Abstract
BACKGROUND
The number of long-term survivors of allogeneic hematopoietic stem cell transplantation (allo-HSCT) is increasing; however, few studies have addressed their long-term pulmonary function.
METHODS
We examined 660 baseline and follow-up pulmonary function tests in 89 long-term survivors of pediatric hematologic malignancies and allo-HSCT.
RESULTS
At least one abnormal lung parameter was seen in 40.4% of baseline tests and developed in 64% of post–allo-HSCT tests (median follow-up: 8.9 years). Abnormal baseline values in ratio of forced expiratory volume in 1 second and forced vital capacity (FEV1/FVC), FEV1, residual volume (RV), functional residual capacity (FRC) and FVC were associated with abnormal post–allo-HSCT values. The following pulmonary function values declined significantly with time: FEV1/FVC, forced mid-expiratory flow (FEF25%–75%), total lung capacity (TLC), diffusion capacity corrected for hemoglobin (DLCOcorr), RV, FRC, and RV/TLC. Older age at the time of allo-HSCT was associated with lower FEV1/FVC, FEF25%–75%, and DLCOcorr and higher RV/TLC. Patients who experienced respiratory events within 1 year post–allo-HSCT had lower FEV1 and FVC values and higher RV/TLC from their baseline PFTs. Female patients had reduced FVC, TLC, and RV values but higher FEV1/FVC. Pulmonary dysfunction was also associated with high-risk hematological malignancies and peripheral blood HSC product.
CONCLUSION
Abnormal pulmonary functions in allo-HSCT survivors are prevalent, which underscore the need for risk-adapted continual monitoring and improved preventive and management strategies.
Keywords: childhood, hematopoietic stem cell transplantation, leukemia, pulmonary function, survivor
Improved allogeneic hematopoietic stem cell transplantation (allo-HSCT) methods have resulted in a growing population of long-term survivors. The risk of long-term adverse effects of allo-HSCT is a major concern, especially among survivors of pediatric cancer.1,2 Pulmonary complications are well-recognized consequences of allo-HSCT and are associated with radiation therapy, chemotherapy, infections, and immune system–mediated lung damage caused by chronic graft-vs-host disease (GVHD).3–7 Pulmonary dysfunction may include restrictive or obstructive defects and abnormalities in diffusion capacity, all of which may improve over time but may never normalize.5–7
To standardize the care of cancer survivors, an expert panel was convened to develop guidelines for ongoing surveillance and follow-up care by American Society of Clinical Oncology.8 However, the recommendations for screening cardiac and pulmonary late effects were not approved, in part, because the panel’s systematic literature review was primarily derived from retrospective or cross-sectional studies describing incidences or prevalences.
Here we report our long-term prospective study of pulmonary function in a large cohort of survivors after allo-HSCT for pediatric hematologic malignancies. We evaluated the natural history and risk factors of pulmonary dysfunction after allo-HSCT. This information should help identify patients at risk and guide treatment strategies.
MATERIALS AND METHODS
Patient Characteristics
This prospective, long-term study was approved by the St. Jude Children’s Research Hospital Institutional Review Board.2 Allo-HSCTs were performed between 1990 and 2005. All patients who survived more than 1 year post–allo-HSCT were eligible. Of the 208 eligible patients, 154 had hematologic malignancies. Among these 154, 44 patients who were too young to perform pulmonary function tests (PFTs) at the time of allo-HSCT (younger than 6 years old) and 7 who had 2 or more allo-HSCTs were excluded from the analysis. Among 103, 8 did not have pre-allo-HSCT PFTs and 6 could not perform interpretable PFTs. Thus, 89 who were 6 years or older at the time of allo-HSCT and had pre and post–allo-HSCT PFTs with evaluable quality and were the subjects of this report.
Patients were classified according to their risk of relapse following allo-HSCT.2 The standard-risk category included patients with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) in first or second remission and patients with chronic myeloid leukemia (CML) in the first chronic phase. The high-risk category included patients with ALL or AML in third or subsequent remission or in relapse, patients with secondary leukemia, patients with CML beyond the first chronic phase, and those with myelodysplastic syndrome (MDS).
Pulmonary Function Testing
PFTs were performed during participants’ annual visits to the institution for at least 10 years post–allo-HSCT and until the patient was at least 18 years old. All PFTs were performed in the same laboratory, per American Thoracic Society guidelines.9
PFTs included spirometry and measurement of lung volumes and single breath carbon monoxide diffusion capacity.9 Spirometry was performed using a pneumotachograph, and lung volumes were determined by the open-circuit nitrogen-washout method. Diffusion capacity for carbon monoxide was measured using the single-breath technique and corrected for hemoglobin concentration (DLCOcorr). For each individual, we calculated the ratio of observed values compared to those predicted on the basis of the patient’s age, sex, height, and race.10–13
Forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), total lung capacity (TLC), and DLCOcorr were considered abnormal if they were less than 80% of the predicted normal values. Forced mid-expiratory flow (FEF25%–75%) was considered abnormal if it was less than 67% of the predicted value. Residual volume (RV) and functional residual capacity (FRC) were classified abnormal if they were larger than 120% of the predicted normal values. FEV1/FVC and RV/TLC were considered abnormal if the ratios were less than 80% and more than 30%, respectively.
