Abstract
Objective
Circulating cell-endothelial cell interaction in sepsis is a rate-determining factor in organ dysfunction, and interventions targeting this process have a potential therapeutic value. In this project, we examined whether curcumin, an active ingredient of turmeric and an anti-inflammatory agent, could disrupt interactions between circulating blood cells and endothelium and improve survival in a murine model of sepsis.
Methods
Mice were subjected to cecal ligation and puncture (CLP) to induce sepsis vs. sham surgery. We studied leukocyte and platelet adhesion in cerebral microcirculation using intravital fluorescent video microscopy technique, blood brain barrier dysfunction using Evans Blue leakage method, P-selectin expression using dual radiolabeling technique and survival in mice subjected to Sham, CLP and CLP with curcumin pre-treatment (CLP+Curcumin).
Results
Curcumin significantly attenuated leukocyte and platelet adhesion in cerebral microcirculation, Evans Blue leakage in the brain tissue and improved survival in mice with CLP. P-selectin expression in mice with CLP+Curcumin was significantly attenuated compared to CLP in various microcirculatory beds including brain. Reduction in platelet adhesion was predominantly via modulation of endothelium by curcumin.
Conclusion
Curcumin pre-treatment modulates leukocyte and platelet adhesion and blood brain barrier dysfunction in mice with CLP via P-selectin expression and improves survival in mice with CLP.
Keywords: Cerebral microcirculation, P-selectin, Barrier dysfunction
Introduction
Microvascular dysfunction due to circulating cell-endothelial cell interaction is the rate determining factor in tissue damage and organ dysfunction in sepsis and septic shock(1-3). The number of organs involved in the multiple organ dysfunction syndrome is directly proportional to the mortality in sepsis (4). Specific agents targeting different factors/cytokines to attenuate microvascular dysfunction and reduce mortality in sepsis have been a investigated with only limited success(5,6). This underscores the need to investigate the role of naturally occurring anti-inflammatory agents.
The phytochemical curcumin (1,7-bis[3-methoxyl-4-hydrophenyl]-1,6-heptadiene-3,5-dione), a polyphenol derived from the root Curcumina Longa , is an active ingredient of turmeric. In addition to its use as a coloring agent and spice, curcumin is used in Ayurvedic and Chinese medicine for thousands of years, and is known for its anti-inflammatory and anti-apoptotic properties in the modern medicine. Curcumin is a potent inhibitor of NF-kappa B activation(7,8). Curcumin prevents the blood brain barrier dysfunction and is a neuroprotective agent in ischemic stroke(9). Curcumin attenuates tissue injury with endotoxemia(10), and in polymicrobial sepsis induced by cecal ligation and puncture (CLP)(11). Reports suggest that curcumin modulates adhesion molecule expression (12,13) but the exact role of curcumin in modulation of cell adhesion in microcirculation in sepsis in vivo is not very well studied.
Sepsis and septic shock are the leading causes of death in non-coronary intensive care units in the United States(14). Sepsis induced encephalopathy (SAE) alone is shown to be an independent predictor of mortality(15), but little is known about pathophysiology of SAE. Several vascular and non-vascular mechanisms are suggested(16,17). We have shown previously that in a laboratory model of murine sepsis induced by CLP, there is a significant increase in leukocyte and platelet adhesion in the cerebral microcirculation accompanied by behavioral and blood brain barrier dysfunction(18,19). In the current study, we tested the effects of curcumin on cerebral microcirculation in mice with CLP to attempt to answer the question of how curcumin acts on the circulating cell-endothelial cell interactions, blood-brain barrier dysfunction and survival.
Material and Methods
Animals
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Wake Forest University School of Medicine, Winston-Salem and performed according to the criteria outlined in the National Institutes of Health guidelines. C57Bl/6 male mice (n=5-7/group for each parameter and 5-7 weeks of age) were used. All mice were purchased from Jackson Laboratories (Bar Harbor, ME).
Cecal ligation and puncture (CLP)
CLP was used to induce sepsis, as previously described(18). Briefly, the animals were anesthetized with intramuscular injection of ketamine hydrochloride (150 mg/kg) and xylazine (7.5mg/kg). Two-centimeter midline incision in the abdomen was made; the cecum was isolated, tightly ligated and perforated three times (upper, middle and lower third) with a 20-gauge needle. Using a cotton swab, fecal contents were squeezed and spread around the cecum. A special precaution was taken to make sure that intestinal obstruction was not caused. The abdominal incision was closed using two layers of sutures (peritoneum and skin). Each mouse then received 1 ml of normal saline subcutaneously for fluid resuscitation.
In the Sham-operated mice, the cecum was isolated, exteriorized and returned to the abdominal cavity without ligation or punctures. The other procedures were identical to the CLP mice.
Intravital video microscopy (IVM)
The cerebral microcirculation of the mice was observed 4 h after CLP or Sham surgery using intravital fluorescent video microscopy. Preliminary experiments revealed no differences in the inflammatory responses at 4 h versus 6 h following CLP, so all subsequent experiments were performed using a post- injury period of 4 h. The mice were anesthetized with ketamine hydrochloride (150 mg/kg i.m.) and xylazine (7.5mg/kg i.m.). Core body temperature was maintained at 35 ± 0.5°C. A tracheotomy was performed to facilitate mechanical ventilation of all the mice with room air. The femoral arterial (to access mean arterial blood pressure monitoring and arterial blood for blood gas sampling) and femoral venous (for intravenous administration of ex-vivo labeled platelets, and acridine orange) cannulations were performed. Head of the mouse was fixed in a stereotaxic frame and a right parietal craniotomy was performed as previously described leaving dura intact(18,20). A cover glass was placed on the cranial window. Artificial cerebrospinal fluid filled the space between the cranial window and the cover glass. The animals were then placed on mechanical ventilator and allowed to stabilize for 30 min prior to observation of cerebral post-capillary venules (just prior to penetration into the cerebral cortex) under an upright microscope.
