Abstract
Previously, a standard theory of systems level memory consolidation was developed to describe how memory recall becomes independent of the medial temporal memory system. More recently, an extended consolidation theory was proposed that predicts seven changes in regional neural activity and inter-regional functional connectivity. Using longitudinal event related functional magnetic resonance imaging of an associate memory task, we simultaneously tested all predictions and additionally tested for consolidation related changes in recall of associate memories at a sub-trial temporal resolution, analyzing cue, delay and target periods of each trial separately. Results consistent with the theoretical predictions were observed though two inconsistent results were also obtained. In particular, while recall-related delay period activity decreased with consolidation as predicted, visual cue activity increased for consolidated memories. Though the extended theory of memory consolidation is largely supported by our study, these results suggest the extended theory needs further refinement and the medial temporal memory system has multiple, temporally distinct roles in associate memory recall. Neuroimaging analysis at a sub-trial temporal resolution, as used here, may further clarify the role of the hippocampal complex in memory consolidation.
Keywords: Consolidation, fMRI, Memory, Long-Term Memory
Introduction
In 1953 a patient with intractable epilepsy, HM, underwent bilateral medial temporal lobe resection which removed much of the mesial surface of the temporal lobes including the majority of the hippocampi and hippocampal gyri (Scoville and Milner, 1957). As a consequence of the surgery HM, and patients with similar medial temporal damage, present with severe anteriograde amnesia for declarative memories as well as retrograde amnesia for declarative information acquired in the years prior to the surgery (Manns and Squire, 2002). However, information acquired earlier in life, including childhood autobiographical memory and lexical information such as the names of objects and meanings of words, are relatively spared (Kensinger et al., 2001). Systems level consolidation theory suggests that the medial temporal lobes, and the hippocampal complex in particular, are necessary for the initial storage and recall of memories but that remote memories are eventually stored elsewhere in neocortex and may be recalled independently of the medial temporal lobe memory system (Squire et al., 1984; Zola-Morgan & Squire, 1990; Squire 1992; Squire and Alverez 1995; Squire and Bayley 2007). To avoid interference between old and new memories, it has been suggested that a fast-learning hippocampal complex initially facilitates learning and recall with new memories slowly stabilized over time, possibly via subsequent recall and reconsolidation events or replay during rest and sleep (McClelland et al., 1995; Hoffman and McNaughton, 2002; Wilson, 2002). This stabilization process incorporates the memory into the slow-learning neocortical networks, which maintain existing knowledge (McClelland et al., 1995; Alverez and Squire, 1994). Though considerable debate continues regarding, for example, the necessity of the hippocampus for remote memories with autonoetic content (i.e episodic memories), or the probability that some semantic memories can be acquired in spite of hippocampal damage, the major elements of the systems level consolidation of non-episodic memory has been well supported (Nadel and Moscovitch, 1997; Vargha-Khadem et al. 1997; Schmolck et al., 2002; Manns et al., 2003).
Additional lesion studies have identified temporally graded retrograde amnesia following lesions to several medial temporal structures including the hippocampus (CA fields and subiculum), entorhinal cortex, and perirhinal cortex (Cho et al., 1993; Bolhuis et al., 1994; Clark et al., 2002; Glenn et al., 2003). However, the effects of lesions to different areas appear to be temporally distinct, with, for example, memories remaining dependent on intact entorhinal cortex for a longer duration than on intact hippocampus (Izquierdo et al., 1997). Thus, while the hippocampus proper remains an important focus for consolidation studies, consolidation related effects occur throughout much of the medial temporal lobes.
Recently, Frankland and Bontempi (2005) proposed extensions to the standard theory of systems level consolidation based on extensive experimental evidence in animal models of memory. Their extended theory can be summarized by seven predictions regarding changes in regional activity and inter-regional functional connectivity within the brain (see Figure 1 and Table 1). First, neural activity in the medial temporal memory region should be decreased during recall of consolidated remote relative to recent memories due to the decreased role of the hippocampus and related structures in consolidated memory recall. Second, neural activity in neocortical memory storage regions should increase during recall of consolidated remote relative to recent memories due to their increased role in storing the memory. Third, neural activity in prefrontal cortex should increase during recall of consolidated remote relative to recent memories due to its role in orchestrating memory retrieval and a possible role in suppressing medial temporal activity for consolidated memories (Laroche et al., 2000). Fourth, absolute magnitude of inter-regional functional connectivity (correlated neural activity) between prefrontal cortex and the medial temporal memory region should increase during recall of consolidated remote relative to recent memories due to the possible suppression (inhibition) of the hippocampus and related structures by the prefrontal cortex. Fifth, inter-regional functional connectivity between prefrontal cortex and neocortical memory storage regions should increase during recall of consolidated remote relative to recent memories due to the increased role of the prefrontal region in coordinating consolidated recall. Sixth, inter-regional functional connectivity between the medial temporal memory regions and neocortical memory storage regions should be decreased during recall of consolidated remote relative to recent memories due to the decreased role of the medial temporal memory regions in coordinating consolidated memory recall. And seventh, inter-regional functional connectivity within the neocortical storage regions should increase during recall of consolidated remote relative to recent memories due to the increased coordinated role these regions in consolidated memory recall. While individually these predictions are based on experimental evidence mostly from animal models, neuroimaging studies of systems level consolidation related changes in humans have been less consistent regarding these predictions and have only recently begun to test all the predictions simultaneously.
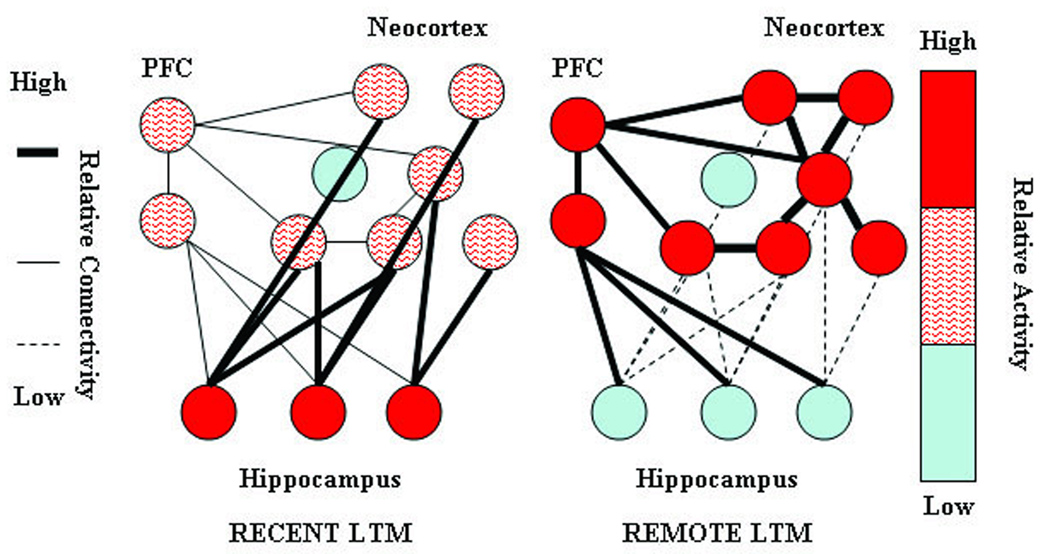
Figure 1. Graphical depiction of the standard model of systems level memory consolidation.
The figure, adapted from Frankland and Bontempi (2005) Figure 5, graphically depicts the seven changes in regional activity and inter-regional connectivity predicted by the standard theory. Node color indicates activity level with red indicating relatively greater activity. Connection line weight indicates strength of functional association with heavier lines indicating stronger functional connectivity.
Table 1.