Statistical Analysis
Longitudinal methods (PROC MIXED) and Cox proportional hazards models (PROC PHREG) with statistical package SAS 9.1.3 as well as Fisher’s exact test were used to analyze the 660 PFTs. Independent analyses were performed for the 9 response variables: FEV1, FVC, FEV1/FVC, FEF25%–75%, RV, FRC, TLC, RV/TLC, and DLCOcorr. Covariates included years post–allo-HSCT, age at allo-HSCT, donor age, sex, race (Caucasian, African American, or other), HSC product (bone marrow or peripheral blood), donor source (sibling, parent, or unrelated), HLA match (3/6–5/6 vs 6/6), pre–allo-HSCT conditioning chemotherapy and radiotherapy (14 Gy vs 12 Gy), T-cell depletion of graft, donor and recipient cytomegalovirus (CMV) status, malignancy status pre–allo-HSCT (standard-risk vs high-risk), and presence or absence of respiratory events within 1 year or more than 1 year post–allo-HSCT, and acute or chronic GVHD.
In Cox proportional hazards models, we defined pulmonary event time as years from allo-HSCT to the time of event for each patient. Pulmonary event time was defined as 0 if the patient experienced the event pre–allo-HSCT. The time was censored at the last follow-up for those patients who had not experienced the abnormalities. Cumulative incidences for the event of interest were obtained using the SAS Macro CIN based on Gray’s approach accounting for competitive risks.
RESULTS
Patient Characteristics
Table 1 summarizes the characteristics of the patients. The median age at allo-HSCT was 12.7 years (range, 6.6–21.3 years) and the median duration of follow-up since HSCT was 8.9 years (range, 1.7–16.4 years). The median number of evaluable PFTs per patient was 8 (range, 2–21).
Table 1.
Patient and graft characteristics
| Characteristic | No. patients | % |
|---|---|---|
| Sex | ||
| Female | 39 | 43.8 |
| Male | 50 | 56.2 |
| Race | ||
| Caucasian | 67 | 75.3 |
| African American | 6 | 6.7 |
| Other | 16 | 18 |
| Primary diagnosis | ||
| AML | 31 | 34.8 |
| ALL | 28 | 31.5 |
| CML | 21 | 23.6 |
| MDS | 9 | 10.1 |
| Disease status at allo-HSCT | ||
| Standard-risk | 59 | 66.3 |
| High-risk | 30 | 33.7 |
| Donor | ||
| Unrelated | 41 | 46.1 |
| Matched sibling | 37 | 41.6 |
| Parent | 11 | 12.3 |
| HSC product | ||
| Bone marrow | 79 | 88.8 |
| Blood stem cell | 10 | 11.2 |
| HLA match | ||
| 6/6 | 70 | 78.6 |
| 5/6 | 8 | 9 |
| 4/6 | 2 | 2.3 |
| 3/6 | 9 | 10.1 |
| T cell–depleted graft | ||
| Yes | 41 | 46.1 |
| No | 48 | 53.9 |
| Cytomegalovirus status (recipient/donor) | ||
| −/− | 27 | 30.3 |
| +/− | 12 | 13.5 |
| −/+ | 15 | 16.9 |
| +/+ | 35 | 39.3 |
| Conditioning chemotherapy | ||
| Cyclophosphamide + cytarabine | 55 | 61.8 |
| Cyclophosphamide + thiotepa | 22 | 24.7 |
| Cyclophosphamide | 8 | 9 |
| Other | 4 | 4.5 |
| TBI dose | ||
| 0 Gy | 3 | 3.4 |
| 12 Gy | 55 | 61.8 |
| 14 Gy | 31 | 34.8 |
| Use of ATG, OKT-3, or alemtuzumab | ||
| Yes | 49 | 55.1 |
| No | 40 | 44.9 |
| Acute GVHD | ||
| none | 45 | 50.6 |
| Grade I–II | 40 | 44.9 |
| Grade III–IV | 4 | 4.5 |
| Chronic GVHD | ||
| None | 66 | 74.2 |
| Limited | 17 | 19.1 |
| Extensive | 6 | 6.7 |
| Respiratory problems | ||
| Within 1 year post–allo-HSCT | 14 | 15.7 |
| More than 1 year post–allo-HSCT | 7 | 7.9 |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; CML, chronic myeloid leukemia; GVHD, graft-versus-host disease; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; No., number; TBI, total body irradiation
Although various conditioning regimens were administered, the vast majority of patients received cyclophosphamide and total body irradiation. Cytarabine was used before 2002 and was replaced by thiotepa thereafter. Acute GVHD was observed in 49.4% and extensive chronic GVHD was limited to only 6.7% of the patients. While 15.7% of the patients developed pulmonary events within 1 year post–allo-HSCT, 7.9% experienced chronic respiratory problems (i.e., wheezing and cough) more than 1 year post–allo-HSCT. The respiratory problems within 1 year post-allo-HSCT include 11 cases of pneumonia, 2 of bronchiolitis obliterans organizing pneumonia and 1 of pleural effusion with pneumothorax.