Circulating cell-endothelial cell interactions
In order to monitor the adhesive interactions between leukocytes/platelets and venular endothelium in the mouse brain, leukocytes were labeled in vivo with acridine orange (0.05% in 100 μL of PBS), and recorded prior to the infusion of platelets, labeled ex vivo with carboxyfluorescein diacetate succinimidyl ester (CFSE) (90 μM). This allowed separate monitoring of both cell types in each venule, as platelets were observed once significant acridine orange fluorescence was no longer detected.
The details of the platelet isolation technique are as outlined previously(18). The blood (0.9 ml) was collected from matched donor mouse in a polypropylene tube containing 0.1 mL acid citrate dextrose buffer (Sigma Chemicals) and centrifuged twice at 120 g for 8 and 3 min. Platelet-rich plasma (PRP) was removed and placed in a clean polypropylene tube between centrifugation steps. Subsequently, this PRP was centrifuged at 550 g for 10min to pellet the platelets. The pellet was then gently re-suspended in 1500 μL PBS (pH 7.4), incubated with the fluorescent dye CFSE at room temperature for 10 min, centrifuged again for 10 min at 550 g and re-suspended in 500 μL PBS, and protected from light until use.
Contamination of the platelet suspension by leukocytes was evaluated from a 25 μL sample to ensure that the leukocyte count did not exceed 0.01%. The platelets (n=100 × 106) were infused over 5 min, yielding <5% of the total platelet count and allowed to circulate for a period of 5 min before videotaping. These platelet isolation procedures are known to have no significant effect on the activity or viability of isolated platelets as assessed by flow cytometry(21).
Post capillary venules (three to five in number) in each mouse brain were recorded to quantify leukocyte and platelet adhesion, with 5-7 mice per group. Each vessel recording was analyzed for one minute. A cell (leukocyte/platelet) was considered adherent if it remained stationary for at least 30 consecutive seconds out of the one minute recording. The mean of the average values of leukocyte (LA) and platelet adhesion (PA) determined in each mouse was used to generate the group mean.
Platelets recordings were re-analyzed to assess the platelet velocity. Estimates of pseudo-shear rate in venules were obtained using measurements of venular diameter (Dv) and the maximal velocity of non-interacting and free flowing platelets (Vplt) according to the following formulation: pseudo shear rate = ((Vplt/1.6)/Dv) × 8/second(22).
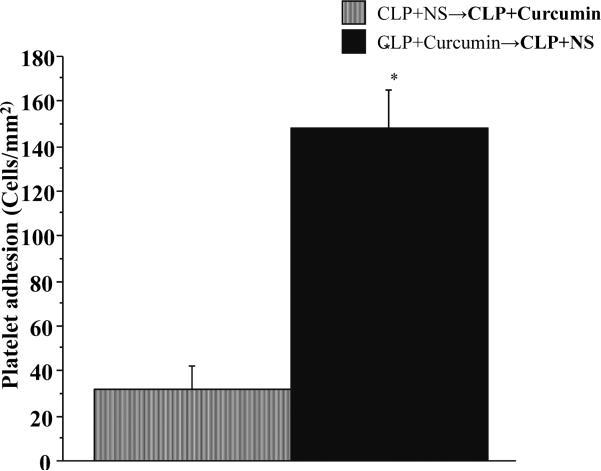
In order to further characterize the anti-inflammatory effects of curcumin on the endothelium vs. platelets, we pre-treated the platelet donor as well as experimental mice with either curcumin or vehicle (normal saline: NS) for 48 hours prior to CLP. We subjected the platelet donor mice to cecal ligation and puncture for four hours before blood collection and platelet isolation according to the protocol mentioned above. Then we infused the platelets from CLP+Curcumin donor mice into CLP+NS experimental mice and CLP+NS donor mice into CLP+curcumin experimental mice. The results are depicted in Figure 7.
Figure 7. The impact of Curcumin on platelet adhesion was mainly on the endothelial side compared to the platelet side.
Intravital fluorescent microscopy showed that platelet adhesion in the brain post-capillary venules of vehicle pre-treated experimental CLP mice when infused with platelets from curcumin pre-treated CLP mice (CLP+Curcumin →CLP+NS) was significantly higher compared to curcumin pre-treated experimental CLP mice infused with platelets from vehicle pre-treated CLP mice (CLP+NS →CLP+Curcumin).
Evans blue leakage in the brain (EB leakage)
Blood brain barrier dysfunction was monitored using the leakage of Evan's blue dye method as described in literature(19,23). The mice were injected with 4ml/kg of 2% Evans blue (EB) intravenously within 15 minutes of injury, and allowed to circulate for four hours. At the end of four hours, after obtaining a blood sample the left ventricle was perfused with normal saline at 110 mmHg until clear fluid appeared in the right atrial incision. Brain tissue was harvested, weighed and homogenized and sonicated in 50% trichloroacetic acid (TCA) and centrifuged at 10,000 rpm for twenty minutes. The supernatant obtained was read on spectrophotometer at 610 nm wavelength read against a concentration curve.
The blood sample obtained was centrifuged and diluted in TCA and then processed and read the same fashion as the brain tissue as stated above. EB leakage was expressed as ng/gm brain tissue/microliter serum activity.
In vivo quantification of P-selectin expression
The P-selectin expression in different regional vascular beds was quantified using dual radiolabeled mAb technique as described previously(24). The P-selectin binding mAb (RB40.34; BD Pharmingen Cat no: 553742) was labeled with 125I and the non-binding control mAb (an irrelevant rat IgG1 mAb; BD Pharmingen Cat no: 553993) was labeled with 131I using the iodogen method(25). Briefly, 250 μg of protein were incubated with 250 μCi of sodium iodine-125 or sodium iodine-131 along with 125 μg of sodium iodogen at 4°C for 5 min. Phosphate-buffered saline (PBS, pH 7.4) was added to bring the volume to 2.5 ml. Using gel filtration on Sephadex PD-10 column(GE healthcare: product code: 17-0851-01), the radiolabeled mAb was separated from free 125I or 131I. The column was equilibrated and then eluted with PBS containing 1% bovine serum albumin. Two fractions of 2.5 mL were collected, the second of which contained the radiolabeled mAb. Absence of free 125I or 131I was was ensured by extensive dialysis of the protein-containing fraction. Radiolabeled mAbs were stored at 4°C until use.
