Abstract
The recent reports of the effect of 2,3-diphosphoglycerate (2,3-DPG) on hemoglobin affinity for oxygen suggested that this substance may play a role in man's adaptation to acidosis and alkalosis.
A study of the effect of induced acidosis and alkalosis on the oxyhemoglobin dissociation curve of normal man was therefore carried out, and the mechanisms involved in the physiological regulation of hemoglobin oxygen affinity examined.
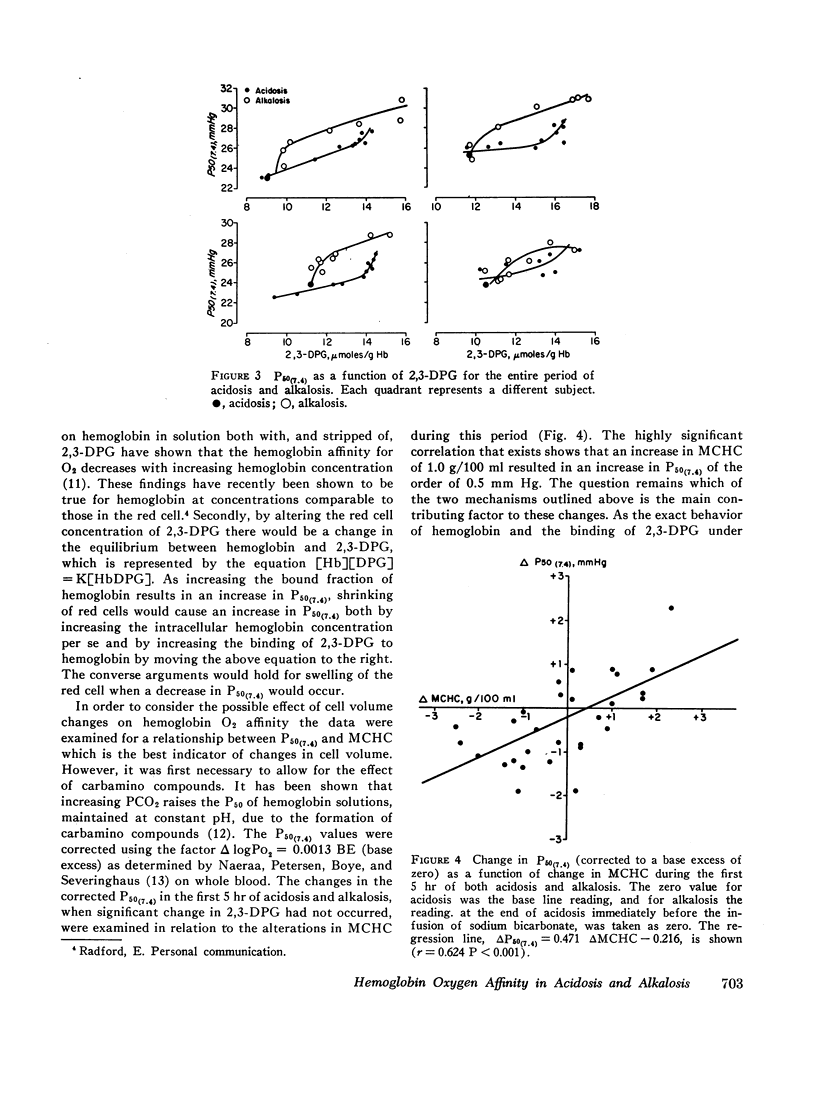
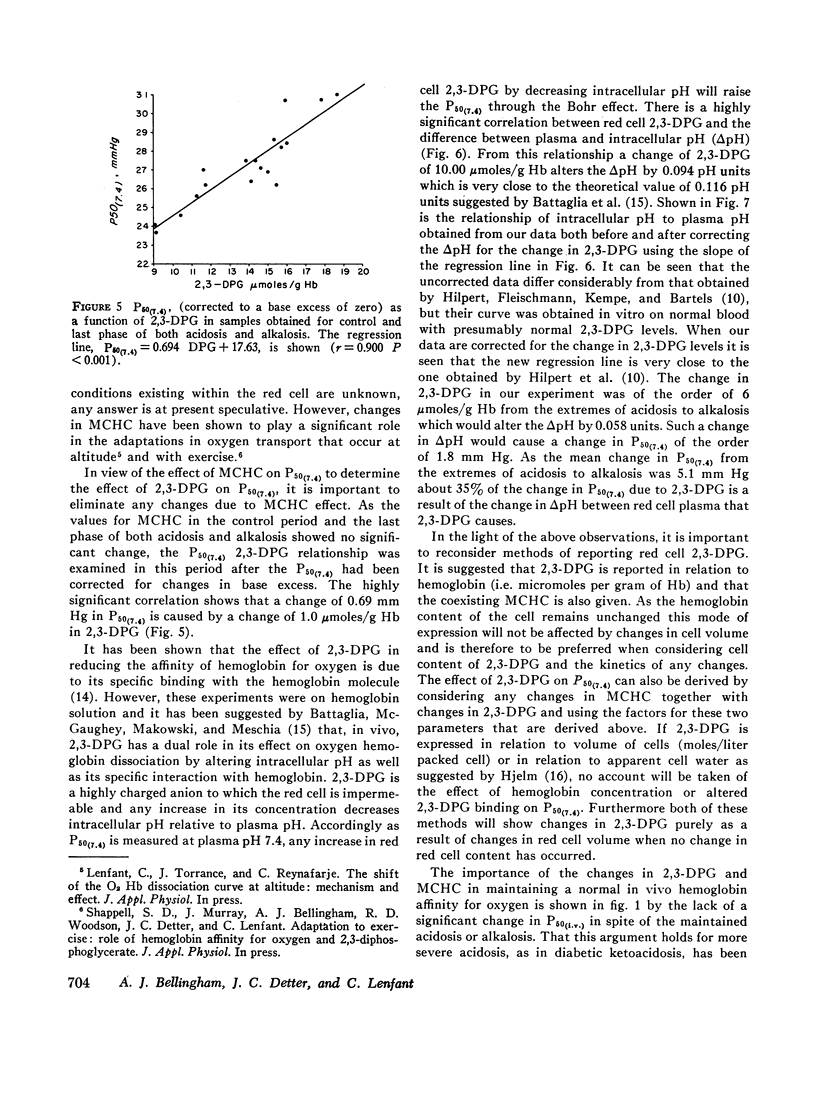
In acute changes of plasma pH there was no alteration in red cell 2,3-DPG content. However, there were changes in hemoglobin oxygen affinity and these correlated with changes in mean corpuscular hemoglobin concentration (MCHC). With maintained acidosis and alkalosis, red cell 2,3-DPG content was altered and correlated with the changes in hemoglobin oxygen affinity. Both of these mechanisms shift the hemoglobin oxygen dissociation curve opposite to the direct pH (Bohr) effect, and providing the rate of pH change is neither too rapid nor too large, they counteract the direct pH effect and the in vivo hemoglobin oxygen affinity remains unchanged.
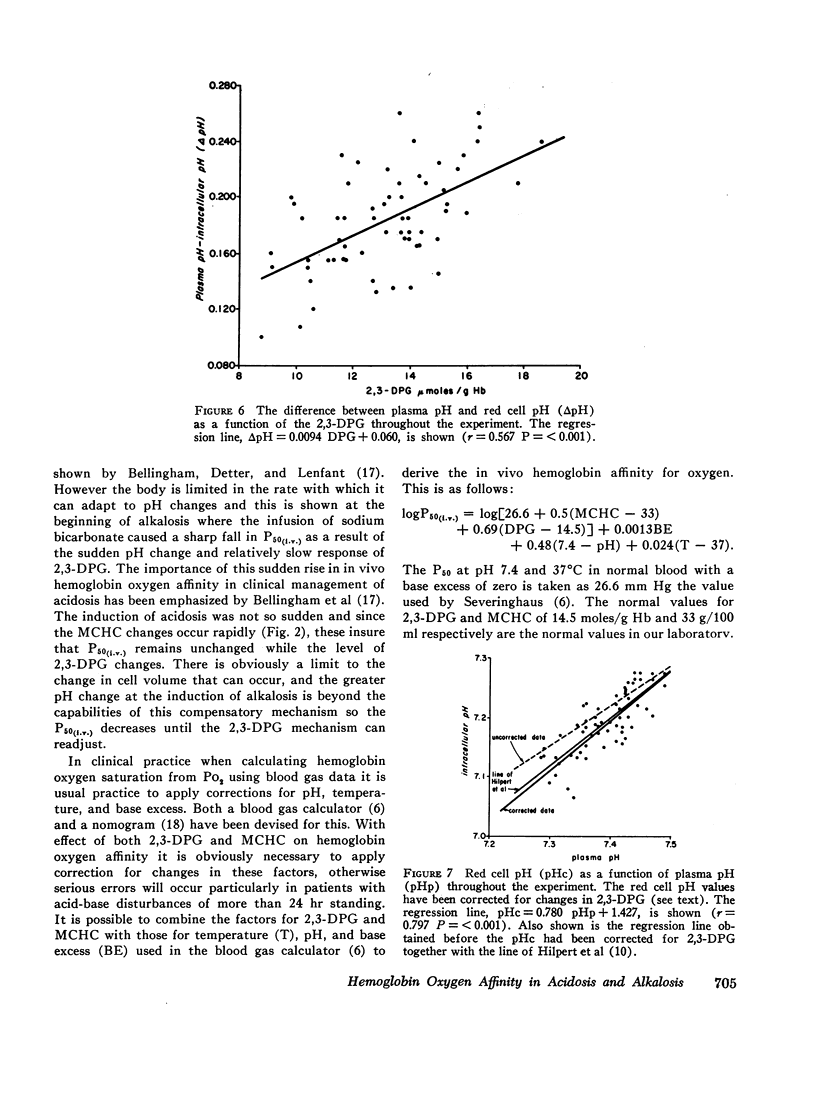