Prevalence of Pulmonary Dysfunction
Abnormal lung parameters (i.e., obstruction, air trapping, restriction, or reduced diffusion capacity) were preexisting in 12.4% to 40.4% of patients pre–allo-HSCT (Table 2), and respiratory dysfunction developed in 15.7% to 64% post–allo-HSCT. Patients with abnormal pre–allo-HSCT values of FEV1/FVC (p=0.0083), FEV1 (p=0.011), RV (p<0.0001), FRC (p=0.0056) and FVC (p=0.0025) were more likely to have abnormal post–allo-HSCT values when compared with patients with normal pre–allo-HSCT PFT values.
Table 2.
Proportional hazards analysis of pulmonary function in 89 survivors of childhood hematologic malignancies who received allogeneic HSCT
| Tests | % of Patients below predicted values | Risk Factors | P value | Hazard Ratio | |
|---|---|---|---|---|---|
| Pre-transplant | Post-transplant* | ||||
| Obstructive values | |||||
| FEV1/FVC | 18.0% | 22.5% | Male | 0.048 | 2.1 |
| FEV1 | 22.5% | 36.0% | Respiratory event (<1yr after HSCT) | 0.0007 | 3.226 |
| FEF25%–75% | 16.9% | 49.4% | Respiratory event (<1yr after HSCT) | 0.0074 | 2.703 |
| Peripheral blood product | 0.029 | 2.489 | |||
| High risk group | 0.042 | 1.786 | |||
| Older recipient age (years) | 0.038 | 1.082 | |||
| Air trapping values | |||||
| RV | 40.4% | 28.1% | |||
| RV/TLC | 23.6% | 38.2% | |||
| FRC | 20.2% | 15.7% | |||
| Restrictive values | |||||
| FVC | 15.7% | 39.3% | Peripheral blood product | 0.027 | 2.716 |
| Respiratory event (<1yr after HSCT) | 0.028 | 2.257 | |||
| Acute GVHD | 0.034 | 1.942 | |||
| TLC | 12.4% | 43.8% | Younger donor age (years) | 0.0025 | 1.035 |
| Cytarabine use | 0.031 | 0.463 | |||
| Diffusion values | |||||
| DLCOcorr | 19.1% | 64.0% | Respiratory problem (>1yr after HSCT) | <0.0001 | 8.065 |
| High risk group | 0.0006 | 2.558 | |||
| Positive recipient CMV antibody | 0.036 | 1.704 | |||
| Older recipient age (years) | 0.0051 | 1.102 | |||
| Cytarabine use | 0.0025 | 0.399 | |||
The post-transplant abnormal group does not include those who were also abrnormal pre-transplant.
CMV indicates cytomegalovirus; DLCOcorr, diffusion capacity corrected for hemoglobin; FEF25–75%, forced mid-expiratory flow; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FRC, functional residual capacity; GVHD, graft versus host disease; HSCT, hematopoietic stem cell transplantation; RV, residual volume; TLC, total lung capacity
Obstructive Pulmonary Defects
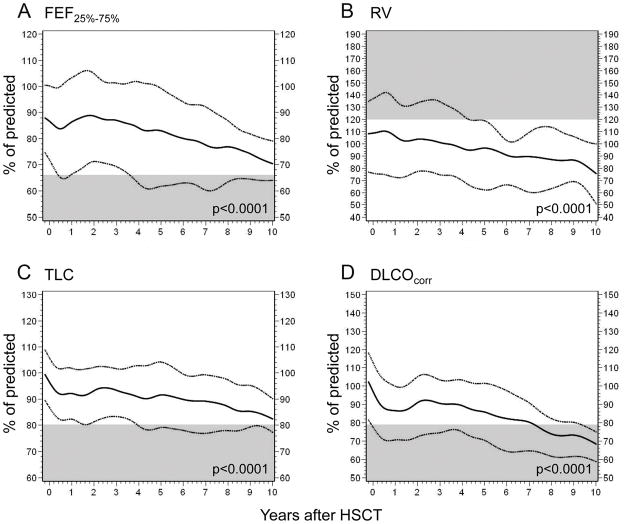
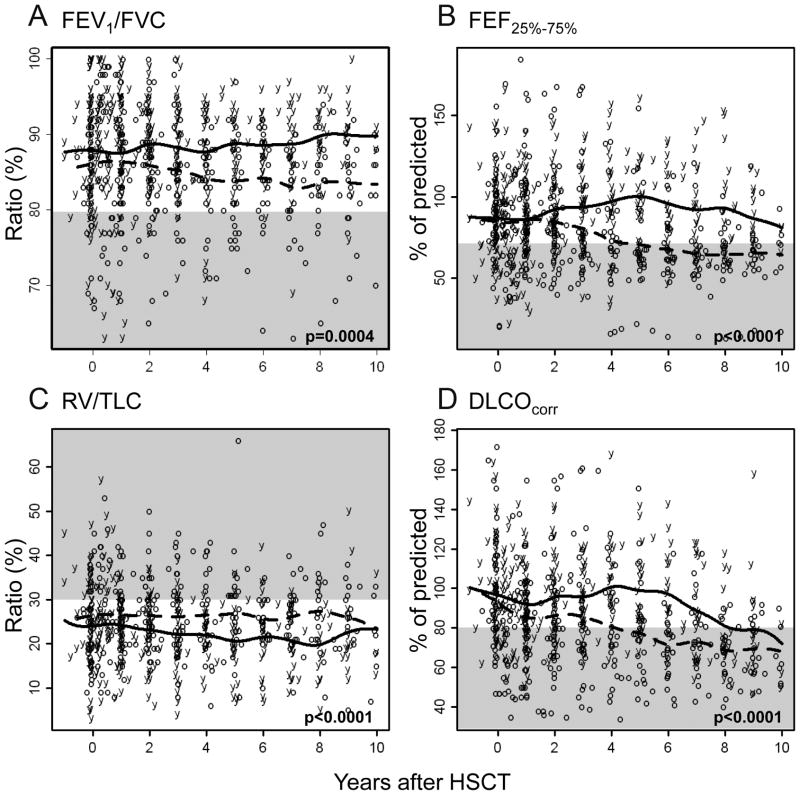