The dual radiolabeled mAb procedure was performed 4 h post-CLP or sham surgery. The left external jugular vein and carotid artery were cannulations were performed for venous and arterial access. A mixture of 10 μg of 125I–labeled P-selectin mAb and a dose (0.5–5.0 μg depending on 131I decay) of non-binding mAb was injected slowly via the jugular venous cannula and allowed to circulate for 5 min. After obtaining a blood sample from the carotid catheter, bicarbonate-buffered saline was isovolemically exchanged by infusion through the jugular vein with simultaneous withdrawal of blood/buffer from the carotid catheter. Subsequently, copious flushing with bicarbonate-buffered saline through the carotid catheter was performed, after transection of the inferior vena cava at the thoracic level. Liver, kidney, brain, and intestinal tissue were harvested and weighed.
The method for calculating P-selectin expression has been described previously(24,26). The 125I (binding mAb) and 131I (non-binding mAb) activities in different tissues and in 50 μl samples of cell free plasma were counted in a 14800 Wizard 3 gamma-counter (Wallac, Turku, Finland), with automatic correction for background activity and spillover. A 2 μl aliquot of the radiolabeled mAb mixture was assayed to determine total injected activity of each labeled mAb. The radioactivities remaining in the tube used to mix the mAbs and the syringe used to inject the mixture were subtracted from the total injected activity. The accumulated activity of each mAb in an organ was expressed as the percent of the injected activity (ng mAb) per gram of tissue. P-selectin expression was calculated by subtracting the accumulated activity per gram tissue of the non-binding mAb (131I IgG isotype) from the activity of the binding anti-P-selectin mAb (125I RB40.34). Studies have shown previously, that mAbs retain their functional activity following radio-iodination as evidenced by a similar effectiveness of labeled and unlabeled mAbs to block white blood cell adherence in rat mesenteric venules(26). It has also been shown that constitutive and induced expression of P-selectin is not detectable in different organs of P-selectin deficient mice (24).
Curcumin pre-treatment
Curcumin was purchased from Sigma-Aldrich (Cat no: 28260). Curcumin was dissolved in 5N NaOH and titrated to pH 7.4 using 1N HCl, and then diluted with normal saline to the concentration of 10 mg/ml solution. This method of preparation was used in literature(9,27).
We pre-treated mice with 100mg/kg intraperitoneal administration of curcumin the dose used in murine model of inflammation(27,28). The CLP and Sham mice without curcumin pre-treatment received 200 microliter of normal saline intraperitoneally. The exact duration of pre-treatment for cell adhesion in cerebral microcirculation is unknown. We used 24 hours, 48 hours and 72 hours of pre-treatment with curcumin prior to induction of injury in our preliminary experiments (data not shown). There was no protective effect in animals pre-treated for 24 hours. Since there was no statistically significant difference in cell adhesion in groups pre-treated for 48 vs. 72 hours, we used 48 hours as an optimal duration of pre-treatment and used this time frame for further experiments.
Experimental protocol
Mice (C57Bl/6) were divided in three treatment groups as follows: 1) Sham, 2) Cecal ligation and puncture (CLP) and 3) CLP with curcumin pre-treatment for 48 hours as described above. After initial injury, mice were allowed to awaken and then re-anaesthetized for cannulations, tracheotomy, craniotomy (for IVM experiments) or cannulations alone (for dual radiolabeling) or transcardiac perfusion of the circulation (for EB leakage) and further procedures were performed for a specific read out at four hours from the initial injury. In addition, we subjected the platelet donor and recipient mice to cecal ligation and puncture in a separate group of mice and infused platelets from curcumin pre-treated post-CLP mice into vehicle (normal saline: NS) pre-treated post-CLP experimental mice and vice versa.
Survival study
In a separate group of experiments, mice were subjected to 1) Sham (n=7), 2) CLP (n=10) and 3) CLP (n=10) with curcumin pre-treatment as described above. All mice were allowed free access to food and water and were monitored every six to twelve hours and treated with either curcumin or vehicle (physiological saline) every 24 hours. The mice were monitored for seven days to specifically look for long term adverse effects of curcumin.
Statistical analysis
The data were analyzed using a one-way ANOVA with Fisher's (post hoc) test in all but one statistical comparison. All values are reported as means ± SE. Statistical significance was set at p < .05. Survival analysis was performed using log-rank test with SAS software. The survival curve was plotted using Kaplan-Meier curve.
Results
Duration for curcumin pre-treatment
Table 1 shows the leukocyte and platelet adhesion in mice with Sham, CLP, and CLP with curcumin pre-treatment for 24 and 48 hours prior to CLP. Both, leukocyte and platelet adhesion are significantly increased in CLP vs. Sham operated mice. Leukocyte and platelet adhesion in mice with 24 hour pre-treatment with curcumin did not show significant difference from that with CLP alone. However, both the leukocyte and platelet adhesion were significantly decreased in mice pre-treated with curcumin for 48 hours compared to CLP alone.
Table 1.
Time course for mice with curcumin pre-treatment: Leukocyte and platelet adhesion in mice in Sham, CLP alone and CLP pre-treatment with curcumin for 24 and 48 hours prior to CLP.
| Experimental group | Leukocyte Adhesion | Platelet Adhesion |
|---|---|---|
| Sham | 3.410 ± 2.1 | 7.1 ± 4.6 |
| CLP | 156.1 ± 7.6* | 104.7±14.8* |
| CLP+curcumin 24 hrs | 157.2 ± 1.9* | 113.1 ± 18.6* |
| CLP+curcumin 48 hrs | 41.8 ± 12.3*# | 15.8 ± 4.9# |
Leukocyte and platelet adhesion /mm2 in mice with Sham, CLP, CLP with curcumin pre-treatment for 24 and 48 hours. Values are expressed as mean ± SEM.
p<0.05 vs. Sham
p<0.05 vs. CLP
Mean arterial pressure and venular pseudo wall shear rates
Mean arterial pressures (MAP) and venular wall shear rates (WSR) in all the groups are shown in Table 2. There was a significant reduction of mean arterial pressure in mice subjected to CLP compared to Sham counterparts. However, mice with CLP+Curcumin had significantly increased blood pressure compared to CLP alone. Although higher than CLP mice, the MAP of CLP+Curcumin mice remained significantly lower than Sham mice.