Seven predictions of the extended theory of systems level consolidation.
| Systems Level Consolidation should lead to: | |
|---|---|
| 1 | Decreased hippocampal activity |
| 2 | Increased neocortical (lateral temporal) activity |
| 3 | Increased prefrontal activity |
| 4 |
Increased connectivity between prefrontal and hippocampal region (inhibitory) |
| 5 |
Increased connectivity between prefrontal and lateral temporal regions (excitatory) |
| 6 |
Decreased connectivity between hippocampal and lateral temporal regions |
| 7 | Increased connectivity within lateral temporal regions |
Because of an important theoretical debate regarding systems level consolidation of non-semantic, purely episodic memories (c.f., Nadel and Moscovitch 1997), the primary focus of these consolidation studies has been the hippocampus proper. An alternative theory to the standard model, Multiple Trace Theory, agrees that the hippocampal complex is not necessary for consolidated semantic memories. However, Multiple Trace Theory posits that episodic memories with a strong autonoetic component are never consolidated to memory systems outside the hippocampus, regardless of their age. Several studies have now closely examined the relative activity in the hippocampal complex during recent versus remote recall and have yielded considerably mixed results with some studies showing greater hippocampal activity for remote memory recall (Haist et al., 2001; Niki and Luo, 2002; Maguire and Frith, 2003; Piefke et al., 2003; Douville et al., 2005), some studies showing greater hippocampal activity for recent memory recall (Gilboa et al., 2004; Piolino et al., 2004; Rekkas and Constable, 2005), and others showing little or no effect in the region (Maguire et al., 2001; Bernard et al., 2004; Steinvorth et al., 2006). Recent studies using experimentally induced memories which allow for greater control over age and strength of the memory trace have also yielded inconsistent results with reduced (Takashima et al., 2006), greater (Bosshardt et al., 2005), or equivalent (Stark and Squire, 2000) hippocampal complex activation during recall of more remote memories. However, several issues potentially confound the interpretation of many of these studies, including differences in familiarity between targets and foils, use of familiarity versus recall for response, use of blocked experimental designs, use of linguistic stimuli which may have pre-existing consolidated representations, or low remote memory accuracy during testing.
In the current study, we use event-related functional magnetic resonance imaging (fMRI) in a longitudinal experiment to simultaneously test each of the seven predictions of the full extended theory of systems level consolidation for non-autonoetic associate memories. We examine recent and remote recall for abstract nonlinguistic visual-to-auditory paired associate memories. We trained twelve young, cognitively normal, right handed subjects to accurately recognize the abstract auditory associate of each of 18 non-namable visual images. Functional imaging data were collected immediately following the initial training session (the recent session), and 28 days after initial training (the remote session) while subjects performed the associate memory task. The 28 day consolidation period was chosen based on a recent study suggesting that consolidation effects are visible with fMRI in as little as 2 days and are fairly stable after 30 days (Takashima et al., 2006). The stimuli and associations were novel to the subjects, thus avoiding potential activations of pre-existing consolidated memories. Foils were drawn from targets of other items, thus avoiding experimentally induced differences in stimulus familiarity, which may alter activation levels in distinct hippocampal complex structures (Eichenbaum et al., 2007). We separately modeled the hemodynamic response to the visual cue, delay period, and auditory probe components of the task allowing detailed analysis of temporal as well as spatial information during subcomponents of the trials (Zarahn et al., 1999; Zarahn 2000). To our knowledge, this is the first attempt to test for consolidation related changes in the within-trial temporal dynamics of memory recall related neural activity in humans. Since such finer scale temporal changes are outside the scope of the extended standard theory in its current formulation, the current experiment is in a unique position not only to test the predictions of the extended theory of systems level memory consolidation but also to provide additional constraining data for its further refinement.
METHODS
Subjects
Twenty-three, young, healthy, right handed paid volunteers with normal hearing and normal or corrected to normal vision were initially recruited for the experiment. All participants were given a medical examination and gave informed consent in accordance with the procedures of the National Institutes of Health. Prior to scanning, subjects were trained to associate stimulus pairs to 90% accuracy. Of these 23 subjects, six were excluded for failing to learn the task to sufficient accuracy within the 1.5 hour initial training session (these six were over 80% accurate but less than 90%), two were excluded for failing to return for the scheduled follow-up session, two were excluded for poor performance inside the scanner environment, and one was excluded for excessive motion during scanning. Only the remaining 12 subjects (7 female, mean ± std age = 25.75 ± 3.44yrs) were analyzed further.
Stimuli
The experiment consisted of 18 pairs of visual and auditory stimuli created as part of a larger experiment (see Supplementary Material). The 18 visual stimuli were generated using low pass filters implemented in Photoshop (Adobe Inc.) from nine photographs of common animals and nine non-nameable fractal images (used with permission from www.fractalrecursions.com). The resulting images were not identifiable and subjects were not aware of the nature of the stimuli. The 18 auditory stimuli were created using a modified sine wave speech process (Remez et al., 2001), implemented in Matlab (MathWorks) based on a linear predictive coding algorithm from nine spoken animal names and nine nonwords matched to the animal names for length in phonemes, syllable number, and mean phoneme transition frequency. The resulting sounds were not identifiable as speech and subjects were not aware of the linguistic nature of the stimuli. Animal pictures were paired with the spoken name of the animal and fractal images were randomly paired with a spoken nonword.
Task
Subjects were trained to associate the image and sound pairs outside the scanner. During training, subjects were presented with all eighteen correct image-sound pairings three times each in a random order and then performed a paired associate recognition memory task with feedback. In this associate memory task, an image was presented on a laptop computer followed by a 2 second delay period then a sound. Subjects responded using the keyboard if the sound was the correct pair of the image. Feedback on the subjects’ performance was presented after each trial. There were 36 trials in each test and each image appeared twice, once correct and once with an incorrect sound taken from another experimental pair. This presentation-test cycle was iterated a minimum of four times until the subjects achieved a 90% accuracy level on the test. Subjects failing to achieve criterion in 1.5 hours (approximately 12 runs) were excluded from the study. After achieving the accuracy criterion, subjects were placed in the scanner within 30min and given one more presentation of the correct pairings with the scanner running to acclimate them to auditory stimuli in the presence of scanner noise and to equate the time between last training and initial testing across subjects.
Data were then collected while subjects performed 72 delayed paired associate trials, described below, distributed over three scanning runs. Each run consisted of 24 trials and runs were balanced such that each visual cue and each auditory target occurred at least once and at most twice per run and four times overall. Each pairing was presented correctly twice. Incorrect parings were different for each run and had minimal overlap with incorrect parings from the training session. Preliminary testing on a different subject cohort suggested that some retraining was necessary to achieve acceptable accuracy levels after 28 days. Thus, subjects returned approximately two weeks after the initial training day and were tested on their recall of the pairs outside the scanner in a single 36 item test. This test was structured identically to the testing during initial training as described above though without feedback. Subjects were then retrained, as before, to again achieve 90% accuracy. Subjects returned exactly 28 days after the initial training/scanning session and again performed 72 delayed paired associate trials structured as in the first scanning session though with different trial orders. After this remote memory session subjects underwent additional testing not discussed further here.
In each scanned trial, an image was presented for two seconds followed by a five to eight second delay (mean 6.5s) consisting of a white fixation cross on a black screen, followed by a sound with duration of approximately 1 second. The sound was the correct pair of the image in 50% of the trials. Subjects responded via button press, during the first 3 seconds of a 4 to 18 second inter-trial interval (ITI). The long ITI durations essentially represent null “baseline” events to better identify the evoked response (Friston et al., 1999). The fixation cross was presented during the ITI. Auditory foils were taken from the targets of other images and no novel images or sounds were presented. The trial ISI and ITI timings were selected to minimize the correlation between the expected hemodynamic response regressors and maximize the regression tolerance (mean tolerance = 0.63) to better observe the evolution of the task related BOLD signal over time.
Imaging
Functional imaging data were collected with a 3 Tesla GE system (General Electric, Milwaukee, WI), using the ASSET EPI parallel imaging sequence (acceleration factor of 2) and an eight channel receive only head coil. Thirty-two T2*-weighted axial slices (TR = 2000ms, TE = 30ms) were collected for each volume in an interleaved order with a 2.6mm slice thickness, 1.2mm slice gap, and a 2.5mm by 2.5mm within plane resolution (96 by 96 Matrix, 240mm FOV). Each scanning run consisted of 232 volumes and the first four were excluded to allow for tissue saturation effects. Two structural images were acquired at the first scanning session. Both structural images were T1 weighted SPGR images. One image was collected with the same voxel size at the same slices as the functional images (TE 2.4ms, Flip angle 70°). A second, full brain image was also acquired at higher resolution, optimized to maintain short acquisition time (220mm FOV, 224 by 224 Matrix, 1mm by 1mm in plane resolution, 1.3mm slice thickness, 128 slices, TE 2.7ms, Flip angle 12°, Prep time 450ms).
Data Analysis
Analysis of the functional imaging data was performed using the SPM2 software package (http://www.fil.ion.ucl.ac.uk/spm) and additional routines implemented in Matlab (MathWorks). Images were corrected for slice acquisition time effects, realigned within each run then across sessions and runs using a six parameter rigid body model and the mean of the resulting images was calculated. The low resolution T1 anatomical image was similarly coregistered to the mean EPI image and the high resolution T1 image was coregistered to this low resolution T1 image. The high resolution anatomical image was segmented into separate gray matter, white matter and CSF components and the gray matter image was normalized to a gray matter template image included in SPM2 which is in MNI atlas space. The resulting normalization parameters were applied to the coregistered EPI images which were then resliced to 2.5mm isotropic voxels using 7th degree B-splines. EPI images were smoothed with an 8mm FWHM Gaussian kernel so the residual images from the analysis would better approximate a Gaussian random field.
