Abstract
The hallmark of social insects is their caste system: reproduction is primarily monopolized by queens, whereas workers specialize in the other tasks required for colony growth and survival. Pheromones produced by reining queens have long been believed to be the prime factor inhibiting the differentiation of new reproductive individuals. However, there has been very little progress in the chemical identification of such inhibitory pheromones. Here we report the identification of a volatile inhibitory pheromone produced by female neotenics (secondary queens) that acts directly on target individuals to suppress the differentiation of new female neotenics and identify n-butyl-n-butyrate and 2-methyl-1-butanol as the active components of the inhibitory pheromone. An artificial pheromone blend consisting of these two compounds had a strong inhibitory effect similar to live neotenics. Surprisingly, the same two volatiles are also emitted by eggs, playing a role both as an attractant to workers and an inhibitor of reproductive differentiation. This dual production of an inhibitory pheromone by female reproductives and eggs probably reflects the recruitment of an attractant pheromone as an inhibitory pheromone and may provide a mechanism ensuring honest signaling of reproductive status with a tight coupling between fertility and inhibitory power. Identification of a volatile pheromone regulating caste differentiation in a termite provides insights into the functioning of social insect colonies and opens important avenues for elucidating the developmental pathways leading to reproductive and nonreproductive castes.
Keywords: queen pheromone, inhibitory pheromone, social insect, honest signal, egg volatile
Reproductive division of labor based on castes is a major transition in the evolution of social insects (1, 2). Regulation of the number of fertile queens requires communication between reproductive and nonreproductive individuals, often through pheromones (3–7). Queens of many social insect species produce a variety of pheromones that profoundly influence the behavior, development, and physiology of colony members. Since the discovery more than 50 y ago of the queen honey bee substance that inhibits the queen rearing behavior of workers (8), there has been very little progress in the chemical identification of inhibitory queen pheromones. In termites, which evolved eusociality independently of Hymenoptera, the existence of queen pheromones inhibiting the differentiation of supplementary queens has been suggested for many decades (5–7), but to date no active compounds have been identified. Although reproductive-specific hydrocarbons (9) and proteinaceous compounds (10) have recently been proposed as possible inhibitory pheromones in termites, there is no evidence of inhibitory effect of either of these classes of compounds.
Colonies of termites can contain several types of reproductive individuals, depending on their developmental origin (Fig. S1). Termite colonies are typically founded by a monogamous pair of primary reproductives (adult winged forms), one king and one queen. In many species, especially in the subterranean termites (Rhinotermitidae), secondary reproductives, also called neotenics, are produced from within the colony upon the death of the primary king and/or queen (11, 12). These neotenic individuals can either differentiate from nymphs to become “nymphoid” reproductives with wing pads or they can differentiate from workers to become “ergatoid” reproductives without wing pads.
Lower termites of the genus Reticulitermes provide good subjects for obtaining large numbers of female reproductives, facilitating the identification of reproductive inhibitory pheromones, because the primary queen is often replaced by many female neotenics (11–13; Fig. 1A). In Reticulitermes and other lower termites it has long been observed that the presence of fertile female reproductives prevents the differentiation of new female neotenics (14, 15). To test for an inhibitory effect of female reproductives on the differentiation of new neotenics and to determine how such an effect is mediated, we used Reticulitermes speratus, a species widely distributed in central Japan. We now report that the presence of fertile female neotenics inhibits the development of new female neotenics by means of a volatile pheromone produced by both fertile reproductives and by the eggs they lay.
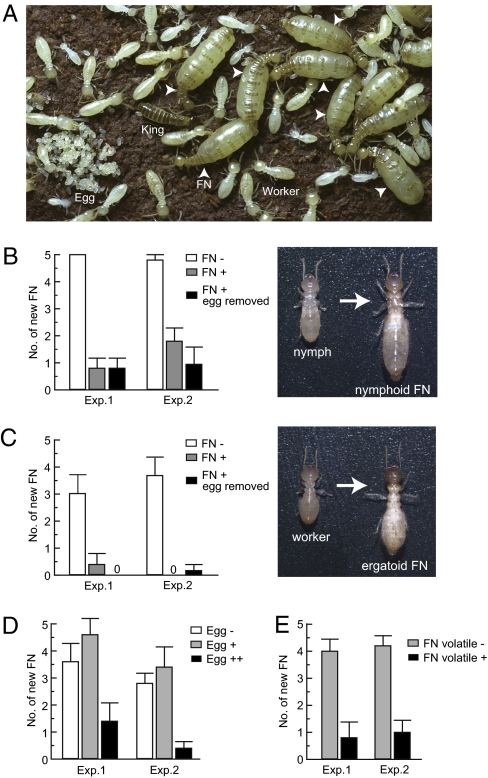
Fig. 1.