Table 2.
Mean arterial blood pressure (MAP) and venular wall shear rate (WSR) in different experimental groups.
| Experimental group | MAP (mmHg) | WSR (s-1) |
|---|---|---|
| Sham | 58.20±2.59 | 502.39±57.29 |
| CLP | 44.40±2.4* | 204.12±14.62* |
| CLP+Curcumin | 50.6±1.07# | 386.68±35.33# |
Mean arterial blood pressure (MAP) and venular wall shear rate (WSR) in different experimental groups are depicted here. Values are expressed as mean ± SEM.
p<0.05 vs. Sham
p<0.05 vs. CLP
Similarly, the WSR in cerebral venules of CLP mice were significantly lower than in their Sham counterparts. Curcumin treatment improved venular WSR significantly mice subjected to CLP. There was no significant difference in the WSR of mice with CLP+Curcumin vs. Sham mice. The pH, PCO2 and PaO2 in three groups were not significantly different from each other (data not shown).
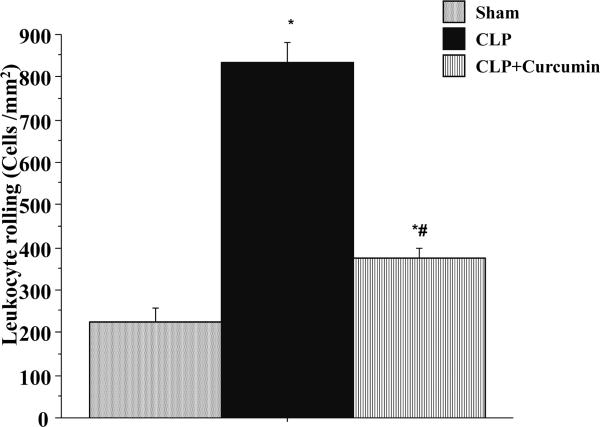
Leukocyte rolling
Figure 1 depicts the leukocyte rolling in cerebral venules of mice subjected to Sham and CLP with (CLP+Curcumin) or without (CLP) curcumin pre-treatment. Mice with CLP exhibited a significant increase in the number of rolling leukocytes compared to the Sham mice. Pre-treatment with curcumin (CLP+Curcumin) significantly attenuated the CLP-induced leukocyte rolling compared to CLP alone. Leukocyte rolling in the CLP+Curcumin mice remained significantly higher than that of the Sham mice.
Figure 1. CLP induced leukocyte rolling in the cerebral microcirculation is significantly decreased in curcumin pre-treated mice.
Intravital fluorescent microscopy showed that mice subjected to CLP had significantly increased leukocyte rolling when compared to Sham operated controls (*p<0.05). Curcumin pre-treatment significantly prevented the leukocyte rolling after CLP (#p<0.05).
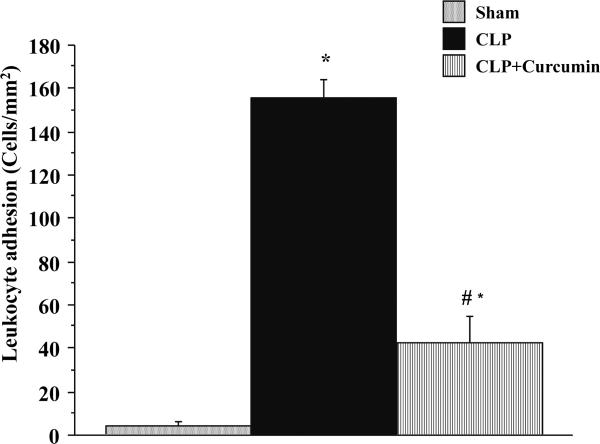
Leukocyte adhesion
Figure 2 depicts the leukocyte adhesion responses in cerebral venules of mice subjected to Sham and CLP with (CLP+Curcumin) or without (CLP) curcumin pre-treatment. Similar to the leukocyte rolling, mice with CLP exhibited a profound increase in the number of adherent leukocytes compared to the Sham mice. Pre-treatment with curcumin (CLP+Curcumin) significantly decreased the CLP-induced recruitment of adherent leukocytes compared to CLP alone. Leukocyte adhesion in the CLP+Curcumin mice remained significantly higher than that of the Sham mice.
Figure 2. CLP induced leukocyte adhesion in the cerebral microcirculation is significantly decreased in curcumin pre-treated mice.
Intravital fluorescent microscopy showed that mice subjected to CLP had significantly increased leukocyte adhesion when compared to Sham operated controls (*p<0.05). Curcumin pre-treatment significantly prevented the leukocyte adhesion after CLP (#p<0.05).
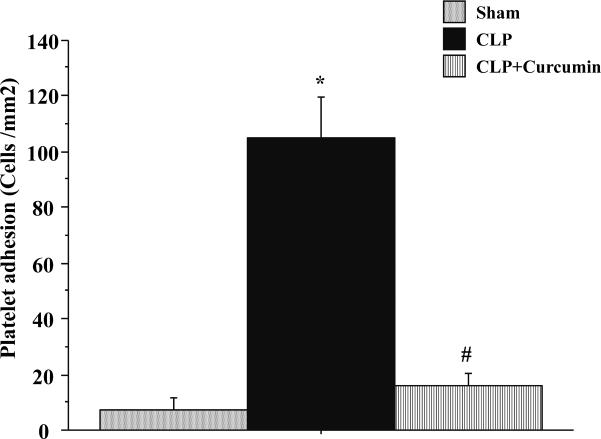
Platelet adhesion
Figure 3 depicts the platelet adhesive responses in mice subjected to Sham, CLP and CLP+Curcumin. These platelets were isolated from matched platelet donor mice. The pattern of responses for platelet adhesion between groups was nearly identical to that observed for leukocytes. There was a significant recruitment of adherent platelets in cerebral venules of the mice subjected to CLP compared to Sham. CLP+Curcumin group had significant reduction in platelet adhesion compared to CLP. There was no significant difference in platelet adhesion of mice with Sham vs. with CLP+Curcumin.