Individual subject analyses were performed using a restricted maximum likelihood multiple linear regression as implemented in SPM2. Images were band-pass filtered using a discrete cosine transform to remove noise and a canonical hemodynamic response was used to approximate the expected response to the visual cue, mid-point of the delay period, and auditory response components of correct trials. Because the contrast of interest was the comparison of the evoked responses between sessions and the use of the event related design, no separate baseline or control condition (aside from the null ITI) was needed to identify these within session evoked response. Separate regressors were used to model incorrect trials. To further ensure avoidance of the potentially confounding effects of accuracy and sensitivity in the imaging analyses, correct trials with reaction times 2.5 standard deviations beyond the run mean or greater than 3 seconds were scored as incorrect. Additionally individual runs where the 95% confidence interval on the within run d' value crossed 0 were excluded from the analysis. This excluded one recent run from one subject and one remote run from a different subject. Subject motion (6 parameters per-volume), and image run means were also removed. Removing the voxel means of each scan effectively removes the main effect of scanning session. Paired t-tests corrected for non-sphericity and subject specific effects, were used in the second level random effects analysis. The non-sphericity correction was applied to avoid confounding effects of variance differences in the longitudinal design. To control for alpha inflation due to multiple comparisons, we set an initial uncorrected voxel-level threshold of p < 0.001, then applied a cluster level threshold at p < 0.05 corrected for search volume and the initial 0.001 threshold (Friston et al., 1994; Poline et al., 1997). This procedure rigorously and accurately limits the false positive rate due to multiple comparisons and is more sensitive than voxel-level statistics (Poline et al., 1997).
Because of the regional specificity of the predictions, univariate activation analyses were restricted to anatomical regions defined a priori in common atlas space (Maldjian et al., 2003). We created the individual bilateral regions of interest (ROI) in the following manner. The medial temporal ROI for testing prediction one included the bilateral hippocampus and parahippocampal gyri. Given the numerous medial temporal foci involved in consolidation effects, the resolution of the EPI images relative to the size of these medial temporal structures, and the potential inaccuracies in post-normalization overlap, this generous ROI including most of the medial temporal region, is appropriate. To better localize consolidation related changes in this region and identify which portions of the medial temporal memory network were active, likely locations of effects were then identified using a probabilistic cytoarchitectonic atlas created from postmortem histology (Amunts et al., 2005) in conjunction with the SPM Anatomy Toolbox (Eickhoff et al., 2005). These probabilities, based on post-normalization overlap of multiple subjects offer a potentially more realistic interpretation of localization across subjects than discrete labels. Local maxima of test statistics within activated regions of interest were assigned to an anatomical region if their probability of being a region exceed a conservative threshold of 80%. For localization, the CA fields, dentate gyrus, and subiculum definitions within the atlas were combined into a single hippocampus region. While these atlas-based probabilistic localizations are likely inferior to manual segmentation of high-resolution images from individual subjects, the fast, whole-brain images necessary to test all seven predictions reduce the applicability of these manual methods.
Because of the visual-then-auditory nature of the associate memories, the likely sensory storage regions are the fusiform and inferior temporal gyri for visual memories and the middle and superior temporal gyri for auditory memories. Thus, the “neocortical region” for testing prediction two consisted of two ROIs, one combining the fusiform and inferior temporal gyri, and one combining the middle and superior temporal gyri. The prefrontal mask for testing prediction three was restricted to the bilateral inferior frontal gyri (see supplementary material for graphical depiction of anatomical ROIs).
Functional connectivity analysis was performed by calculating the change in inter-regional correlation coefficients of the residual time series between sessions. Eleven ROI time-series were created for each subject from 7.5mm cubes centered on each of the 11 maxima or sub-maxima of the between condition changes that went in the predicted direction from the above univariate analysis (see Table 2). Thus, the connectivity ROIs were selected by locating statistical peaks within the a priori defined anatomical regions of interest. The time-series data from ROI voxels were band pass filtered and mean centered at each voxel within each session thus removing the main effect of session. For each ROI, the principal eigenvector was extracted from each run independently and concatenated. The expected task effect and motion effects were removed from each ROI time-series via regression using the design matrix from the univariate analysis. To avoid relationships due to global noise signals such as heartbeat, respiration or other volume-wise confounds and reduce any effect of differences in between session signal to noise ratios, the design matrices were augmented with noise estimates based on time-series from ROIs in the first and second ventricles. The residual entered into the correlation analysis contains the trial-to-trial task variability without the consistent task effect (Horwitz et al., 2005). Robust between session changes in the inter-regional correlation coefficients were identified by converting the within condition correlation coefficients in each subject to Z scores using Fisher's Z transform, normalizing the between condition change in Z, and using the Z test, Bonferoni corrected for multiple comparisons, across subjects to identify non-zero changes. Because the main effects of task and session were removed prior to the correlation analysis, the analysis is not redundant with the activation analysis.
Table 2.
Consolidation related changes in regional functional connectivity. Changes in functional connectivity between regions are indicated by the Fisher’s Z score for the change in correlation coefficient above the main diagonal and by the associated p value corrected for multiple comparisons below the main diagonal. Changes that were not statistically robust are indicated by the – symbol. Connectivity changes that were not relevant to the extended theory of systems level consolidation were not tested and have been left blank in the table.
| Hippocampal Complex | Lateral Temporal | Interior Frontal Gyrus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI Coordinates |
−30, −40, −12 | −32, −30, −15 | −30, −32, −12 | −30, −40, −12 | −52, −58, −15 | −60, −40, −15 | −65, −42, −2 | 40, 38, 12 | 48, 40 −12 | 52, 28, −8 | −45, 12, 28 |
| −30, −40, −12 | --- | --- | --- | --- | --- | 3.77 | --- | ||||
| −32, −30, −15 | −3.60 | --- | --- | --- | 3.61 | --- | --- | ||||
| −30, −32, −12 | --- | --- | --- | --- | --- | --- | --- | ||||
| 28, −40, −8 | --- | --- | --- | --- | --- | --- | --- | ||||
| −52, −58, −15 | --- | 0.014 | --- | --- | 3.51 | --- | −5.00 | --- | --- | --- | |
| −60, −40, −15 | --- | --- | --- | --- | 0.020 | --- | −5.27 | --- | --- | 5.30 | |
| −65, −42, −2 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | |
| 40, 38, 12 | --- | --- | --- | --- | <.001 | <.001 | --- | ||||
| 48, 40 −12 | --- | 0.013 | --- | --- | --- | --- | --- | ||||
| 52, 28, −8 | 0.007 | --- | --- | --- | --- | --- | --- | ||||
| −45, 12, 28 | --- | --- | --- | --- | --- | <.001 | --- | ||||
RESULTS
Behavioral data
Subjects included in the analysis required 7.43 ± 2.31 training iterations on average to reach the 90% accuracy criterion. Mean accuracy levels at the 14 day unscanned reminder session was 70.14% ± 11%. While this accuracy was quite variable across subjects, at least some portion of the memories remained intact for all subjects as only 1.85 ± 0.80 additional training sessions were required to return all subjects to 90% accuracy (minimum 1 maximum 3). This was considerably less than the 7.3 training iterations required initially.
Response latencies and accuracies were recorded for each scanned trial. Behaviorally, subject accuracy was well above chance and comparable for recent memories and remote memories, (mean accuracy (% correct ± std) recent 80.32% ± 6.54% remote 75.93% ± 7.26%, t11 = 1.64, p = 0.13) though there was a trend for task sensitivity (d') to be greater in the recent than remote session (mean d'±std recent 1.94 ± 0.50, remote 1.52 ± 0.55, t11 = 2.09, p = 0.06). There was no robust difference between mean reaction time measured from the end of the auditory stimulus (mean RT±std recent 886.93ms ± 184.25ms, remote 840.76ms ± 321.66ms, t11 = 0.55, p = 0.60). Thus performance was comparable, though not equivalent, in the two sessions with a trend to greater accuracy for recent memories.