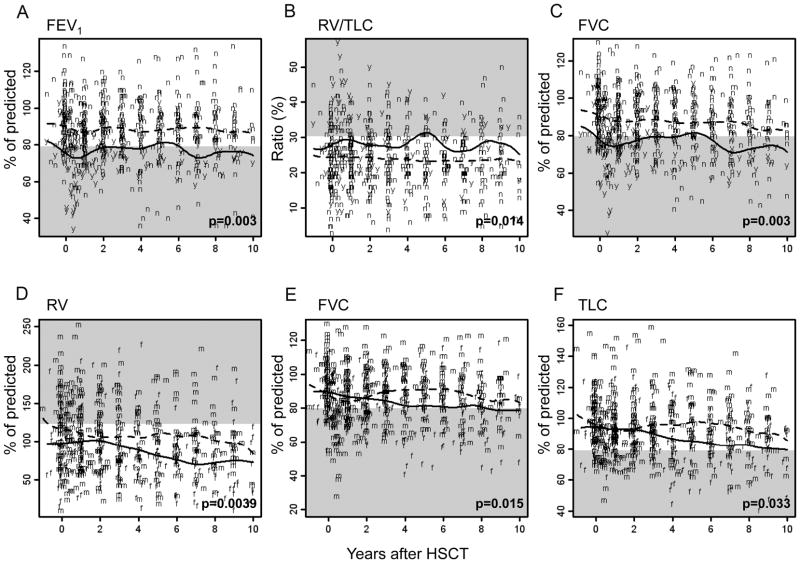
FEV1/FVC ratio, FEV1, and FEF25%–75% values were evaluated to assess obstructive pulmonary defects (Tables 2–3). Longitudinal analyses of the cohort revealed a gradual decline in the FEV1/FVC (p=0.0001; Table 3) and the FEF25%–75% (p<0.0001; Fig. 1a) post–allo-HSCT. Transient recovery occurred 1 to 2 years post–allo-HSCT after the FEF25%–75% declined; however, the values deteriorated again thereafter. The declines in FEV1/FVC and FEF25%–75% were significant in patients older than the median age (12.7 years, p=0.0004 and p<0.0001, respectively; Figs. 2A and 2B). The FEV1/FVC in male patients was worse than that in female patients during the first 4 years post–allo-HSCT (p=0.026; Table 3). Patients who experienced a respiratory event within 1 year post–allo-HSCT showed lower FEV1 values at baseline and throughout follow-up, compared to those values in patients who did not experience any respiratory events (p=0.003; Fig. 3A).
Table 3.
Longitudinal analysis of pulmonary function in 89 survivors of childhood hematologic malignancies who received allogeneic HSCT
| Tests | Factors | P value | Estimates of slope |
|---|---|---|---|
| Obstructive values | |||
| FEV1/FVC | Male | 0.026 | −2.6554 |
| Recipient age (year) | 0.0004 | −0.5444 | |
| Years from HSCT | 0.0001 | −0.2482 | |
| FEV1 | Respiratory event (<1yr after HSCT) | 0.003 | −11.4091 |
| FEF25%–75% | Recipient age (year) | < 0.0001 | −2.4812 |
| Years from HSCT | < 0.0001 | −1.909 | |
| Air trapping values | |||
| RV | Female | 0.0039 | −18.7051 |
| Years from HSCT | < 0.0001 | −3.369 | |
| RV/TLC | Respiratory event (<1yr after HSCT) | 0.014 | 4.1278 |
| Recipient age (year) | < 0.0001 | 0.6059 | |
| Years from HSCT | 0.0039 | −0.2847 | |
| FRC | Years from HSCT | < 0.0001 | −2.347 |
| Restrictive values | |||
| FVC | Respiratory event (<1yr after HSCT) | 0.003 | −11.7654 |
| Female | 0.015 | −6.9235 | |
| TLC | Female | 0.033 | −6.6557 |
| Years from HSCT | < 0.0001 | −1.179 | |
| Diffusion values | |||
| DLCOcorr | Years from HSCT | < 0.0001 | −2.544 |
| Recipient age (year) | < 0.0001 | −2.015 | |
DLCOcorr indicates diffusion capacity corrected for hemoglobin; FEF25–75%, forced mid-expiratory flow; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FRC, functional residual capacity; GVHD, graft versus host disease; HSCT, hematopoietic stem cell transplantation; RV, residual volume; TLC, total lung capacity
Figure 1.