Figure 3. CLP induced platelet adhesion in the cerebral microcirculation is significantly decreased in curcumin pre-treated mice.
Intravital fluorescent microscopy showed that mice subjected to CLP had significantly increased platelet adhesion when compared to Sham operated controls (*p<0.05). Curcumin pre-treatment significantly prevented the platelet adhesion after CLP (#p<0.05).
Evans blue leakage
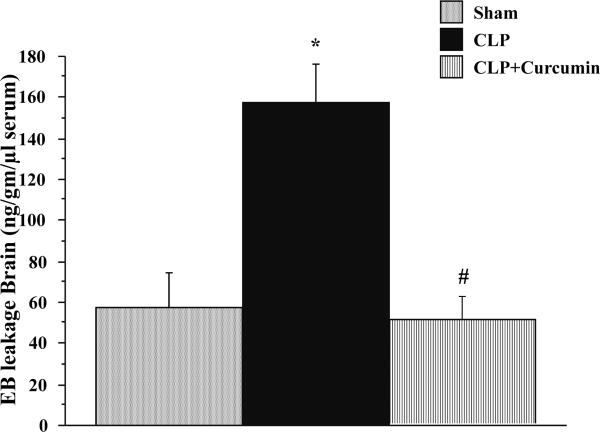
Figure 4 depicts the results for Evans blue (EB) leakage in brain tissue in different groups. CLP produced a significant increase in EB leakage in CLP mice compared to Sham. Curcumin pre-treatment significantly prevented the CLP-induced increase in EB leakage as evidenced by decrease in EB leakage in CLP+Curcumin group. There was no significant difference in the EB leakage of mice subjected to Sham vs. CLP+Curcumin.
Figure 4. Curcumin pre-treatment significantly decreased CLP-induced blood brain barrier dysfunction in mice.
Evans Blue Dye leakage in mice with CLP was significantly increased compared to Sham operated mice (*p<0.05). CLP-induced dye leakage was significantly decreased in curcumin pre-treated mice (#p<0.05).
P-selectin expression
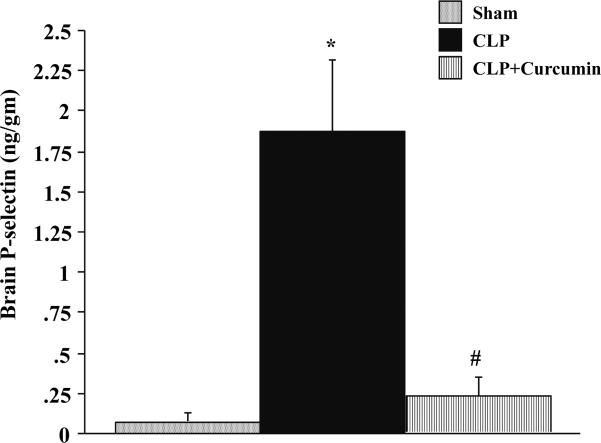
P-selectin levels in the brain tissue of mice subjected to Sham, CLP and CLP+Curcumin were measured four hours after injury as shown in Figure 5. There was a significant increase in the P-selectin expression of brain tissue of mice subjected to CLP compared to Sham mice. Moreover, there was a significant attenuation of P-selectin expression in the brain of curcumin pre-treated mice subjected to CLP. There was no significant difference between the P-selectin levels in the brain tissue of mice subjected to Sham vs. CLP+Curcumin.
Figure 5. CLP induced P-selectin expression in the brain is significantly decreased in curcumin pre-treated mice.
P-selectin expression measured with dual radiolabeling technique showed that mice subjected to CLP had significantly increased P-selectin expression in the brain when compared to Sham operated controls (*p<0.05). Curcumin pre-treated mice showed significantly decreased P-selectin expression after CLP (#p<0.05).
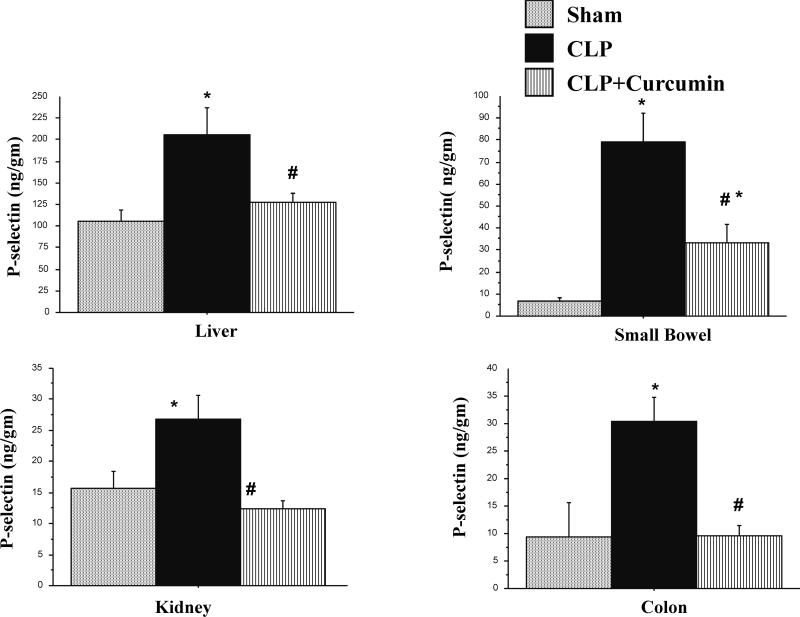
The P-selectin expression in other microcirculatory beds is shown in Figure 6. P-selectin expression was significantly increased in the liver, kidney, small bowel and colon of mice subjected to CLP compared to Sham mice. Moreover, mice with CLP with curcumin pre-treatment showed significantly lower P-selectin levels in all these organs compared to CLP mice alone. This decrease in P-selectin expression in the liver, kidney and colon tissue in CLP+Curcumin mice was all the way down to the levels seen in Sham mice.
Figure 6. CLP induced P-selectin expression in the liver, kidney, and intestines are significantly decreased in curcumin pre-treated mice.