Univariate activation analysis
Predictions One through Three of the extended theory concern between-session changes in activity intensity in individual regions and are thus testable using univariate statistics. The results for Prediction One, decreased medial temporal response during remote memory relative to recent memory recall, were ambiguous. Two medial temporal regions (peak MNIx,y,z = −30 −40 −12, t11=5.31, K=26, pK=0.005(corrected), volume = 406.3µl; peak MNIx,y,z = 28 −40 −8, t11 = 5.16, K=12 pK=0.035(corrected), volume = 187.5µl) were identified as more active in the recent than remote condition during the delay period of the task (see Figure 2a–f) confirming the prediction. We then localized the cluster peaks using the probabilistic atlas as described above. Two sub-peaks within the left hemisphere cluster [(MNIx,y,z = −32 −30 −15, t11 = 4.86) and (MNIx,y,z = −30, −32 −12, t11 = 5.27)] were identified as being in the hippocampus region with a minimum of 90% probability. The first peak (−32, −30, −15) was active during the delay of the recent condition (t11 = 2.20, p=0.025) but not active in the remote condition (t11 = −1.31, p=0.108). The second peak (−30, −32, −12) was not active during the delay in the recent session (t11 =1.35, p=0.104) and less active than fixation during the remote condition (t11 =−2.65, p=0.011). No peaks from the right hemisphere cluster were identified as within the hippocampus and were instead localized to the parahippocampal gyrus.
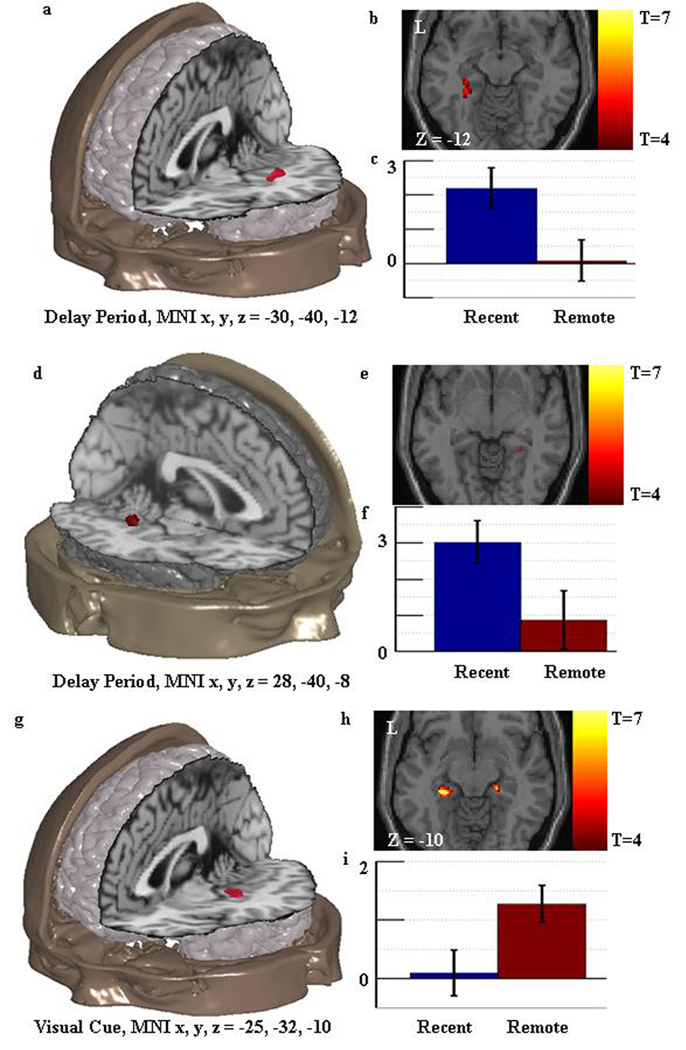
Figure 2. Univariate activation results relevant to prediction one: consolidation related decreased activity in the hippocampal complex.
Figure 2a shows the location of the consolidation related decrease in activity found during the delay period in the vicinity of the left hippocampus. This result is consistent with the extended theory. Figure 2b shows an axial slice through the region with the associated t values. Figure 2c shows the standardized regression coefficients for the peak of the region in the recent and remote memory condition with standard error bars. Figure 2d–f shows similar information for the consolidation related decrease in activity found in the right hippocampal complex during the delay period. This result is consistent with the extended theory. Figure 2g–i shows similar information for the consolidation related increase in activity found in the hippocampal complex during the visual cue. This result is inconsistent with the extended theory.
Critically however, a cluster within the medial temporal ROI was identified as more active in the remote than recent condition during the visual cue period of the task (peak MNIx,y,z = −25, −32, −10, t11 = 5.76, K = 28, pK = 0.007(corrected)), volume = 437.5µl; see Figure 2g–i) contradicting the theoretical prediction. The single peak of this cluster (MNIx,y,z = −25 −32 −10, t11 = 5.76) was assigned to the hippocampus region with an 80% probability. This peak was not active above fixation in the recent session (t11 =0.05, p=0.481) but was active in the remote session (t11 =3.55, p=0.002).
Prediction Two of the extended theory, increased neocortical (in this case lateral temporal) response during remote memory relative to recent memory recall, was confirmed during the visual cue in the fusiform/inferior temporal ROI (peak MNIx,y,z = −52, −58, −15, t11 = 7.62, K = 23, pK = 0.015(corrected), volume = 359.4µl; Figure 3a–c). The peak of this cluster was active during the remote but not recent session (Recent t11 = 0.31, p = 0.381; Remote t11 = 3.81, p = 0.001). Prediction Two was also confirmed during the delay period in the middle/superior temporal ROI (MNIx,y,z = −60, −40, −15, t11 = 6.26, K = 25, pK = 0.022(corrected), volume = 390.6µl; Figure 3d–f). Again, the peak of this cluster was active in the remote but not recent session (Recent t11 = 0.13, p = 0.450; Remote t11 = 2.87, p = 0.0076). An additional subpeak of this cluster was also identified (MNIx,y,z = −65, −42, −2, t11 = 5.97). No differences in the fusiform/inferior temporal ROI were seen during the delay or auditory target portions of the task and no differences in the middle/superior temporal ROI were seen during the visual cue or auditory target periods of the task. No lateral temporal regions showed a greater response during the recent than remote session in any task period.
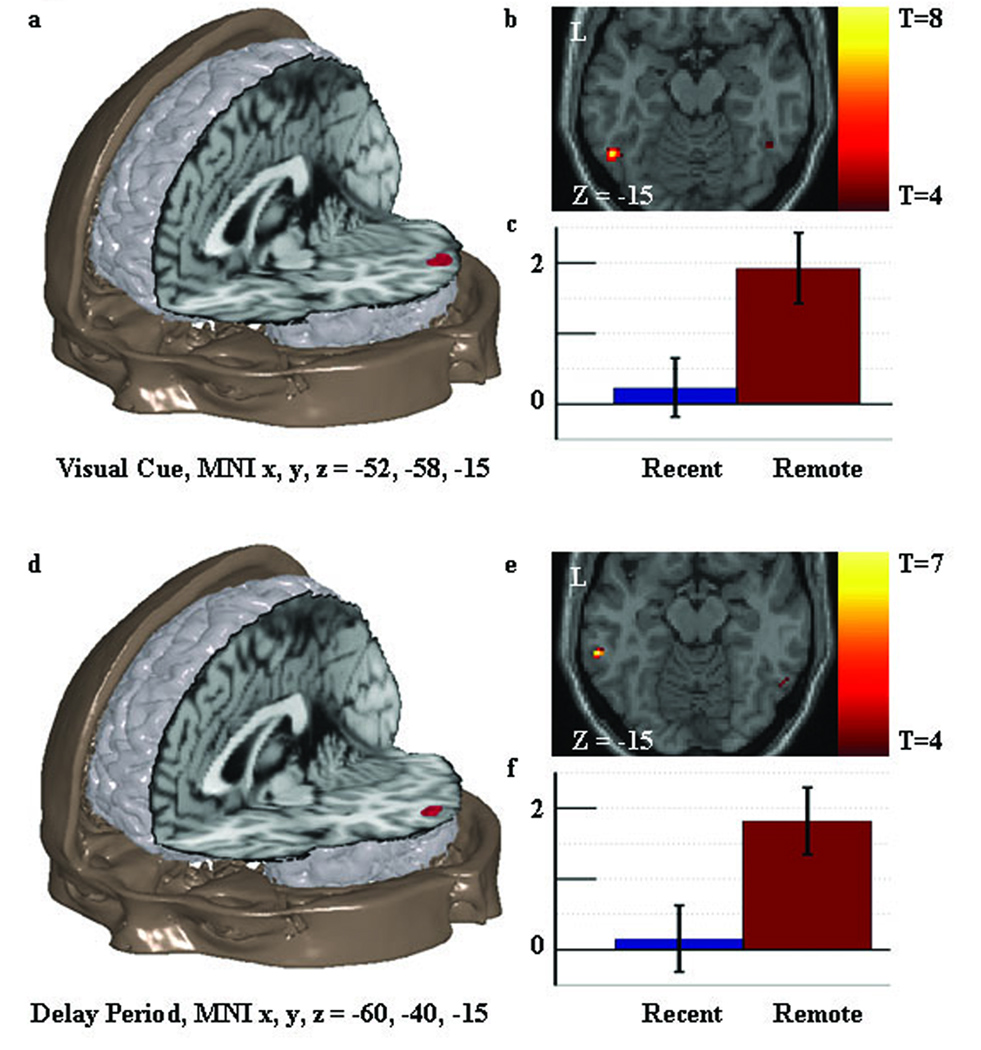
Figure 3. Univariate activation results relevant to prediction two: consolidation related increased activity in the lateral temporal cortex.