Changes in pulmonary function over time post–allo-HSCT. The solid lines depict the 50th percentile, and the dotted lines represent the interquartile ranges (25th percentile-75th percentile). Abnormal ranges are shown in gray.
Figure 2.
Comparison of changes in long-term pulmonary function over time on the basis of age at allo-HSCT. Raw data and 50th percentiles are plotted for patients younger than 12.7 years at allo-HSCT (solid lines and “y” letters) and those older than 12.7 years (dotted lines and “o” letters). Abnormal ranges are shown in gray.
Figure 3.
Changes in pulmonary function over time on the basis of respiratory event within 1 year post–allo-HSCT (a–c) and sex (d–f). Raw data and 50th percentiles are plotted. Solid lines depict patients with respiratory events (“y” letters) or female sex (“f” letters), and dotted lines represent patients without respiratory events (“n” letters) or male sex (“m” letters). Abnormal ranges are shown in gray.
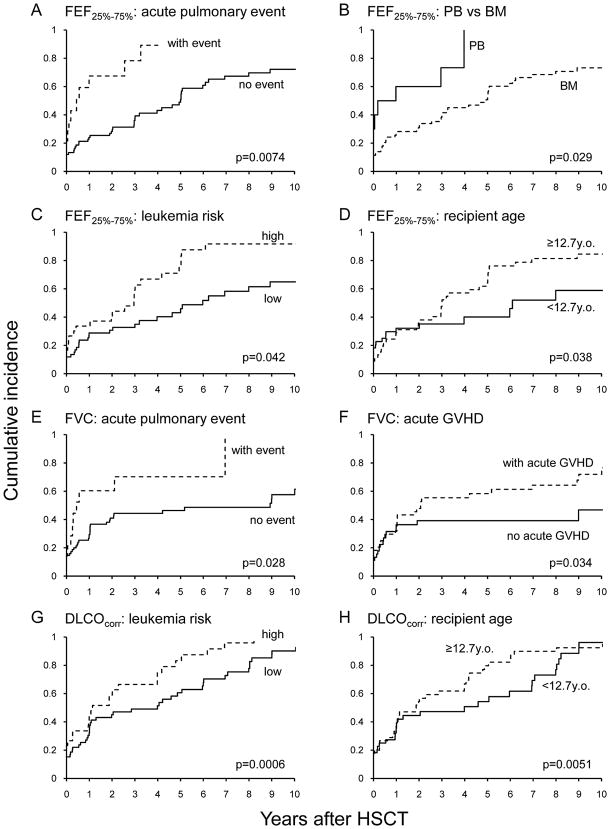
Abnormal FEV1/FVC and FEF25%–75% pre-allo-HSCT were observed in 18% and 16.9% of patients, respectively. Among patients who did not have pre–allo-HSCT abnormalities, abnormal FEV1/FVC and FEF25%–75% were observed post–allo-HSCTs in 22.5% and 49.4%, respectively (Table 2). Proportional hazards analyses demonstrated that male patients were at higher risk of abnormal FEV1/FVC (p=0.048). Patients who experienced a respiratory event within 1 year post–allo-HSCT (p=0.0074; Fig. 4A), received a peripheral blood HSC product (p=0.029; Fig. 4B), had high-risk hematologic disease (p=0.042; Fig. 4C), and were older at allo-HSCT (p=0.038; Fig. 4D) were also at higher risk of abnormal FEF25%–75%. Abnormal FEV1 was more often in patients who experienced a respiratory event within 1 year post–allo-HSCT (p=0.0007; Table 2).
Figure 4.
Cumulative incidence of abnormal FEF25%–75% (A–D), FVC (E and F), and DLCOcorr (G and H).
Pulmonary Air Trapping
We examined RV, RV/TLC, and FRC to evaluate air trapping (Tables 2–3). For the cohort, all 3 air trapping indicators decreased over time post–allo-HSCT (p<0.0001 for RV and FRC; p=0.0039 for RV/TLC; Table 3 and Fig. 1B). Female patients had more decline in RV (p=0.0039; Fig. 3D) and similar tendency in FRC (p=0.058). Older age at allo-HSCT (p<0.0001; Fig. 2C) and a respiratory event within 1 year post–allo-HSCT (p=0.014; Fig. 3B) were associated with higher RV/TLC at the baseline PFT and throughout the follow-up.