It is also shown that approximately 35% of the change in hemoglobin oxygen affinity resulting from an alteration in red cell 2,3-DPG, is explained by effect of 2,3-DPG on the red cell pH.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Battaglia F. C., McGaughey H., Makowski E. L., Meschia G. Postnatal changes in oxygen affinity of sheep red cells: a dual role of diphosphoglyceric acid. Am J Physiol. 1970 Jul;219(1):217–221. doi: 10.1152/ajplegacy.1970.219.1.217. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yu C. I. The oxygenation of hemoglobin in the presence of 2,3-diphosphoglycerate. Effect of temperature, pH, ionic strength, and hemoglobin concentration. Biochemistry. 1969 Jun;8(6):2567–2571. doi: 10.1021/bi00834a046. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. Intracellular organic phosphates as regulators of oxygen release by haemoglobin. Nature. 1969 Feb 15;221(5181):618–622. doi: 10.1038/221618a0. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- Chanutin A., Curnish R. R. Effect of organic and inorganic phosphates on the oxygen equilibrium of human erythrocytes. Arch Biochem Biophys. 1967 Jul;121(1):96–102. doi: 10.1016/0003-9861(67)90013-6. [DOI] [PubMed] [Google Scholar]
- HILPERT P., FLEISCHMANN R. G., KEMPE D., BARTELS H. THE BOHR EFFECT RELATED TO BLOOD AND ERYTHROCYTE PH. Am J Physiol. 1963 Aug;205:337–340. doi: 10.1152/ajplegacy.1963.205.2.337. [DOI] [PubMed] [Google Scholar]