Figure 3a shows the location of the consolidation related increase in activity found in the fusiform-inferior temporal ROI during the visual cue. This result is consistent with the extended theory. Figure 3b shows an axial slice through the region with the associated t values. Figure 3c shows the standardized regression coefficients for the peak of the region in the recent and remote memory condition with standard error bars. Figure 3d–f shows similar information for the consolidation related increase in activity found in the middle-superior temporal ROI during the delay period.
Prediction Three of the extended theory indicating increased prefrontal response during remote memory relative to recent memory recall was also confirmed. During the visual cue, remote memory recall produced a larger relative BOLD response within the inferior frontal gyrus ROI, in the vicinity of BA 44 and 46 (MNIx,y,z = −45, 12, 28, t11 = 7.15, K = 23, pK = 0.022(corrected), volume = 359µl; Figure 4a–c) and in the vicinity of BA 47 (MNIx,y,z = 40 38 12, t11 = 6.14, K=24, pK=0.019(corrected), volume = 375µl. During the delay period, remote memory recall produced a larger relative BOLD response in the inferior frontal gyrus ROI, in the vicinity of BA 47 (MNIx,y,z = 48 40 −12, t11=5.70 K = 31 pK = 0.005(corrected), volume = 484µl; Figure 4d–f). No differences in this ROI were seen in the auditory target portion of the task and no inferior frontal gyrus regions were more active in the recent than the remote session during any task period.
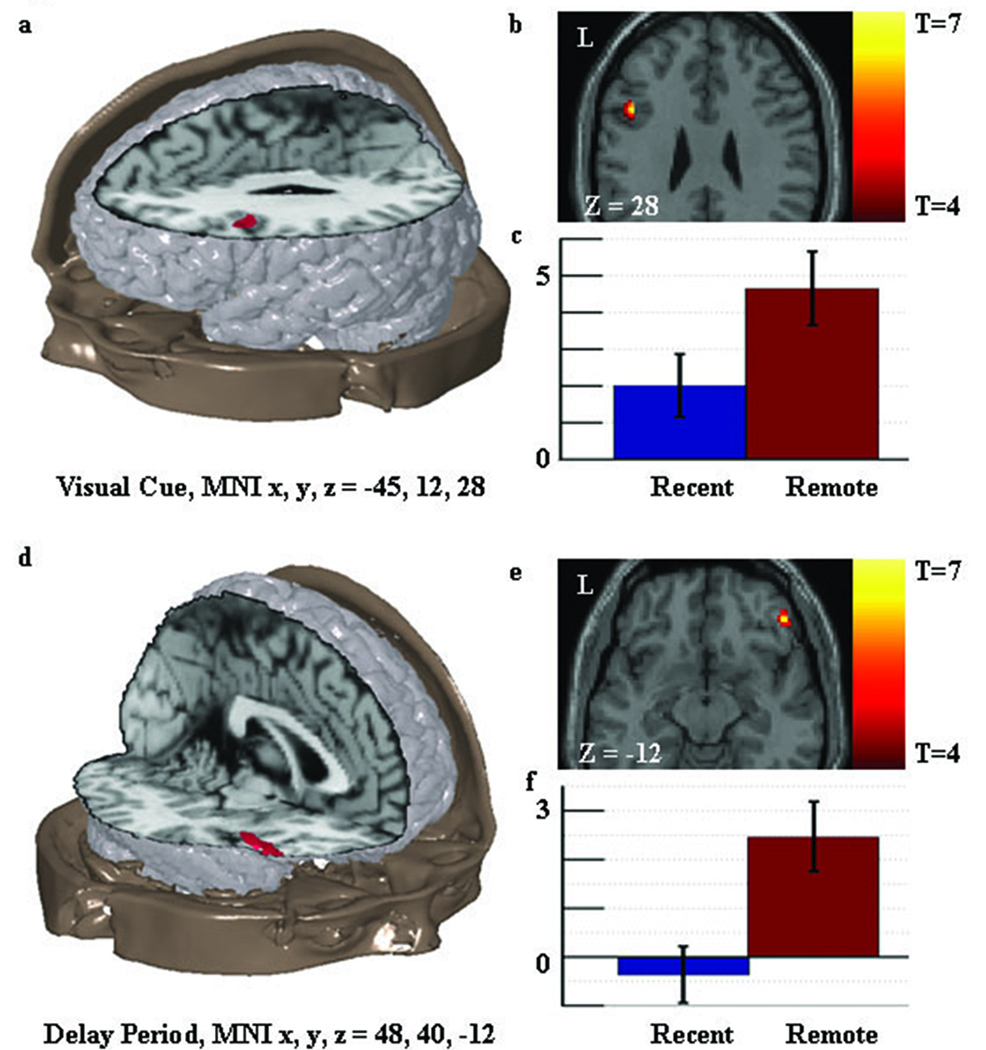
Figure 4. Univariate activation results relevant to prediction three: consolidation related increased activity in the prefrontal cortex.
Figure 4a shows the location of the consolidation related increase in activity found in the inferior frontal ROI during the visual cue. This result is consistent with the extended theory. Figure 4b shows an axial slice through the region with the associated t values. Figure 4c shows the standardized regression coefficients for the peak of the region in the recent and remote memory condition with standard error bars. Figure 4d–f shows similar information for the consolidation related increase in activity found in the inferior frontal ROI during the delay period.
Functional connectivity analysis
We tested for the predicted changes in functional connectivity magnitudes between the hippocampal complex, lateral temporal, and frontal regions identified in the univariate analyses. Peaks and sub-peaks from these ROIs were selected and analyzed using a correlated residuals analysis. The changes are summarized in Table 2. The functional connectivity of the left hippocampal region identified as more active during the visual cue in the remote than recent session was analyzed separately.
Prediction Four, increased connectivity between the medial temporal memory network and the prefrontal cortex was confirmed. Functional connectivity was greater during the remote than the recent recall condition between a right inferior frontal region and a region in the vicinity of the left perirhinal/parahippocampal cortex ((MNI x,y,z = 52, 28, −8 with MNI x, y, z = −30, −40, −12, Z11 = 3.77, p = 0.007(corrected)) and between another right inferior frontal region and a region in the vicinity of the left hippocampus (MNI x, y, z = 48, 40, −12 with MNI x, y, z = −32, −30, −15, Z11 = −3.61, p=0.013(corrected)).
The results for Prediction Five, increased functional connectivity between the prefrontal and neocortical storage regions, were ambiguous. As predicted, functional connectivity between the left dorsal prefrontal ROI and the ventral left middle temporal ROI was greater in the remote than recent condition (MNI x,y,z = −45, 12, 28, with MNI x,y,z = −60, −40, −15, Z11= 5.30, p < 0.001 corrected). However, functional connectivity was greater in the recent than remote condition between a right inferior frontal and two lateral temporal regions (MNI x,y,z = 40, 38, 12, with MNI x,y,z = −52, −58, −15, Z11 = −5.00, p < 0.001 corrected; and MNI x,y,z = 40, 38, 12, with MNI x,y,z = −60, −40, −15, Z11 = −5.27, p < 0.001 corrected). Thus, for Prediction Five, the left inferior frontal region conformed to the prediction while the right inferior frontal region did not.
Prediction Six, decreased connectivity between the neocortical storage regions and the hippocampal complex, was confirmed. Functional connectivity was greater in the recent than remote condition between the lateral temporal region and a region in the vicinity of the hippocampus (MNI x,y,z = −52, −58, −15 with MNI x,y,z = −32, −30, −15, Z11 = −3.60, p = 0.014 corrected).
Prediction Seven, increased functional connectivity within the neocortical (lateral temporal) region, was confirmed. Functional connectivity was greater in the remote than the recent condition between the inferior temporal/fusiform gyrus region and the middle temporal gyrus (MNI x, y, z = −52, −58, −15, with MNI x, y, z = −60, −40, −15, Z11 = 3.51, p=0.020 corrected). No decreases in functional connectivity were observed within these regions.
DISCUSSION
In the current study, we compared BOLD response for correct recent versus correct remote retrieval of non-autonoetic, associate memories. We used this data to explicitly test each of seven specific hypotheses derived from current systems level consolidation theory. The experimental analysis allowed us to compare not only the coarse-grain (between session) temporal dynamics, but finer-grain (within trial sub-component) dynamics as well. In general, we confirmed five of the seven predictions of the extended theory and found at least some evidence for the remaining two.