Female neotenics and eggs suppress the differentiation of new female neotenics. (A) Termite royals with workers and brood. Female neotenics (FN) are indicated by arrowheads. (B) Presence of current FN significantly suppressed the differentiation of new female (nympoid) reproductives from nymphs in comparison with controls (P < 0.001, Tukey's HSD). Removal of eggs did not significantly reduce the inhibitory effect (P = 0.42). (C) Presence of current functional neotenics significantly suppressed the differentiation of new female (ergatoid) reproductives from workers (P < 0.001, Tukey's HSD). Removal of eggs did not significantly reduce the inhibitory effect (P = 0.97). (D) Continuous addition of 100 eggs per day (Egg ++) suppressed the differentiation of new female neotenics from workers (P < 0.01), but 20 eggs per day (Egg +) did not have a significant effect compared with controls (P = 0.34). (E) Volatiles of current female neotenic reproductives through stainless steel mesh significantly suppressed the differentiation of new female neotenics from nymphs (P < 0.001). Values denote the mean ± SEM of five replications.
Results and Discussion
In a first experiment we compared the number of female neotenics produced in groups containing nymphs, workers, and a female neotenic with similar groups without a female neotenic. In these experiments some female and male neotenics were produced from nymphs (nymphoid reproductives) but not from workers (ergatoid reproductives). The differentiation of new female nymphoid reproductives was greatly suppressed by the presence of a functional female reproductive (colony: F1,26 = 0.69, P = 0.41; treatment: F2,26 = 64.83, P < 0.0001, two-way ANOVA; Fig. 1B). By contrast, there was no significant influence of the presence of female reproductives on the differentiation of male nymphoid reproductives (treatment: F2,26 = 1.37, P = 0.27). In a second experiment we similarly investigated the inhibitory power of female neotenics but used experimental units containing only workers. Under such conditions some workers differentiated into ergatoid reproductives. As in the previous experiment, the presence of a fertile female reproductive greatly suppressed the differentiation of new female ergatoid reproductives (colony: F1,26 = 0.14, P = 0.71; treatment: F2,26 = 35.17, P < 0.0001; Fig. 1C) but not male ergatoid reproductives (treatment: F2,26 = 0.64, P = 0.53). Thus, female neotenics exerted a strong sex-specific effect on the differentiation of new neotenics regardless of developmental origin.
To determine whether the inhibition of female reproductive differentiation was mediated directly by the female reproductives and/or via the brood she produced we conducted two experiments. First, we tested whether female neotenics continued to have an inhibitory effect when their eggs were removed every 24 h. Female neotenics without brood significantly inhibited the differentiation of female nymphoid reproductives in colonies containing nymphs and workers (Fig. 1B) as well as the differentiation of female ergatoid reproductives in colonies containing workers only (Fig. 1C). Second, we tested whether eggs also had an inhibitory effect by comparing the production of new female neotenics in colonies without female reproductives where eggs were absent or continuously added at a rate of 100 eggs per day or 20 eggs per day. There was a significant difference between treatments (F2,26 = 16.42, P < 0.0001), as well as a significant colony effect (F1,26 = 4.76, P = 0.04; Fig. 1D). A posteriori comparisons showed a significant difference between colonies receiving 100 eggs per day and colonies without eggs (P < 0.01) but no significant difference between colonies receiving 20 eggs per day and colonies without eggs [P = 0.34, Tukey's honestly significant difference (HSD) test]. Thus, both female reproductives and their eggs, in sufficiently large numbers, exert a strong inhibitory influence on the differentiation of new female neotenics.