- Hjelm M. The mode of expressing the content of intracellular components of human erythrocytes with special reference to adenine nucleotides. Scand J Haematol. 1969;6(1):56–64. doi: 10.1111/j.1600-0609.1969.tb01804.x. [DOI] [PubMed] [Google Scholar]
- Lenfant C., Ways P., Aucutt C., Cruz J. Effect of chronic hypoxic hypoxia on the O2-Hb dissociation curve and respiratory gas transport in man. Respir Physiol. 1969 Jun;7(1):7–29. doi: 10.1016/0034-5687(69)90066-8. [DOI] [PubMed] [Google Scholar]
- MARGARIA R. The contribution of hemoglobin to acid-base equilibrium of the blood in health and disease. Clin Chem. 1957 Aug;3(4 Pt 2):306–318. [PubMed] [Google Scholar]
- ROBINSON M. A., LODER P. B., DE GRUCHY G. C. Red-cell metabolism in non-spherocytic congenital haemolytic anaemia. Br J Haematol. 1961 Jul;7:327–339. doi: 10.1111/j.1365-2141.1961.tb00343.x. [DOI] [PubMed] [Google Scholar]
- SEVERINGHAUS J. W. Oxyhemoglobin dissociation curve correction for temperature and pH variation in human blood. J Appl Physiol. 1958 May;12(3):485–486. doi: 10.1152/jappl.1958.12.3.485. [DOI] [PubMed] [Google Scholar]
- Severinghaus J. W. Blood gas calculator. J Appl Physiol. 1966 May;21(3):1108–1116. doi: 10.1152/jappl.1966.21.3.1108. [DOI] [PubMed] [Google Scholar]
- Torrance J., Jacobs P., Restrepo A., Eschbach J., Lenfant C., Finch C. A. Intraerythrocytic adaptation to anemia. N Engl J Med. 1970 Jul 23;283(4):165–169. doi: 10.1056/NEJM197007232830402. [DOI] [PubMed] [Google Scholar]