P-selectin expression measured with dual radiolabeling technique showed that mice subjected to CLP had significantly increased P-selectin expression in the liver, kidney, small bowel and colon tissue when compared to Sham operated controls (*p<0.05). Curcumin pre-treated mice showed significantly decreased P-selectin expression after CLP (#p<0.05). The P-selectin expression in small intestine in CLP+Curcumin remained significantly higher than the SHAM operated mice.
Figure 7 depicts the effect of curcumin on endothelium vs. platelets in mice with CLP. Please note that the platelet adhesion of vehicle pre-treated experimental mice (CLP+Curcumin →CLP+NS) remained significantly higher compared to curcumin pre-treated counterparts (CLP+NS→CLP+Curcumin) in spite of the fact that the platelets in vehicle treated experimental mice (CLP +NS) were pre-treated with curcumin and the platelets in curcumin treated experimental mice were treated with vehicle.
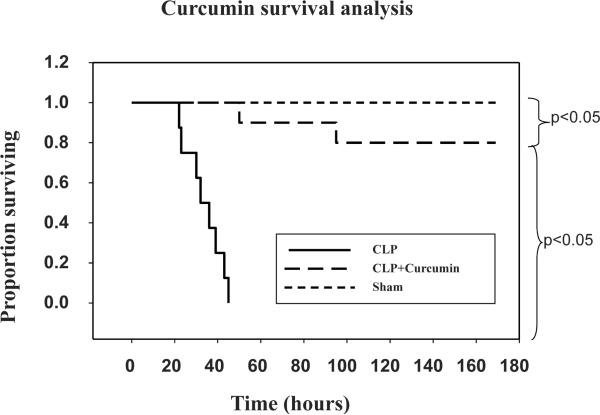
Figure 8 depicts Kaplan-Meier survival curve in mice subjected to Sham, CLP and CLP+Curcumin. Although significantly lower than the Sham mice, mice with CLP+Curcumin pre-treatment survived significantly longer than those with CLP alone.
Figure 8. Curcumin pre-treatment improves survival in mice with CLP.
Kaplan-Meier survival curve of the mice subjected to Sham, CLP and CLP+Curcumin. Pre-treatment of mice with curcumin prior to CLP showed significant improvement in survival compared to mice with CLP alone. Survival of mice with CLP+Curcumin was significantly lower than that of sham mice.
Discussion
Sepsis and septic shock are the leading causes of death in critically ill patients(29). Mortality in sepsis and septic shock is directly related to the number of organ systems affected such as lung, liver, kidney and brain(4). Circulating cell-endothelial cell interactions are the rate limiting step in the cytokine-induced inflammatory process(2). Evidence suggests that septic encephalopathy alone is associated with higher mortality in critically ill patients(15). The exact mechanism behind pathophysiology of sepsis induced encephalopathy is unknown, but many cellular and non cellular mechanisms are implicated(16,30). We have shown previously, that there is a significant increase in leukocyte and platelet adhesion in cerebral microcirculation in mice with CLP via modulation of P-selectin expression. Moreover, there was a significant decrease in the cell adhesion in response to treatment with P-selectin, ICAM-1 and CD-18 monoclonal antibodies, in mice with CLP(18). In the current study, we demonstrate, for the first time that curcumin, when provided as a pre-treatment for 48 hours, markedly attenuates microvascular injury events and improves survival in mice with sepsis induced by cecal ligation and puncture. This is supported by reductions both in leukocyte/platelet adhesion and in vascular leakage. We further implicate attenuation of P-selectin expression as one of the mechanism associated with the physiologic event of curcumin.
Curcumin is a polyphenol derived from Curcumina longa, a member of ginger family is used in ancient Ayurvedic and Chinese medicine for centuries. Curcumin is known for its anti-inflammatory and anti-apoptotic properties in the modern medicine(8,31). Curcumin is known to be neuroprotective in laboratory models of ischemic stroke(27,32). Evidence suggests that curcumin modulates adhesion molecule expression in endothelial cells(13), more specifically in cerebrovascular endothelial cells(12). While protective effect of curcumin in experimental model of sepsis via up-regulation of nuclear receptor PPAR-γ was demonstrated by Siddiqui et al(11), the effect of curcumin on adhesive response in microvasculature in sepsis remains unknown. Current study demonstrates anti-inflammatory and anti-thrombogenic properties of curcumin in a clinically relevant model of sepsis, namely cecal ligation and puncture. Additionally, this study also demonstrates the effect of curcumin on improving survival in mice with CLP.
A variety of factors may be responsible for this increase in the leukocyte and platelet adhesion in mice with CLP. Decreased MAP and/or cerebral venular wall shear rate as shown in Table 2 may be responsible for this increase for instance. We have shown recently that in mice without septic injury, the venular wall shear rates required to induce leukocyte and platelet adhesion in cerebral microcirculation are much lower (51-100s-1) (19). The lowest venular wall shear rates reached in the current study were much higher than that (CLP: 204.12±14.62). This indicates, that the increased cell adhesion in mice with CLP can not be explained by low venular wall shear rates alone. Curcumin increased the MAP and WSR significantly compared to the CLP mice alone. While we can not conclusively rule out the possibility that the decreased cell adhesion in curcumin pre-treated mice was due to increased wall shear rates, this finding itself is significant from the stand point of shock reversal. Since the curcumin per-se was not associated with increased blood pressure over a wide range of dose(33), this effect of curcumin can only be attributed to its anti-inflammatory property and the exact mechanism warrants further investigation.
The recruitment of the circulating cells involves a series of adhesive interactions mediated by adhesion molecules expressed on the surface of endothelium and circulating cells(1,34). The adhesion molecule, especially P-selectin expression is up-regulated in mice subjected to CLP(35). In our previous work, we have shown that the increased leukocyte and platelet adhesion in the cerebral microcirculation in mice subjected to CLP occurs via up-regulation of P-selectin expression(18,19,36,37). Moreover, we have also shown that the cell adhesion is significantly decreased in the cerebral microcirculation of septic mice in response to treatment with P-selectin monoclonal antibody treatment(18).