Restrictive Pulmonary Defects
FVC and TLC were measured to evaluate restrictive abnormalities (Tables 2–3). TLC values significantly declined with longer interval from allo-HSCT (p<0.0001), though a slight recovery occurred between the second and third year post–allo-HSCT (Fig. 1C). Remarkable sex differences were seen in FVC (p=0.015; Fig. 3E) and TLC (p=0.033; Fig. 3F): male patients recovered their values to the baseline at 2 years and remained relatively stable thereafter; female patients had steady decline over time post–allo-HSCT. From baseline and throughout follow-up, FVC values were persistently lower in patients who experienced a respiratory problem within 1 year post–allo-HSCT (p=0.003; Fig. 3C).
Baseline FVC and TLC was abnormal in 15.7% and 12.4% of the patients, respectively (Table 2). These measures became abnormal for the first time post–allo-HSCT in 39.3% and 43.8% of patients, respectively. Proportional hazards analyses demonstrated that the patients who received a peripheral blood HSC product (p=0.027), experienced a respiratory problem within 1 year post–allo-HSCT (p=0.028; Fig. 4E), and had acute GVHD (p=0.034; Fig. 4F) were at higher risk of abnormal FVC values (Table 2). Abnormal TLC values were associated with younger donor age (p=0.0025). The use of cyclophosphamide with cytarabine was significantly associated with better TLC values compared to the use of cyclophosphamide with thiotepa or other chemotherapy agents (p=0.031).
Diffusion Capacity Changes
We assessed DLCOcorr to evaluate diffusion capacity (Tables 2–3). Longitudinal analyses showed a rapid decline immediately post–allo-HSCT and then transient stabilization in diffusion capacity 2 to 3 years post–allo-HSCT; however, DLCOcorr worsened thereafter (p<0.0001; Fig. 1D). Older age at allo-HSCT (p<0.0001; Fig. 2D) was associated with progressive worsening of DLCOcorr.
Low DLCOcorr was seen in 19.1% of patients pre–allo-HSCT; in 64% of patients, this abnormality was seen only during post–allo-HSCT (Table 2). Proportional hazards model revealed that patients with respiratory problems beyond 1 year post–allo-HSCT (p<0.0001), high-risk hematologic disease (p=0.0006; Fig. 4G), positive CMV titer (p=0.036), and older age at allo-HSCT (p=0.0051; Fig. 4H) were at higher risk of abnormal DLCOcorr. The use of cyclophosphamide with cytarabine was significantly associated with better DLCOcorr compared to the use of cyclophosphamide with thiotepa or other chemotherapy agents (p=0.0025).
DISCUSSION
We examined the effects of allo-HSCT on pulmonary function in survivors of pediatric hematologic malignancies. We used 2 analytic approaches: longitudinal analyses, which examined in detail changes in pulmonary function over time, and proportional hazards analyses, which took into account pre-existing lung conditions and assessed the risk factors associated with development of pulmonary dysfunction post–allo-HSCT.
A few large-scale pediatric studies have addressed pulmonary function beyond 5 years post-HSCT.3,4 However, they were limited to cross-sectional analyses without consideration of baseline PFTs and involved heterogeneous patient populations (benign and malignant disease and autologous and allo-HSCT). In this prospective study of a relatively uniform cohort, i.e., survivors of pediatric hematologic malignancies who received allo-HSCT, we showed that 12.4% to 40.4% of the patients had abnormal lung parameters pre–allo-HSCT, and 64% of the patients developed at least one abnormal lung parameter after allo-HSCT. This alarming prevalence of lung abnormalities highlights the vulnerability of this population.
To optimize the cost effectiveness of surveillance strategies, we identified several risk factors that are useful for risk-adapted monitoring and counseling, including the abnormal pre–allo-HSCT PFT, interval from allo-HSCT, patient age, respiratory event after allo-HSCT, sex, leukemia-risk group, and HSC product. The association of the latter 2 risk factors with lung dysfunction is expected, as high-risk disease is often associated with more intensive treatment and complications prior to allo-HSCT than the standard-risk group and peripheral blood HSC product has been associated with higher incidence of chronic GVHD resulting in pulmonary insufficiency.14,15
The natural history of pulmonary dysfunction post–allo-HSCT has not been well characterized. Our cohort showed progressive worsening of pulmonary function over time, i.e., obstructive, air trapping, restrictive, and diffusion measures declined during a median follow-up of 8.9 years. Upon closer examination, we found a transient partial recovery or stabilization of pulmonary function during the second and third year post–allo-HSCT, after the initial decline during the first year. This pattern has been reported in other pediatric studies5–7. In those reports, lung volumes and diffusion capacity at 3 to 6 months post-HSCT were lower than pre-HSCT values; the function partially recovered 12 to 24 months post-HSCT. This partial recovery may mislead clinicians to stop monitoring pulmonary function in these patients; however, our study’s much longer follow-up showed that lung parameters continue to decline after this brief partial recovery, and the declining values had not yet plateaued at last follow-up.