Systems level consolidation increased recall related activity in left lateral temporal regions associated with the modality of the stimuli being remembered and increased connectivity between these regions. While substantial volumes of the lateral temporal lobes were active in individual analysis of both sessions (not shown), the regions identified as increasing with consolidation were only robustly active in the remote memory condition and were not active in the recent memory condition (Figure 3C and F). This finding is consistent with the hypothesis that the memories have been at least partially consolidated to this region. Visual regions (inferior temporal/fusiform gyri) were identified as more active during the visual cue period while polymodal regions (middle temporal) were identified as more active later during the delay period consistent with the visual-then-auditory nature of the task and the supposition that sensory processing regions are involved in long-term memory storage. Connectivity between these two regions increased in the second session even after the main task effect was removed. Systems level consolidation also decreased functional connectivity between the left lateral temporal region and a peak within the medial temporal ROI, which was identified as likely within the hippocampus. Consolidation also increased functional connectivity between the left lateral temporal region and the left prefrontal cortex. This is consistent with the hypothesized reduction of the role of the medial temporal memory network in coordinating recall of sensory information in remote memory and an increase in the role of the prefrontal cortex. Again, the changes in connectivity are beyond that explainable by correlations due to the consistent task effect and thus reflect additional confirmations of the extended theory's hypotheses.
Within the medial temporal lobes, the results were less directly in line with the theoretical predictions; increases and decreases related to one month of experience were observed in this region. Medial temporal regions showing larger BOLD response in the recent than remote session were identified as likely within the hippocampus and perirhinal cortex. This localization is in agreement with other data with greater spatial resolution. Previous lesion studies have focused on the CA fields as a primary location of consolidation related reductions within the hippocampus proper (Bontempi et al., 1999) and the perirhinal cortex is known to be involved in delayed matching paradigms and paired associate memory in particular (Miyashita et al., 1996, 1998; Rolls and Kesner, 2006). A different region also probabilistically localized to the hippocampus was active above fixation baseline in the visual cue period of the remote condition only. Further study using a similar experimental paradigm at a much higher spatial resolution and focused specifically on the hippocampal region (at the expense of other regions) would be necessary to determine if the substructures of the hippocampus are differentially affected by systems consolidation.
Consolidation increased recall-related activity in prefrontal cortex bilaterally. The left prefrontal region was located in the vicinity of the pars opercularis, putative BA 44, an area strongly implicated in phonological and auditory processing (Romanski et al., 1999; Poldrack et al., 2001) and auditory working memory storage (Baddeley, 2003). This region was significantly active for both recent and remote memory, likely indicating its role in the working memory requirements of the task. The region increased activation and increased functional connectivity with the lateral temporal region during remote relative to recent memory recall. The increase in activity and connectivity is consistent with the hypothesis that this region is involved in the coordination of recall related activity as well since the working memory requirements of the task did not differ between sessions. The activation and connectivity results are in agreement with previous reports in nonhuman primates identifying coordinated activity between inferior temporal and prefrontal regions being necessary for memory recall (Eacott and Gaffan 1992; Gutnikov et al, 1997).
The right inferior frontal ROIs were putatively located in right Brodmann's area 47, a region that has been implicated in a number of memory processes including non-spatial working memory (Smith and Jonides, 1999). This region was only robustly active in the remote memory condition. These right frontal regions were more connected to the lateral temporal regions during the recent than remote memory recall session and more connected to the medial temporal memory system, including the putative hippocampus, in the remote than recent session. Since neither this prefrontal ROI nor the lateral temporal ROI were robustly active across subjects in the recent memory condition, it is unclear what greater connectivity in the recent condition indicates. It is possible that the correlation in the recent condition is not related to the trial-to-trial variability but to some other activity unrelated to the task such as “revisiting” the stimulus pairing or evaluating the response during the inter-trial interval.
Brodmann area 47 has been implicated in response suppression, including memory suppression, in both lesion and imaging studies (Aron et al., 2004). While BA47 is not the region implicated as having a direct role suppressing the hippocampus in animal models, it is possible that this region mediates suppression of the hippocampal region via strategic control. This potentially explains the increased connectivity between this region and the inactive portions of the hippocampal complex in the remote memory condition. It also possibly explains the larger connection in the recent session between the right prefrontal region and the lateral temporal region if right prefrontal cortex was suppressing lateral temporal involvement in the not-yet consolidated memory recall. However, this type of causal role cannot be directly determined from the correlation results and is perhaps beyond the types of memory suppression previously reported.
The increases and decreases seen in the medial temporal and other regions may not be completely due to consolidation related effects. The one month consolidation period used in the current study, though previously demonstrated to be of sufficient length to observe consolidation related effects (c.f. Takashima et al., 2006), was unlikely to be sufficient to complete the consolidation process. If considerable learning occurred in the 2 week reminder session, some “remote” memories may be as little as two weeks old. Thus the increase observed in the vicinity of the hippocampus may simply be due to continued consolidation processes or continued learning of the item pairs or other incidental learning.
Numerous studies have observed differential effects within the medial temporal lobes when contrasting encoding and recall related activity (c.f., Zeineh et al., 2003; Eldridge et al., 2005). In the current study, training occurred outside the scanner and subjects achieved a high level of accuracy. During scanned testing, no feedback was given and correct responses to both correct and incorrect pairings were analyzed. Thus any encoding related activity observed was likely to be incidental (e.g., trial ordering, incorrect pairings etc.). There is no a priori reason this incidental encoding should differ between sessions. Still, a reduction in encoding related processing remains a potential explanation for the medial temporal decrease and a potential confound for the current study. It is not clear, however, how this interpretation explains the medial temporal increase seen in the visual cue. An encoding/retrieval interpretation of this increase would suggest that subjects retrieved “more” during the remote condition when they were numerically less accurate and trended toward decreased sensitivity. While this might indicate more “effortful” retrieval related activity, such an interpretation seems contradictory to a previous study showing a positive correlation of medial temporal activity with accuracy during the retrieval phase (Zeineh et al., 2003).
Encoding versus retrieval could also be argued to potentially explain the prefrontal results. The hemispheric encoding retrieval asymmetry (HERA) model has been used to describe differences in prefrontal activity during encoding and retrieval (Tulving et al., 1994, Nyberg et al., 1996). Though not without controversy (c.f Miller et al., 2002), HERA theory states that memory encoding preferentially activates left prefrontal regions while recall activates right prefrontal regions. Thus, the increased right prefrontal activity during the remote condition may simply reflect increased recall related processes rather than consolidation related effects. However, the left prefrontal cortex also increased from the recent to the remote session, contrary to this interpretation. It is not clear how both encoding and retrieval could have increased in the remote session though perhaps a re-encoding of partially forgotten visual stimuli is possible. Further, it is unclear how such an interpretation explains the decreased connectivity between the right prefrontal region and the lateral temporal region, which would presumably increase if the right prefrontal activity reflected recall. Nor does the HERA model encoding/retrieval interpretation seem consistent with the increased left prefrontal and lateral temporal connectivity unless encoding processes also increased for remote memory recall. As with the hippocampal complex results, studying both learning and consolidation simultaneously may help to better disambiguate these issues.
A related alternative explanation is an effect of memory type and/or memory strength. Differential recall effects have been described within the medial temporal lobes with the hippocampus proper associated with explicit memory recall and perirhinal cortex and other structures associated with feelings of familiarity (Brown and Aggleton, 2001). It is possible that a change in the level of explicit recall between the recent and remote sessions may explain the medial temporal increase and decrease observed here. However it is unclear how participants could perform the paired associate memory task with only familiarity information given that foils are always drawn from other experimental items and all stimuli are presented an equal number of times.
Differential medial temporal lobe effects have also been ascribed to memory strength (Squire et al., 2007). Though we analyzed correct trials only, it is possible that the trend to greater accuracy in the recent condition indicates that subjects held stronger memories during this session. Based on the memory strength interpretation, the weaker memory should have resulted in decreased hippocampal activity and increased perirhinal cortex activity. However, we observed an increase and decrease putatively within the hippocampus. Further the decrease we observed occurred during the delay period and the increase during the visual cue. While differential memory strength remains a possible explanation for some of the observed changes, it cannot account for the full pattern of results. Better spatial resolution within the hippocampus would partially help to address this question, though the memory strength hypothesis does not attempt to address the within trial temporal difference observed.