Additional experiments in which female neotenics were confined in double mesh cages revealed that the inhibitory effect of female reproductives was mediated by volatiles. Caged female neotenics were without contact with other colony members, yet they significantly suppressed the differentiation of new female neotenics (treatment: F1,17 = 49.45, P < 0.0001; colony: F1,17 = 0.19, P = 0.67; Fig. 1E).
To identify the volatile inhibitory pheromone produced by female neotenics, we collected a large number of R. speratus colonies and used the two largest for chemical analyses. Headspace-collected volatiles from 100 fully developed female neotenics were analyzed by gas chromatography (Fig. 2A) followed by mass spectrometry (GC-MS; Fig. 2 B and C). We found that the volatiles from female neotenics consist of an ester n-butyl-n-butyrate [nBnB; Chemical Abstracts Service (CAS) no. 109-21-7; Fig. 2B] and an alcohol 2-methyl-1-butanol (2M1B; CAS no. 137-32-6; Fig. 2C) in a 2.14:1 ratio. No other chemical was detected.
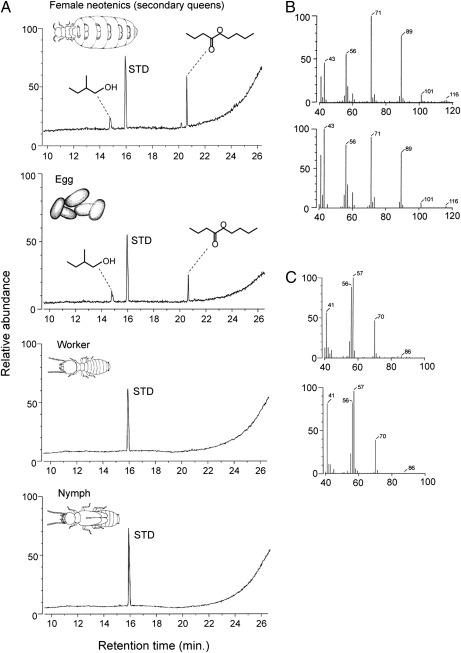
Fig. 2.
Headspace GC-MS analysis. (A) Chromatograms of headspace-collected volatiles of female neotenics, eggs, workers, and nymphs. Female neotenic and egg volatiles consisted of identical compounds, nBnB and 2M1B, whereas workers and nymphs did not have these compounds. STD, toluene as an internal standard. (B) Mass spectra of nBnB from female neotenics (Upper) and of commercial origin (Lower). (C) Mass spectra of 2M1B from female neotenics (Upper) and of commercial origin (Lower).
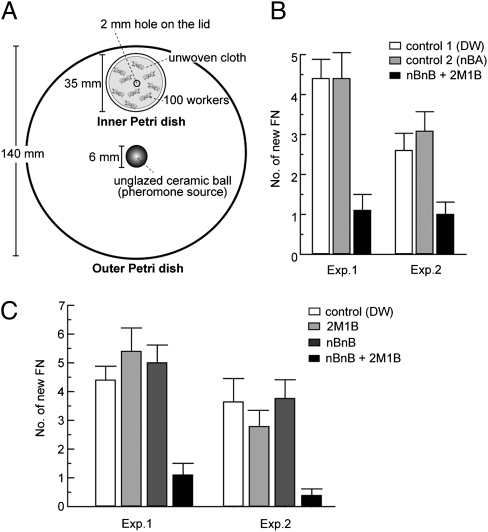
We then addressed whether nBnB and 2M1B are key components of the female reproductive inhibitory pheromone by investigating the effect of both volatiles on the differentiation of new neotenics. To this end we developed a protocol for exposing termites to the volatile compounds gradually and continuously (Fig. 3A), whereby the compounds once absorbed by an unglazed ceramic ball slowly volatilize in the outer Petri dish and then enter the inner Petri dish through a small opening on the lid. A 2:1 blend of commercial nBnB and 2M1B, matching the naturally occurring ratios, strongly suppressed the differentiation of workers into female ergatoid reproductives (colony: F1,38 = 7.95, P < 0.01; treatment: F2,38 = 19.66, P < 0.0001; Fig. 3B), whereas there was no significant influence on the differentiation of male ergatoid reproductives (treatment: F2,38 = 0.51, P = 0.60). The synthetic pheromone blend did not significantly affect survival rates of test termites compared with controls receiving only water (P > 0.05, Tukey's HSD). In addition, each single compound alone had no effect on differentiation of female neotenics compared with a control treatment (P > 0.05, Tukey's HSD; Fig. 3C).