Growth and endocrine sequelae of cancer treatment are worse in children treated at a younger age16, however, data on pulmonary dysfunction and its relationship to age have been inconclusive. Some small studies have suggested that pulmonary function is better if transplantation is performed early in life,7,17 while others have shown no effect of age.3,18 In our cohort, pulmonary dysfunction was strongly associated with older age at allo-HSCT in both longitudinal and proportional hazards models. Remarkably, patients older than the median age (12.7 years) at the time of allo-HSCT did not show any signs of recovery with extended follow-up. We also analyzed longitudinal changes of 155 PFTs in 34 allo-HSCT recipients younger than 6 years (unpublished data). Their PFT values declined until adolescence; however, the values recovered thereafter. The lung growth and/or repair could be associated with hormonal effects and this process might not occur efficiently when damage occurs during puberty and adolescence. Prompt intervention and patient counseling are warranted, including instructions to avoid smoking, preferably live in an area with minimal air pollution, and receive vaccinations to avoid influenza and pneumococcal infections.19,20
Patients who experienced respiratory events within 1 year post–allo-HSCT had lower FVC and FEV1 and higher RV/TLC from the baseline, which persisted throughout follow-up. Likewise, patients with abnormal pre–allo-HSCT PFTs were more likely to have abnormal post–allo-HSCT PFT values. Patients with worse baseline PFTs may be susceptible to infections and other pulmonary events that inhibit the post-HSCT repair. Careful selection of conditioning regimen, such as reduced-intensity conditioning or lung shielding, as well as diligent prophylaxis of pulmonary infection post–allo-HSCT should be considered when baseline PFTs are abnormal.
Some studies have demonstrated a sex difference in pulmonary function soon after HSCT. For example, Wieringa et al.6 showed that girls had a lower mean TLC than boys, and Clark et al.21 reported that male survivors had lower FEV1/FVC values. However, other studies have failed to show a sex difference, and whether some of the observed differences diminish over time remains unknown. We found that female survivors had lower lung volumes (FVC, TLC and RV). All survivors showed worse restrictive values at 1 year post–allo-HSCT, but only in females did those values continue to decline. Male patients more frequently had abnormal obstructive values than did females. Significant sex differences have been observed in the incidence, pathogenesis, severity, and responsiveness to treatment of reactive airway disease and chronic obstructive airway disease.22,23 These results suggest that a better understanding of the hormonal influence, pharmacogenetics, and mechanisms that control the sex disparity in chest wall, diaphragmatic, and lung functions would help improve management strategies.
For preparative regimen, fractionated TBI was used in 86 of the 89 patients. There was no difference in any PFT parameters between those patients who received 12 Gy of TBI versus those who received 14Gy. As actual lung dose has been reduced to 10 Gy by lung shielding as a change in routine practice since April 2001, we also analyzed the effect of actual lung dosages. However, we found no statistically significant effect by lung doses (0, 10, 12 or 14Gy) on any PFT measurements. Although we do not have enough malignant cases treated with chemotherapy-only conditioning, our experience in 38 patients with non-malignant diseases (30 of them were treated with chemotherapy-only conditioning) revealed that busulfan was associated with lower FEV1, FVC and DLCOcorr.24 Recent incorporation of reduced intensity regimen without TBI or busulfan might improve long-term pulmonary sequelae.
Although we have long-term follow-up of median 8.9 years, most of the survivors are still below 30 years old. The patients with chronic respiratory problems are only 7.9%, which are not as prevalent as observed PFT abnormalities. Respiratory illness early in life and decreased lung function during childhood are the strongest predictors of adult chronic obstructive pulmonary disease, which is the fourth leading cause of death in the U.S.25,26 Our longitudinal analysis showed continued declines in PFT values. Therefore, until further longer follow-up of allo-HSCT survivors is achieved, we believe that longitudinal continual surveilance of PFT data is important.
In summary, our longitudinal and proportional hazards analyses of pulmonary function in pediatric survivors of hematologic malignancies who received allo-HSCT revealed a high prevalence of abnormalities that worsened over time. Our findings suggest that continual monitoring of respiratory function and development of preventive measures are important. Risk-adaptive approaches should consider the pre–allo-HSCT PFT values, interval from allo-HSCT, patient age at allo-HSCT, respiratory problems within 1 year post–allo-HSCT, sex, leukemia-risk group, and HSC product. Early detection of pulmonary dysfunction will prompt intervention and patient counseling. Continual follow-up of this cohort and others and further investigation of the effect of surveillance and intervention strategies are warranted to achieve the ultimate goal of minimizing pulmonary morbidity and mortality in this highly vulnerable growing population.
Acknowledgments
We acknowledge the expertise of W. Paul Mackert and Doug Harper in performing the pulmonary function studies, the assistance of Drs. James Tutor and Robert Schoumacher in test interpretation, and the expertise of Dr. Angela McArthur in editorial review of the manuscript.
Supported in part by Cancer Center Core Grant CA21765 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC). Dr. Pui is the American Cancer Society Professor.
Footnotes
The authors have no potential conflicts of interest, including specific financial interests, relationships, or affiliations relevant to the subject of this manuscript.