Task difficulty may also impact the pattern of results in the prefrontal cortex. Again, given the observed trend to decreased sensitivity, recall in the remote condition was likely more difficult. Thus, the increased left prefrontal activity may simply reflect this increased difficulty (Gould et al., 2003). The additional increase in right prefrontal activity could reflect a change in strategy in response to less vivid memories. Such strategy shifts have been observed for delayed matching tasks with longer delays which presumably also reflect some reduction in vividness (McIntosh et al., 1996).
In an attempt to avoid the potentially confounding effects of difficulty, we used a high criterion level during training, excluded subjects who could not accurately learn the pairings quickly, and excluded subjects who could not accurately respond during testing. Furthermore, we examined only correct responses that were given quickly to pairings to which subjects responded correctly during both sessions; incorrect responses were modeled separately and excluded from the analyses here. Thus, the subjects were in essence 100% accurate for the trials actually examined for the all activation effects reported here. While these considerations likely reduced the possible difficulty effects, differences in recall difficulty remains a potential factor influencing the observed findings. Further study using parametric manipulations of difficulty and consolidation should be used to evaluate their independent effects.
A further limitation of the study may arise from the longitudinal design. Contrasting different scanning sessions potentially induces confounds related to uncontrollable changes in subjects and within the scanner itself. Designs where different item pairs trained at different times and tested within a single session would complement the current study. However, several of these confounding effects may be reduced by staggering subject enrolment such that the recent session of one subject occurs after the remote session of another avoiding coherent scanner confounds, removing the main effects of runs (and thus session), correcting for non-sphericity, and examining only correct trials. All of these methods were employed here. In addition, the current design has the advantage of testing the exact same memories, avoiding counter-balancing issues, and avoiding potential interactions between memories from the same category acquired at different times.
Though the opposing consolidation related effects within the medial temporal region observed here may or may not be completely reconcilable with the extended theory, they potentially explain previous contradictory imaging results. Previous human imaging studies of systems level consolidation have identified consolidation-related decreases in the hippocampal complex while others have reported consolidation related increases. Most early studies and even several later studies employed blocked designs where the BOLD signal from multiple temporally adjacent trials (including potentially incorrect trials) is combined to observe effects. Previous event related designs have used short trials and thus were unable to disambiguate differential effects occurring at sub-trial time-scales. A lack of temporal resolution, combined with the limited spatial resolution of fMRI, may account for previous inconsistent results if the two effects observed here became spatio-temporally blurred together in previous analyses. Unfortunately, the BOLD signal measured by fMRI has a lower limit to its temporal resolution that is orders of magnitude above that of neural activity. Elongating that neural activity through longer trials as was done here may alter the nature of that activity and thus alter the resulting fMRI data. For example, increasing the trial length likely increased working memory demands which has been shown to alter hippocampal activity (Hannula and Ranganath 2008). Further study with imaging methods such as MEG which provide superior temporal resolution may help to test the robustness of the observed difference in the trial subcomponents and clarify the temporal pattern of effects.
The goal of the current experiment was to test the predictions of the extended theory of systems level consolidation in a situation where there would be little theoretical disagreement regarding the predicted outcome. To achieve adequate response accuracy levels, we used a large number of training exposures to the experimental pairs which potentially removed the majority of the autonoetic consciousness or “episodic” quality from these associate memories (Tulving, 1985). Though it is not possible to integrate the knowledge of these nonlinguistic pairings into the broader semantic knowledge base, the associates memories examined here are best described as semantic-like or semanticized memories rather than episodic memories. Multiple Trace Theory agrees with the standard theory that the hippocampal memory system should be less involved in storage and recall of these memories as they age (Nadel and Moscovitch, 1997). However, the current study does have strong consequences for the debate between these two theories. Human imaging studies using a prospective memory paradigm to test the predictions of Multiple Trace Theory for presumably purely episodic memories have argued that a hippocampal increase or decrease would respectively provide evidence in favor or against the theory (cf. Bossardt et al, Takashima et al., 2006; Nadel et al., 2007). In the current study examining associate memories, both relative increases and decreases were observed in the hippocampal complex region though with potentially different spatial foci and a different intra-trial temporal profile. This suggests that definitive evidence for or against either theoretical position may not be obtainable using human functional neuroimaging until the stability, cause, and time course of these relative changes are more fully characterized.
CONCLUSION
We simultaneously tested all seven predictions of the extended model of systems level consolidation including changes in regional activity and interregional functional connectivity using longitudinal functional neuroimaging and a visual-to-auditory paired associate memory task. In general, our results support the predictions of the extended theory. Evidence for each of the seven predictions was observed though evidence complicating the interpretation of two predictions was also identified. Acknowledging a division of labor within the hippocampal complex as well as differences between the left and right prefrontal cortex may potentially reconcile these two contradictory results with the extended theory. Differences among the trial subcomponents were also observed. Previous imaging studies with block designs or event related designs which treat a trial as a single entity were unable to resolve these differences. Thus, previous conflicting results within the hippocampal memory system may have been the result of inadequate temporal resolution. These relatively short-term (i.e., seconds) dynamics are outside the scope of the current extended theory and provide additional information for its future development. The results also indicate caution is required in interpreting hippocampal increases or decreases in activation seen in human functional neuroimaging as definitive support for or against theories of remote episodic memory recall and suggest more detailed analysis of both the long and short-term consequences of memory consolidation are needed.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the intramural program of the National Institute on Deafness and Other Communication Disorders, the National Institutes of Health, and a National Institutes of Health Intramural Research Training Award to JFS. The authors would also like to acknowledge support from the National Institute on Aging (R01AG025526 [GEA]), the Evelyn F. McKnight Brain Institute [GEA], and the State of Arizona [GEA, KC]. We thank L. Talagala, J. Black, and R. Hill for their assistance with data acquisition, A. R. Braun for assistance with medical evaluations, J. Cooper for granting permission to use the fractal images, and A. R. Braun, L. Ungerleider, M. Mishkin, E. Murray, S. Goldinger, P. Killeen, and three anonymous reviewers for comments on earlier versions of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alverez P, Squire LR. Memory consolidation and the medial temporal lobe: a simple network model. Proc. Natl. Acad. Sci. USA. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. and Embryo. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cog. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory and language: an overview. J. of Comm. Disorders. 2003;36:189–208. doi: 10.1016/s0021-9924(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Bernard FA, Bullmore E, Graham K, Thompson S, Hodges J, Fletcher PC. The hippocampal region is involved in successful recognition of both remote and recent famous faces. Neuroimage. 2004;22:1704–1714. doi: 10.1016/j.neuroimage.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Stewart CA, Forrest EM. Retrograde amnesia and memory reactivation in rats with ibotenate lesions to the hippocampus or subiculuim. Q. J. Exp. Psychol. B. 1994;47:129–150. [PubMed] [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- Bosshardt S, Degonda N, Schmidt CF, Goesiger P, Nitsch RM, Hock C, Henke K. One month of human memory consolidation enhances retrieval-related hippocampal activity. Hippocampus. 2005;15:1026–1040. doi: 10.1002/hipo.20105. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus. Nature Rev. Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Cho YH, Beracochea D, Jaffard R. Extended temporal gradient for the retrograde and anterograde amnesia produced by ibotenate entorhinal cortex lesions in mice. J. Neurosci. 1993;13:1759–1766. doi: 10.1523/JNEUROSCI.13-04-01759.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Zola SM, Squire LR. Anterograde amnesia and temporally graded retrograde amnesia for a nonspatial memory task after lesions of hippocampus and subiculum. J. Neurosci. 2002;22:4663–4669. doi: 10.1523/JNEUROSCI.22-11-04663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douville K, Woodard JL, Seidenberg M, Miller SK, Leveroni CL, Nielson KA, Franczak M, Antuono P, Rao S. Medial temporal lobeactivity for recognition of recent and remote famous names: an event related fMRI study. Neuropsychologia. 2005;43:693–703. doi: 10.1016/j.neuropsychologia.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D. Inferotemproal-frontal disconnection: The uncinate fascicle and visual associative learning in monkeys. Eur. J. Neurosci. 1992;4:1320–1332. doi: 10.1111/j.1460-9568.1992.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The Medial Temporal Lobe and Recognition Memory. Ann. Rev. Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras M-H, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoachitectonic areas revisited. Neuroimage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Engle SA, Zeineh MM, Bookheimer S, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. J. Neurosci. 2005;25:3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat. Rev. Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Ding H, Takahashi E, Suzuki A, Kida S, Silva AJ. Stability of recent and remote contextual fear memory. Learn. Mem. 2006;13:451–457. doi: 10.1101/lm.183406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum. Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Zarahn E, Josephs O, Henson RNA, Dale AM. Stochastic designs in event-related fMRI. Neuroimage. 1999;10:607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb. Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Nesbitt C, Mumby DG. Perirhinal cortex lesions produce variable patterns of retrograde amnesia in rats. Behav. Brain Res. 2003;141:183–193. doi: 10.1016/s0166-4328(02)00377-7. [DOI] [PubMed] [Google Scholar]