Fig. 3.
Suppression of the differentiation of new female neotenics by a blend of synthetic nBnB and 2M1B. (A) Experimental setup for the inhibitory bioassay of synthetic volatiles. (B) Inhibitory effect of an artificial pheromone blend. A 2:1 mixture of nBnB and 2M1B suppressed the differentiation of new female neotenics (P < 0.001, Tukey's HSD), whereas n-butyl acetate, an ester (control 2), did not (P = 0.82) compared with control 1 (distilled water). (C) Comparison of inhibitory effect of the individual compounds and a 2:1 mixture of nBnB and 2M1B. The blend significantly suppressed the differentiation of new female neotenics (P < 0.001, Tukey's HSD), whereas neither nBnB alone (P = 0.97) nor 2M1B alone (P = 0.99) had a significant effect compared with the control (distilled water). Values denote the mean ± SEM of five replications.
In contrast to female neotenics, workers and nymphs did not produce any nBnB or 2M1B (Fig. 2A). Interestingly, however, eggs emitted both volatiles (Fig. 2A). Egg volatiles might be transferred from queens through egg marking, as has been shown in some ant species (16), or could be emitted by the eggs themselves. It is known that an antibacterial protein lysozyme, which also functions as an egg recognition signal, is synthesized in eggs (17), implying that termite eggs are biosynthetically active. If the volatiles are transferred from female reproductives to the egg surface, the volatiles should be detected even after the eggs are killed by freezing. However, frozen-killed eggs showed no volatile emission, indicating that the volatiles are actively produced by the eggs themselves (Fig. S2).
In a last experiment we investigated whether the volatile components also emitted by eggs had additional functions. Termite eggs cannot survive without protection by workers (18). Soon after being laid, eggs are carried into nursery chambers where they are groomed by workers and coated with saliva and antibiotic substances. Workers recognize eggs by morphological cues (18–20) and a termite egg recognition pheromone (TERP) consisting of an antibacterial protein lysozyme (17) and a digestive β-glucosidase enzyme (20). These two compounds can only be detected when workers directly touch the egg surface. Our preliminary experiments showed that workers aggregated around egg piles confined in a stainless steel mesh cage, suggesting that workers are attracted to eggs by volatile cues. To determine whether the egg volatiles nBnB and 2M1B are used by workers for egg location, we conducted bioassays using dummy eggs made of glass beads (17, 20). Dummy eggs coated with TERP together with nBnB and 2M1B were carried into egg piles at a significantly higher rate than dummy eggs without nBnB and 2M1B (Fig. 4A). Without TERP, dummy eggs were not recognized as eggs, even when coated with nBnB and 2M1B, indicating that egg volatiles act as an attractant but not as a recognition pheromone (Fig. 4A).
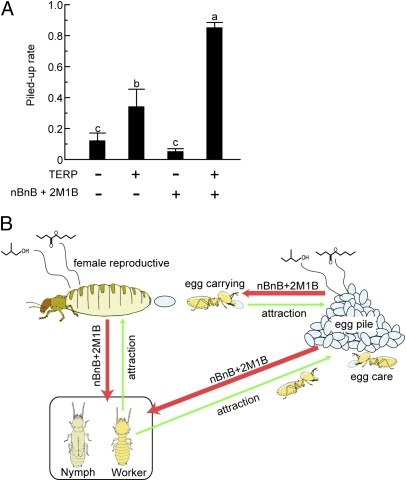
Fig. 4.
Role of egg volatiles as an attractant pheromone. (A) Addition of nBnB and 2M1B to TERP (consisting of lysozyme and β-glucosidase) significantly increased the rate at which dummy eggs were piled up. Dummy eggs coated only with nBnB and 2M1B were not carried into egg piles. Different letters indicate significant differences among treatments (P < 0.05, Tukey's HSD). (B) Proposed functions of nBnB and 2M1B from female reproductives and eggs. These volatiles serve as an attractant to locate eggs and female reproductive and also act to inhibit the differentiation of female neotenics from nymphs and workers. Values denote the mean ± SEM of 15 replications (five replications × three colonies).