References
- 1.Socie G, Salooja N, Cohen A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101:3373–3385. doi: 10.1182/blood-2002-07-2231. [DOI] [PubMed] [Google Scholar]
- 2.Leung W, Ahn H, Rose SR, et al. A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore) 2007;86:215–224. doi: 10.1097/MD.0b013e31812f864d. [DOI] [PubMed] [Google Scholar]
- 3.Cerveri I, Zoia MC, Fulgoni P, et al. Late pulmonary sequelae after childhood bone marrow transplantation. Thorax. 1999;54:131–135. doi: 10.1136/thx.54.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmeister PA, Madtes DK, Storer BE, Sanders JE. Pulmonary function in long-term survivors of pediatric hematopoietic cell transplantation. Pediatr Blood Cancer. 2006;47:594–606. doi: 10.1002/pbc.20531. [DOI] [PubMed] [Google Scholar]
- 5.Cerveri I, Fulgoni P, Giorgiani G, et al. Lung function abnormalities after bone marrow transplantation in children: has the trend recently changed? Chest. 2001;120:1900–1906. doi: 10.1378/chest.120.6.1900. [DOI] [PubMed] [Google Scholar]
- 6.Wieringa J, van Kralingen KW, Sont JK, Bresters D. Pulmonary function impairment in children following hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2005;45:318–323. doi: 10.1002/pbc.20304. [DOI] [PubMed] [Google Scholar]
- 7.Leneveu H, Bremont F, Rubie H, et al. Respiratory function in children undergoing bone marrow transplantation. Pediatr Pulmonol. 1999;28:31–38. doi: 10.1002/(sici)1099-0496(199907)28:1<31::aid-ppul6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 9.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 11.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 12.Weng TR, Levison H. Standards of pulmonary function in children. Am Rev Respir Dis. 1969;99:879–894. doi: 10.1164/arrd.1969.99.6.879. [DOI] [PubMed] [Google Scholar]
- 13.Miller A, Thornton JC, Warshaw R, Anderson H, Teirstein AS, Selikoff IJ. Single breath diffusing capacity in a representative sample of the population of Michigan, a large industrial state. Predicted values, lower limits of normal, and frequencies of abnormality by smoking history. Am Rev Respir Dis. 1983;127:270–277. doi: 10.1164/arrd.1983.127.3.270. [DOI] [PubMed] [Google Scholar]
- 14.Schultz KR, Green GJ, Wensley D, et al. Obstructive lung disease in children after allogeneic bone marrow transplantation. Blood. 1994;84:3212–3220. [PubMed] [Google Scholar]
- 15.Champlin RE, Schmitz N, Horowitz MM, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT) Blood. 2000;95:3702–3709. [PubMed] [Google Scholar]
- 16.Oberfield SE, Chin D, Uli N, David R, Sklar C. Endocrine late effects of childhood cancers. J Pediatr. 1997;131:S37–S41. doi: 10.1016/s0022-3476(97)70009-x. [DOI] [PubMed] [Google Scholar]
- 17.Quigley PM, Yeager AM, Loughlin GM. The effects of bone marrow transplantation on pulmonary function in children. Pediatr Pulmonol. 1994;18:361–367. doi: 10.1002/ppul.1950180604. [DOI] [PubMed] [Google Scholar]
- 18.Nysom K, Holm K, Hesse B, et al. Lung function after allogeneic bone marrow transplantation for leukaemia or lymphoma. Arch Dis Child. 1996;74:432–436. doi: 10.1136/adc.74.5.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avol EL, Gauderman WJ, Tan SM, London SJ, Peters JM. Respiratory effects of relocating to areas of differing air pollution levels. Am J Respir Crit Care Med. 2001;164:2067–2072. doi: 10.1164/ajrccm.164.11.2102005. [DOI] [PubMed] [Google Scholar]
- 20.Ljungman P, Engelhard D, de la Camara R, et al. Vaccination of stem cell transplant recipients: recommendations of the Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant. 2005;35:737–746. doi: 10.1038/sj.bmt.1704870. [DOI] [PubMed] [Google Scholar]
- 21.Clark JG, Schwartz DA, Flournoy N, Sullivan KM, Crawford SW, Thomas ED. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med. 1987;107:648–656. doi: 10.7326/0003-4819-107-5-648. [DOI] [PubMed] [Google Scholar]
- 22.Carter R, Nicotra B, Huber G. Differing effects of airway obstruction on physical work capacity and ventilation in men and women with COPD. Chest. 1994;106:1730–1739. doi: 10.1378/chest.106.6.1730. [DOI] [PubMed] [Google Scholar]
- 23.Matsubara S, Swasey CH, Loader JE, et al. Estrogen determines sex differences in airway responsiveness after allergen exposure. Am J Respir Cell Mol Biol. 2008;38:501–508. doi: 10.1165/rcmb.2007-0298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motosue MS, Inaba H, Pan J, et al. Pulmonary function in pediatric survivors of non-malignant disorders after allogeneic hematopoietic stem cell transplantation. Blood. 2008;112:744a. [Google Scholar]
- 25.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston ID, Strachan DP, Anderson HR. Effect of pneumonia and whooping cough in childhood on adult lung function. N Engl J Med. 1998;338:581–587. doi: 10.1056/NEJM199802263380904. [DOI] [PubMed] [Google Scholar]