- Gould RL, Brown RG, Owen AM, Ffytche DH, Howard RJ. fMRI BOLD response to increasing task difficulty during successful paired associates learning. Neuroimage. 2003;20:1006–1019. doi: 10.1016/S1053-8119(03)00365-3. [DOI] [PubMed] [Google Scholar]
- Gutnikov SA, Ma YY, Gaffan D. Temporo-frontal disconnection impairs visual-visual association learning but not configural learning in Macaca monkeys. Eur. J. Neurosci. 1997;9:1524–1529. doi: 10.1111/j.1460-9568.1997.tb01507.x. [DOI] [PubMed] [Google Scholar]
- Haist F, Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat. Neurosci. 2001;4:1139–1145. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational binding. J. Neurosci. 2008;28:116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi S, Miyashita Y. Formationof mnemonic neural responses to visual paired acssociates in inferotemporal cortex is impaired by perirhinal and entorhinal lesions. Proc. Natl. Acad. Sci. USA. 1996;93:739–743. doi: 10.1073/pnas.93.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL. Coordinated reactivation of distributed memory traces in primate neocortex. Science. 2002;297:2070–2073. doi: 10.1126/science.1073538. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Warner B, Fitzer J, Tagamets MA, Husain FT, Long TW. Investigating the neural basis for functional and effective connectivity: Application to fMRI. Phil. Trans. Roy. Soc. Lond. B. 2005;360:1093–1108. doi: 10.1098/rstb.2005.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Quillfeldt JA, Zanatta MS, Quevedo J, Schmitz PK, Medina JH. Sequential role of hippocampus and amygdala entorhinal cortex and parietal cortex in formation and retrieval of memory for inhibitory avoidance in rats. E. J. Neurosci. 1997;9:786–793. doi: 10.1111/j.1460-9568.1997.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lechner HA, Squire LR, Byrne JH. 100 years of consolidation—remembering Muller and Pilzecker. Learn. Mem. 1999;6:77–87. [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J. Neurosci. 2003;23:5302–5307. doi: 10.1523/JNEUROSCI.23-12-05302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Henson RN, Mummery C, Frith CD. Activity in prefrontal cortex, not hippocampus, varies parametrically with the increasing remoteness of memories. Neuroreport. 2001;12:441–444. doi: 10.1097/00001756-200103050-00004. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Grady CL, Haxby JV, Ungerleider LG, Horwitz B. Changes in limbic and prefrontal functional interactions in a working memory task for faces. Cereb. Cortex. 1996;6:571–584. doi: 10.1093/cercor/6.4.571. [DOI] [PubMed] [Google Scholar]
- Miller MB, Kingstone A, Gazzaniga MS. Hemispheric encoding asymmetry is more apparent than real. J. Cogn. Neurosci. 2002;14:702–708. doi: 10.1162/08989290260138609. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Miyashita Y. Inferior temporal cortex: Where visual perception meets memory. Annu. Rev. Neurosci. 1993;16:245–263. doi: 10.1146/annurev.ne.16.030193.001333. [DOI] [PubMed] [Google Scholar]
- Miyashita Y. Cognitive memory: Cellular and network machineries and their top-down control. Science. 2004;306:435–440. doi: 10.1126/science.1101864. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Okuno H, Tokuyama W, Ihara T, Nakajima K. Feedback signal from medial temporal lobe mediates visual associative mnemonic codes of inferotemporal neurons. Cogn. Brain Res. 1996;5:81–86. doi: 10.1016/s0926-6410(96)00043-2. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Naya Y, Sakai K, Miyashita Y. Activity of primate inferotemporal neurons relater to a sought target in a pair-association task. Proc. Natl. Acad. Sci. USA. 1996;93:2664–2669. doi: 10.1073/pnas.93.7.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya Y, Yoshida M, Miyashita Y. Backward spreading of memory retrieval signal in the primate temporal cortex. Science. 2001;291:661–664. doi: 10.1126/science.291.5504.661. [DOI] [PubMed] [Google Scholar]
- Naya Y, Yoshida M, Miyashita Y. Forward processing of long-term associative memory in monkey inferotemporal cortex. J. Neurosci. 2003;23:2861–2871. doi: 10.1523/JNEUROSCI.23-07-02861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki K, Luo J. An fMRI study on the time-limited role of the medial temporal lobe in long-term topographical autobiographic memory. J. Cogn. Neurosci. 2002;14:500–507. doi: 10.1162/089892902317362010. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Cabeza R, Tulving E. PET studies of encoding and retrieval: the HERA model. Psychonomic Bull. Rev. 1996;3:35–148. doi: 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- O'Mara S. Controlling hippocampal output: The central role of the subiculum in hippocampal information processing. Beh. Brain Res. 2006;174:304–312. doi: 10.1016/j.bbr.2006.08.018. [DOI] [PubMed] [Google Scholar]
- O'Mara SM, Commins S, Anderson M, Gigg J. The subiculum: A review of form, physology and function. Prog. Neurobiol. 2001;64:129–155. doi: 10.1016/s0301-0082(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss P, Zilles K, Markowitsch H, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Piolino P, Giffard-Quillon G, Desgranges B, Chetelat G, Baron J, Eustache F. Re-experiencing old memories via hippocampus: a PET study of autobiographical memory. Neuroimage. 2004;22:1371–1383. doi: 10.1016/j.neuroimage.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, Gabrieli JDE. Relations between the neural bases of dynamic auditory processing and phonological processing: Evidence from fMRI. J. Cog. Neurosci. 2001;13:687–697. doi: 10.1162/089892901750363235. [DOI] [PubMed] [Google Scholar]
- Poline J-B, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activation in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Rekkas PV, Constable RT. Evidence that autobiographic memory retrieval does not become independent of the hippocampus: an fMRI study contrasting very recent with remote events. J. Cogn. Neurosci. 2005;17:1950–1961. doi: 10.1162/089892905775008652. [DOI] [PubMed] [Google Scholar]
- Remez RE, Pardo JS, Piorkowski RL, Rubin PE. On the bistability of sinewave analogs of speech. Psych. Sci. 2001;12:24–29. doi: 10.1111/1467-9280.00305. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog. in Neorobio. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat. Neurosci. 1999;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, Eichenbaum H. Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory. J. of Neurosci. 2006;26:4852–4859. doi: 10.1523/JNEUROSCI.0659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Miyashita Y. Neural organization for the long-term memory of paired associates. Nature. 1991;354:152–155. doi: 10.1038/354152a0. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992;99:195. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr. Opin. Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Bayley PJ. The neuroscience of remote memory. Curr. Opin. Neurobiol. 2007;17:185–196. doi: 10.1016/j.conb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat. Rev. Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Episodic memory, semantic memory and amnesia. Hippocampus. 1998;8:205–211. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Stark CEL, Squire LR. fMRI activity in the medial temporal lobe during recognition memory as a function of study-test interval. Hippocampus. 2000;10:329–337. doi: 10.1002/1098-1063(2000)10:3<329::AID-HIPO13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Steinvorth S, Corkin S, Halgren E. Ecphory of autobiographical memories: an fMRI study of recent and remote memory retrieval. Neuroimage. 2006;30:285–298. doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Petersson K, Rutters F, Tendolkar I, Jensen O, Zwarts M, McNaughton BL. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc. Natl. Acad. Sci. USA. 2006;103:756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature. 1999;401:699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Can. Psycho. 1985;25:1–12. [Google Scholar]
- Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc. Natl. Acad. Sci. USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG. Functional brain imaging studies of cortical mechanisms for memory. Science. 1995;270:769–775. doi: 10.1126/science.270.5237.769. [DOI] [PubMed] [Google Scholar]
- Wilson MA. Hippocampal memory formation, plasticity, and the role of sleep. Neurobiol. Learn. Mem. 2002;78:565–569. doi: 10.1006/nlme.2002.4098. [DOI] [PubMed] [Google Scholar]
- Zarahn E. Testing for neural responses during temporal components of trials with BOLD fMRI. NeuroImage. 2000;11:783–796. doi: 10.1006/nimg.2000.0560. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, D’Esposito M. Temporal isolation of the neural correlates of spatial mnemonic processing with fMRI. Cognit. Brain Res. 1999;7:255–268. doi: 10.1016/s0926-6410(98)00029-9. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan SM, Squire LR. The primate hippocampal formation: evidence for a time-limited role in memory storage. Science. 1990;250:288–290. doi: 10.1126/science.2218534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.