The finding that the same chemicals behave as a female reproductive inhibitory pheromone and an egg attractant has important evolutionary implications. It has long been controversial whether queens chemically manipulate workers by queen pheromones or provide honest signals of their reproductive state that are used by other colony member to adaptively change their development, physiology, and behavior (3, 21–23). The most logical pathway for the dual function of the present pheromone system in R. speratus is that it was first produced as an attractant by eggs. Later, colony members possibly used the amount of this attractant as a means to assess the reproductive output of female reproductives and decide whether to produce new female neotenics. The female reproductives would then have been selected to produce the pheromone themselves to further inhibit the differentiation of new female reproductives. The pheromone would thus have evolved from a cue providing information on the fecundity of female reproductives to a signal produced by functional reproductives themselves that inhibited the differentiation of new female reproductives. In our experiments there was no significant difference in the inhibitory power of female reproductives in colonies where eggs were removed or not removed (P > 0.05, Tukey's HSD test; Fig. 1 B and C). However, our laboratory colonies were considerably smaller (ca. 100 individuals) than colonies in the field (up to 500,000 individuals) (24), and it remains to be investigated whether there are additive inhibitory effects of the female- and egg-produced pheromones, which may provide a mechanism of honest signaling of reproductive status.
Although the possible existence of volatile queen pheromones has previously been suggested for both lower termites (6, 7) and higher termites (25), no volatiles have been identified as termite queen pheromones to date. This is most likely because the amount of volatiles emitted from individual queens is too small to be analyzed. We were able to take advantage of the fact that in R. speratus primary queens are relatively short-lived and are replaced early in the colony life cycle by many female neotenics produced parthenogenetically (13). Thus, colonies of this species contain many fully developed female neotenics, providing sufficient quantities of volatiles for successful chemical identification. Primary queens most likely use the same volatiles to prevent the differentiation of female neotenics, but future studies are needed to test this hypothesis. Identification of potential king pheromones is another important topic for future research.
The inhibitory pheromone consists of two small, aromatic compounds, containing five and eight carbons each with molecular weights of 88.15 and 144.21 g/mol, respectively. Two recent studies reported reproductive-specific cuticular hydrocarbons (C29–C33) (9) and proteinaceous compounds (molecular weights of 7,000–24,000) (10) in termites, although the functions of these chemicals are unknown. Our results suggest that investigation of volatiles produced by female reproductives and their eggs, and potentially kings, could be fruitful in identifying additional pheromones regulating caste differentiation in termites and possibly other social insects.
The termite female reproductive pheromone seems to act directly on colony members, influencing their developmental trajectory. If so, this would be a different mode of action than the honey bee queen substance that acts indirectly on developing larvae by affecting the behavior of workers who care for the brood (8). Our identification of a termite female reproductive pheromone suppressing differentiation of new female neotenics opens up exciting new possibilities to investigate how exocrine signals interact with the endocrine system to regulate caste differentiation in social insects. Recent studies have begun to shed light on the molecular basis for division of labor and caste determination in termites (26–29). Characterization of the pheromone receptors and their downstream targets, as well as the elucidation of pheromone biosynthesis, should provide important insights into how reproductive and nonreproductive developmental pathways are regulated in social insect colonies.
Materials and Methods
Inhibition Assay with Female Neotenics.