Curcumin is known to modulate adhesion molecule response in endothelial cells in vitro(12,13), but the role of curcumin in modulation of adhesion molecules in sepsis is unknown. In the current study, in addition to re-confirming a significant up-regulation of P-selectin expression in various microcirculatory beds in mice subjected to CLP, we also show that curcumin pre-treatment significantly attenuates of P-selectin in the cerebral microcirculation as well as other microcirculatory beds (Figures 5 and 6) in a clinically relevant model of sepsis. Thus, the attenuation of leukocyte rolling/ adhesion and platelet adhesion seen in curcumin pre-treated mice with CLP seems to occur via modulation of P-selectin expression.
In order to further characterize the modulation of endothelial vs. platelet induced adhesion in our model; we pre-treated the experimental and platelet donor mice with curcumin. It is evident from Figure 7, that the curcumin pre-treated experimental mice with CLP, when infused with platelets from vehicle pre-treated mice with CLP (CLP+NS→CLP+Curcumin) afforded a significant attenuation of platelet adhesion compared with the vehicle pre-treated experimental mice with CLP when infused with platelets from curcumin pre-treated CLP mice (CLP+Curcumin→CLP+NS). In other words, the curcumin pre-treatment of endothelium (the experimental mice) showed significant decrease in platelet adhesion compared to that of platelets (the donor mice) in mice with CLP. This data supports the finding that curcumin modulates adhesion molecule expression in the cerebro-vascular endothelial cells in vitro (4) (12). However, the exact molecular mechanism of this modulation needs to be further investigated.
Barrier dysfunction is responsible for organ dysfunction which in turn is implicated in increased morbidity and mortality in sepsis and septic shock. Blood brain barrier (BBB) dysfunction in sepsis has been demonstrated in literature(23,38). Curcumin is known to decrease BBB dysfunction in the ischemia reperfusion model of injury(9), but its role in sepsis induced BBB dysfunction is unknown. We have shown previously that P-selectin immunoblockade significantly decreases EB leakage in the CLP model of sepsis(19). In order to assess the functional impact of down-regulation of cell adhesion and P-selectin expression with curcumin, we studied the impact of curcumin on the barrier function with and without curcumin pre-treatment in mice subjected to CLP. In this study we show that there was a significant decrease in the BBB dysfunction in CLP mice pre-treated with curcumin. This is the first report, to our knowledge, demonstrating a protective role for curcumin on blood brain barrier dysfunction in vivo, in a laboratory model of sepsis.
Multiple organ dysfunction syndrome (MODS) associated with increased morbidity and mortality in sepsis and septic shock is a sequential phenomenon, with different organ system failure occurring at different time points after onset of septic shock with the development of maximal degree of respiratory dysfunction at 1.8±4.7 days to hepatic dysfunction at 4.7±5.5 days(4). In the current study, the protective effect of curcumin is not seen unless we pre-treat mice with curcumin for at least 48 hours (Table 1), indicating delayed effect. Agents like curcumin, with slow onset of action have a role as an adjuvant therapy to decrease the impact of tissue injury after the septic shock has set in. Furthermore, the clinical impact of curcumin in critically ill patients may differ significantly from the one seen in experimental model of sepsis. Curcumin is known for its high safety profile(39). The exact role of curcumin and its efficacy need to be further investigated in the clinical settings.
A major limitation of this study is that we pre-treated the mice with curcumin. It will be necessary to study the effects of curcumin on the modulation of tissue injury leading to MODS after the septic injury has set in to fully assess curcumin's anti-inflammatory potential in sepsis. Additionally, the exact mechanism of modulation of adhesion molecule expression by curcumin needs to be investigated further. Anti-inflammatory and neuroprotective properties demonstrated in this study further support the need for a clinical study to determine the effect of naturally occurring curcumin in human sepsis.
Conclusion
In conclusion, our study supports the anti-inflammatory and neuroprotective properties of curcumin in a clinically relevant model of sepsis. We demonstrate the anti-adhesive properties of curcumin via modulation of P-selectin expression and reduction in blood brain barrier dysfunction in a laboratory model of sepsis. We also demonstrate improved survival in curcumin pre-treated mice with CLP. Our findings suggest that clinical studies focused on the anti-inflammatory effects of curcumin are needed to determine the exact role and efficacy of curcumin in critically ill patients with sepsis and septic shock.
References
- 1.Lush CW, Kvietys PR. Microvascular dysfunction in sepsis. Microcirculation. 2000;7:83–101. doi: 10.1038/sj.mn.7300096. [DOI] [PubMed] [Google Scholar]
- 2.Jung U, Norman KE, Scharffetter-Kochanek K, Beaudet AL, Ley K. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J Clin Invest. 1998;102:1526–33. doi: 10.1172/JCI119893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–77. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 4.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–52. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Abraham E, Wunderink R, Silverman H, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273:934–41. [PubMed] [Google Scholar]
- 6.Abraham E, Reinhart K, Opal S, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290:238–47. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Dhawan S, Hardegen NJ, Aggarwal BB. Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem Pharmacol. 1998;55:775–83. doi: 10.1016/s0006-2952(97)00557-1. [DOI] [PubMed] [Google Scholar]
- 8.Jobin C, Bradham CA, Russo MP, et al. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163:3474–83. [PubMed] [Google Scholar]
