral construct inhibits glioma cell invasion, tumor growth, and angiogenesis. Oncogene. 2003;22:5967–5975. doi: 10.1038/sj.onc.1206535. [DOI] [PubMed] [Google Scholar]
- 36.Margheri F, D'Alessio S, Serrati S, Pucci M, Annunziato F, Cosmi L, et al. Effects of blocking urokinase receptor signaling by antisense oligonucleotides in a mouse model of experimental prostate cancer bone metastases. Gene Ther. 2005;12:702–714. doi: 10.1038/sj.gt.3302456. [DOI] [PubMed] [Google Scholar]
- 37.Gartel AL, Kandel ES. RNA interference in cancer. Biomol Eng. 2006;23:17–34. doi: 10.1016/j.bioeng.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. RNAi-mediated inhibition of cathepsin B and uPAR leads to decreased cell invasion, angiogenesis and tumor growth in gliomas. Oncogene. 2004;23:8486–8496. doi: 10.1038/sj.onc.1207879. [DOI] [PubMed] [Google Scholar]
- 39.Lakka SS, Gondi CS, Yanamandra N, Dinh DH, Olivero WC, Gujrati M, et al. Synergistic down regulation of urokinase plasminogen activator receptor and matrix metalloproteinase-9 in SNB19 glioblastoma cells efficiently inhibits glioma cell invasion, angiogenesis, and tumor growth. Cancer Res. 2003;63:2454–2461. [PubMed] [Google Scholar]
- 40.Jung JW, Hwang SY, Hwang JS, Oh ES, Park S, Han IO. Ionising radiation induces changes associated with epithelial-mesenchymal transdifferentiation and increased cell motility of A549 lung epithelial cells. Eur J Cancer. 2007;43:1214–1224. doi: 10.1016/j.ejca.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 41.Gong J, Traganos F, Darzynkiewicz Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem. 1994;218:314–319. doi: 10.1006/abio.1994.1184. [DOI] [PubMed] [Google Scholar]
- 42.Pulukuri SM, Gondi CS, Lakka SS, Jutla A, Estes N, Gujrati M, et al. RNA Interference-directed Knockdown of Urokinase Plasminogen Activator and Urokinase Plasminogen Activator Receptor Inhibits Prostate Cancer Cell Invasion, Survival, and Tumorigenicity in Vivo. J Biol Chem. 2005;280:36529–36540. doi: 10.1074/jbc.M503111200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Aalinkeel R, Nair MP, Sufrin G, Mahajan SD, Chadha KC, Chawda RP, et al. Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res. 2004;64:5311–5321. doi: 10.1158/0008-5472.CAN-2506-2. [DOI] [PubMed] [Google Scholar]
- 44.Friedrich B, Jung K, Lein M, Turk I, Rudolph B, Hampel G, et al. Cathepsins B, H, L and cysteine protease inhibitors in malignant prostate cell lines, primary cultured prostatic cells and prostatic tissue. Eur J Cancer. 1999;35:138–144. doi: 10.1016/s0959-8049(98)00273-1. [DOI] [PubMed] [Google Scholar]
- 45.Sternlicht MD, Bergers G. Matrix metalloproteinases as emerging targets in anticancer therapy: status and prospects. Emerging Ther Targets. 2000;4:609–633. [Google Scholar]
- 46.Lakka SS, Rajan M, Gondi CS, Yanamandra N, Chandrasekar N, Jasti SL, et al. Adenovirus-mediated expression of antisense MMP-9 in glioma cells inhibits tumor growth and invasion. Oncogene. 2002;21:8011–8019. doi: 10.1038/sj.onc.1205894. [DOI] [PubMed] [Google Scholar]
- 47.Kunigal S, Lakka SS, Gondi CS, Estes N, Rao JS. RNAi-mediated downregulation of urokinase plasminogen activator receptor and matrix metalloprotease-9 in human breast cancer cells results in decreased tumor invasion, angiogenesis and growth. Int J Cancer. 2007;121:2307–2316. doi: 10.1002/ijc.22962. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Gondi CS, Lakka SS, Dinh D, Olivero W, Gujrati M, Rao JS. Downregulation of uPA, uPAR and MMP-9 using small, interfering, hairpin RNA (siRNA) inhibits glioma cell invasion, angiogenesis and tumor growth. Neuron Glia Biology. 2004;1:165–176. doi: 10.1017/s1740925x04000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tummalapalli P, Spomar D, Gondi CS, Olivero WC, Gujrati M, Dinh DH, et al. RNAi-mediated abrogation of cathepsin B and MMP-9 gene expression in a malignant meningioma cell line leads to decreased tumor growth, invasion and angiogenesis. Int J Oncol. 2007;31:1039–1050. [PMC free article] [PubMed] [Google Scholar]
- 50.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 51.Mohr S, McCormick TS, Lapetina EG. Macrophages resistant to endogenously generated nitric oxide-mediated apoptosis are hypersensitive to exogenously added nitric oxide donors: dichotomous apoptotic response independent of caspase 3 and reversal by the mitogen-activated protein kinase kinase (MEK) inhibitor PD 098059. Proc Natl Acad Sci USA. 1998;95:5045–5050. doi: 10.1073/pnas.95.9.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woessmann W, Zwanzger D, Borkhardt A. ERK signaling pathway is differentially involved in erythroid differentiation of K562 cells depending on time and the inducing agent. Cell Biol Int. 2004;28:403–410. doi: 10.1016/j.cellbi.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Bacus SS, Gudkov AV, Lowe M, Lyass L, Yung Y, Komarov AP, et al. Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene. 2001;20:147–155. doi: 10.1038/sj.onc.1204062. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem. 2000;275:39435–39443. doi: 10.1074/jbc.M004583200. [DOI] [PubMed] [Google Scholar]
- 55.Bhoopathi P, Chetty C, Kunigal S, Vanamala SK, Rao JS, Lakka SS. Blockade of tumor growth due to matrix metalloproteinase-9 inhibition is mediated by sequential activation of beta1-integrin, ERK, and NF-kappaB. J Biol Chem. 2008;283:1545–1552. doi: 10.1074/jbc.M707931200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Gondi CS, Kandhukuri N, Kondraganti S, Gujrati M, Olivero WC, Dinh DH, et al. RNA interference-mediated simultaneous down regulation of urokinase-type plasminogen activator receptor and cathepsin B induces caspase-8-mediated apoptosis in SNB19 human glioma cells. Mol Cancer Ther. 2006;5:3197–3208. doi: 10.1158/1535-7163.MCT-05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mo YY, Beck WT. DNA damage signals induction of fas ligand in tumor cells. Mol Pharmacol. 1999;55:216–222. doi: 10.1124/mol.55.2.216. [DOI] [PubMed] [Google Scholar]
- 58.Chetty C, Bhoopathi P, Lakka SS, Rao JS. MMP-2 siRNA induced Fas/CD95-mediated extrinsic II apoptotic pathway in the A549 lung adenocarcinoma cell line. Oncogene. 2007;26:7675–7683. doi: 10.1038/sj.onc.1210584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kunigal S, Lakka SS, Joseph P, Estes N, Rao JS. Matrix metalloproteinase-9 Inhibition Down-Regulates Radiation-Induced Nuclear Factor-{kappa}B Activity Leading to Apoptosis in Breast Tumors. Clin Cancer Res. 2008;14:3617–3626. doi: 10.1158/1078-0432.CCR-07-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 61.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 62.Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2665–2672. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]