Termite colonies of R. speratus were collected from pine forests in Okayama, western Japan, from June to September 2009. We used different colonies for each experiment, that is, 15 field-collected colonies for bioassays and 2 field-collected colonies for chemical analysis. After finding young larvae and eggs, which indicate the presence of royal chambers nearby, we very carefully cut nest wood into ca. 30-cm segments using a chainsaw and then brought the pieces of wood into the laboratory. We extracted all reproductives from royal chambers and as many eggs as possible using an aspirator. To examine the inhibitory effect of fertile female neotenics on the differentiation of new female neotenics, we maintained workers and nymphs either with or without fully developed female neotenics collected from natural colonies. We placed 100 workers and 10 fourth-instar nymphs (five males and five females) together with a mature female neotenic in a 35-mm Petri dish lined with a moist unwoven cloth made of 100% pulp. Only workers and nymphs were placed in a Petri dish in the control treatment. To examine differentiation of new female neotenics from workers, we used groups with only 100 workers. The groups were held at 25 °C and checked daily for the presence newly differentiated female neotenics. Any neotenics produced were removed to prevent them from inhibiting the differentiation of additional neotenics. We compared the total number of neotenics produced over 3 wk. We made five replications for each treatment and repeated the experiment using two colonies (experiments 1 and 2). To ascertain the influence of queen volatiles on the differentiation of new female neotenics, we maintained workers and nymphs with a fertile female neotenic confined in a double stainless steel mesh cage, which prevented direct contact between the female neotenic and the test termites. Five fully developed female neotenics were confined in a double mesh cage (40-mm diameter × 10-mm height) together with 20 workers to care for the reproductive. The cage was located at the center of a 90-mm Petri dish lined with a moist unwoven cloth. We placed 100 workers and 10 fourth-instar nymphs (five males and five females) in the Petri dish and maintained them for 3 wk. Only 20 workers were confined in a cage in the control treatment. We made five replications for each treatment and repeated the experiment using two colonies.
Inhibition Assay with Eggs.
To investigate the inhibitory effect of eggs on the differentiation of new female neotenics, we maintained workers with different quantities of eggs. We placed groups of 100 workers in 35-mm Petri dishes lined with moist unwoven cloth and then assigned them to one of the following treatments: (i) 20 eggs were added to the Petri dish every day (20 eggs per day), (ii) 1,000 eggs were initially placed in the Petri dish, and then 100 eggs were added every day (1,000 + 100 eggs per day), or (iii) no eggs added (control treatment). Because eggs have a fragile chorion we handled them carefully by using a sterilized nylon paint brush moistened with distilled water. One month later, the number of newly differentiated female neotenics was compared among treatments. We made five replications for each treatment and repeated the experiment using two colonies (experiments 1 and 2).
Headspace GS-MS
We used the two largest of the field colonies collected in Okayama from May to July 2009 (colony code: TK090624A and GI090730B) for chemical analysis. One of these colonies had 208 female neotenics (all of them were nymphoid) and one king. The other had 103 female neotenics and one king. All of the female neotenics were physogastric with greatly distended abdomens, characterized by dorsal plates that were separated by massive enlargement of the ovaries. We placed 700 mg (ca. 100 individuals) of the female neotenics in a 22-mL glass vial (22-CV; Chromacol). The vial was sealed with aluminum crimp caps fitted with a silicon septum (20-AC-ST3; Chromacol) and was kept at 25 °C for 30 min. Headspace sampling of volatiles (2 mL) was then collected using a syringe (GASTIGHT #1005; Hamilton). GC-MS analyses of headspace-collected volatiles were performed on a JEOL JMS-700 mass spectrometer combined with a GC instrument (JEOL) equipped with a ZB-1 column (60 m × 0.32 mm i.d., 3-μm film thickness; Phenomenex). Column temperature was raised from 40 °C (5-min hold) at 10 °C/min to 250 °C. The injection was splitless, with helium as carrier gas (1.5 mL/min). MS data were obtained under the following conditions: ionization current, 100 μA; ionization energy, 70 eV; accelerating voltage, 6 kV; scan range, 10–300 m/z. The gas chromatographic system and high-resolution mass spectrometer were both controlled using JMS-700 M Station software. Candidate compounds were identified from the National Institute of Standards and Technology/Environmental Protection Agency/National Institutes of Health (NIST08) Mass Spectral Library and were purchased from Sigma-Aldrich and Wako Pure Chemical Industries. Compounds were identified according to retention time and mass spectra in comparison with synthetic standards. Headspace GC-MS analysis was conducted for 500 mg eggs (ca. 10,000 eggs), 700 mg nymphs, and 700 mg workers for each of the two colonies.
Inhibition Assay with Artificial Pheromone.