- 9.Jiang J, Wang W, Sun YJ, Hu M, Li F, Zhu DY. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood-brain barrier damage. Eur J Pharmacol. 2007;561:54–62. doi: 10.1016/j.ejphar.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 10.Memis D, Hekimoglu S, Sezer A, Altaner S, Sut N, Usta U. Curcumin attenuates the organ dysfunction caused by endotoxemia in the rat. Nutrition. 2008;24:1133–8. doi: 10.1016/j.nut.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqui AM, Cui X, Wu R, et al. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-gamma. Crit Care Med. 2006;34:1874–82. doi: 10.1097/01.CCM.0000221921.71300.BF. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Gu ZL, Qin ZH, Liang ZQ. Effect of curcumin on the adhesion of platelets to brain microvascular endothelial cells in vitro. Acta Pharmacol Sin. 2008;29:800–7. doi: 10.1111/j.1745-7254.2008.00813.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim YS, Ahn Y, Hong MH, et al. Curcumin attenuates inflammatory responses of TNF-alpha-stimulated human endothelial cells. J Cardiovasc Pharmacol. 2007;50:41–9. doi: 10.1097/FJC.0b013e31805559b9. [DOI] [PubMed] [Google Scholar]
- 14.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 15.Sprung CL, Peduzzi PN, Shatney CH, et al. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med. 1990;18:801–6. doi: 10.1097/00003246-199008000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Mizock BA, Sabelli HC, Dubin A, Javaid JI, Poulos A, Rackow EC. Septic encephalopathy. Evidence for altered phenylalanine metabolism and comparison with hepatic encephalopathy. Arch Intern Med. 1990;150:443–9. doi: 10.1001/archinte.150.2.443. [DOI] [PubMed] [Google Scholar]
- 17.Sharshar T, Gray F, Lorin de la Grandmaison G, et al. Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet. 2003;362:1799–805. doi: 10.1016/s0140-6736(03)14899-4. [DOI] [PubMed] [Google Scholar]
- 18.Vachharajani V, Russell JM, Scott KL, et al. Obesity exacerbates sepsis-induced inflammation and microvascular dysfunction in mouse brain. Microcirculation. 2005;12:183–94. doi: 10.1080/10739680590904982. [DOI] [PubMed] [Google Scholar]
- 19.Vachharajani V, Vital S, Russell J. Modulation of circulating cell-endothelial cell interaction by erythropoietin in lean and obese mice with cecal ligation and puncture. Pathophysiology. 2009;17(1):9–18. doi: 10.1016/j.pathophys.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa M, Cooper D, Russell J, et al. Molecular determinants of the prothrombogenic and inflammatory phenotype assumed by the postischemic cerebral microcirculation. Stroke. 2003;34:1777–82. doi: 10.1161/01.STR.0000074921.17767.F2. [DOI] [PubMed] [Google Scholar]
- 21.Tailor A, Granger DN. Hypercholesterolemia promotes P-selectin-dependent platelet-endothelial cell adhesion in postcapillary venules. Arterioscler Thromb Vasc Biol. 2003;23:675–80. doi: 10.1161/01.ATV.0000056742.97580.79. [DOI] [PubMed] [Google Scholar]
- 22.Tsujikawa A, Kiryu J, Yamashiro K, et al. Interactions between blood cells and retinal endothelium in endotoxic sepsis. Hypertension. 2000;36:250–8. doi: 10.1161/01.hyp.36.2.250. [DOI] [PubMed] [Google Scholar]
- 23.Esen F, Erdem T, Aktan D, et al. Effect of magnesium sulfate administration on blood-brain barrier in a rat model of intraperitoneal sepsis: a randomized controlled experimental study. Crit Care. 2005;9:R18–23. doi: 10.1186/cc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eppihimer MJ, Wolitzky B, Anderson DC, Labow MA, Granger DN. Heterogeneity of expression of E- and P-selectins in vivo. Circ Res. 1996;79:560–9. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- 25.Eppihimer MJ, Russell J, Anderson DC, Wolitzky BA, Granger DN. Endothelial cell adhesion molecule expression in gene-targeted mice. Am J Physiol. 1997;273:H1903–8. doi: 10.1152/ajpheart.1997.273.4.H1903. [DOI] [PubMed] [Google Scholar]
- 26.Panes J, Perry MA, Anderson DC, et al. Regional differences in constitutive and induced ICAM-1 expression in vivo. Am J Physiol. 1995;269:H1955–64. doi: 10.1152/ajpheart.1995.269.6.H1955. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Zhao Y, Zheng W, Lu Y, Feng G, Yu S. Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res. 2008;1229:224–32. doi: 10.1016/j.brainres.2008.06.117. [DOI] [PubMed] [Google Scholar]
- 28.Okunieff P, Xu J, Hu D, et al. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys. 2006;65:890–8. doi: 10.1016/j.ijrobp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–40. [PubMed] [Google Scholar]
- 30.Jeppsson B, Freund HR, Gimmon Z, James JH, von Meyenfeldt MF, Fischer JE. Blood-brain barrier derangement in sepsis: cause of septic encephalopathy? Am J Surg. 1981;141:136–42. doi: 10.1016/0002-9610(81)90026-x. [DOI] [PubMed] [Google Scholar]
- 31.Pan R, Qiu S, Lu DX, Dong J. Curcumin improves learning and memory ability and its neuroprotective mechanism in mice. Chin Med J (Engl) 2008;121:832–9. [PubMed] [Google Scholar]
- 32.Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969–85. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- 33.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Altern Complement Med. 2003;9:161–8. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 34.Panes J, Granger DN. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998;114:1066–90. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- 35.Bauer P, Lush CW, Kvietys PR, Russell JM, Granger DN. Role of endotoxin in the expression of endothelial selectins after cecal ligation and perforation. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1140–7. doi: 10.1152/ajpregu.2000.278.5.R1140. [DOI] [PubMed] [Google Scholar]
- 36.Vachharajani V, Vital S, Russell J, Scott LK, Granger DN. Glucocorticoids inhibit the cerebral microvascular dysfunction associated with sepsis in obese mice. Microcirculation. 2006;13:477–87. doi: 10.1080/10739680600777599. [DOI] [PubMed] [Google Scholar]
- 37.Vachharajani V, Vital S, Russell J, Granger DN. Hypertonic saline and the cerebral microcirculation in obese septic mice. Microcirculation. 2007;14:223–31. doi: 10.1080/10739680601139153. [DOI] [PubMed] [Google Scholar]
- 38.Papadopoulos MC, Davies DC, Moss RF, Tighe D, Bennett ED. Pathophysiology of septic encephalopathy: a review. Crit Care Med. 2000;28:3019–24. doi: 10.1097/00003246-200008000-00057. [DOI] [PubMed] [Google Scholar]
- 39.Krishnaraju AV, Sundararaju D, Sengupta K, Venkateswarlu S, Trimurtulu G. Safety and toxicological evaluation of demethylatedcurcuminoids; a novel standardized curcumin product. Toxicol Mech Methods. 2009;19:447–60. doi: 10.1080/15376510903200766. [DOI] [PubMed] [Google Scholar]