The identified compounds of female neotenics and egg volatiles were tested for inhibition of new female neotenics by an assay using a unique protocol. The experimental device consisted of an outer (140-mm diameter) and an inner (35-mm diameter) Petri dish. We placed an unglazed ceramic ball (6-mm diameter) at the center of the outer Petri dish as a pheromone source (Fig. 4A). We placed 100 workers in the inner Petri dish lined with a moist unwoven cloth and made a small opening (2-mm diameter) on the lid. We added 5.0 μL of n-butyl-n-butyrate (Sigma-Aldrich) and 2.5 μL of 2-methyl-1-butanol (Wako Pure Chemical Industries) onto the unglazed ceramic ball every 24 h. The compounds absorbed by the unglazed ceramic ball volatilize once in the outer Petri dish and then enter the inner Petri dish through the small opening on the lid. We prepared two types of negative controls: (i) 5.0 μL/d of distilled water, or (ii) same dosage of n-butyl acetate as a control ester was applied to the unglazed ceramic ball. Comparison of the number of newly differentiated female neotenics was done as described above. Five replications were made for each treatment, and the experiment was repeated by using two colonies.
Egg Attraction Bioassay.
To examine the attractivity of egg-produced chemicals, we used dummy eggs made of glass beads coated with each test chemical as described previously (17, 20). Dummy eggs were made of 0.5-mm diameter glass beads and were coated with each test chemical. Each of the TERP compounds, lysozyme (#L7651; Sigma-Aldrich) and β-glucosidase (#G0395; Sigma-Aldrich), was dialyzed with a dialysis tube (MW3500; BioDesign) against distilled water and lyophilized before being resuspended in 50% glycerol ddH2O with 0.025% Tween 20 to a final concentration of 0.05 mg/μL. Then 2.0 μL of the solution was added to 100 glass beads. We added 0.1 μL of nBnB and 2M1B to the 100 glass beads and vortexed vigorously for 30 s to coat the glass beads evenly with the chemicals. Control beads were coated with TERP alone, egg volatile compounds alone, or with only 50% glycerol. The egg-recognition bioassay was replicated five times for each of the three colonies. Piling rates were arcsine-square-root-transformed and analyzed by two-way ANOVA followed by Tukey's HSD test.
Supplementary Material
Acknowledgments
We thank H. Nakano and T. Yashiro for research assistance; and C. Schal, M. Chapuisat, and K. Tsuji for helpful advice. This work was supported by the Japan Society for the Promotion of Science (K.M.) and the Program for Promotion of Basic Research Activities for Innovative Biosciences (K.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004675107/-/DCSupplemental.
References
- 1.Wilson EO. The Insect Societies. Cambridge, MA: Harvard Univ Press; 1971. [Google Scholar]
- 2.Sherman PW, Lacey EA, Reeve HK, Keller L. The eusociality continuum. Behav Ecol. 1995;6:102–108. [Google Scholar]
- 3.Keller L, Nonacs P. The role of queen pheromones in social insects: Queen control or queen signal? Anim Behav. 1993;45:787–794. [Google Scholar]
- 4.Karlson P, Lüscher M. Pheromones: A new term for a class of biologically active substances. Nature. 1959;183:55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- 5.Light SF. Experimental studies on ectohormonal control of the development of supplementary reproductives in the termite genus Zootermopsis (formerly Termopsis) Univ Calif Publ Zool. 1944;43:413–454. [Google Scholar]
- 6.Lüscher M. Transactions of the Ninth International Congress of Entomology, Amsterdam, 1951. 1952. New evidence for an ectohormonal control of caste determination in termites; pp. 289–294. [Google Scholar]
- 7.Lüscher M. Social control of polymorphism in termites. In: Kennedy JS, editor. Insect Polymorphism. London: Royal Entomological Society of London; 1961. pp. 57–67. [Google Scholar]
- 8.Butler CG. Extraction and purification of ‘queen substance’ from queen bees. Nature. 1959;184:1871. [Google Scholar]
- 9.Liebig J, Eliyahu D, Brent CS. Cuticular hydrocarbon profiles indicate reproductive status in the termite Zootermopsis nevadensis. Behav Ecol Sociobiol. 2009;63:1799–1807. [Google Scholar]
- 10.Hanus R, Vrkoslav V, Hrdý I, Cvačka J, Šobotník J. Beyond cuticular hydrocarbons: Evidence of proteinaceous secretion specific to termite kings and queens. Proc Biol Sci. 2009;277:995–1002. doi: 10.1098/rspb.2009.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorne BL, Traniello JFA, Adams ES, Bulmer M. Reproductive dynamics and colony structure of subterranean termites of the genus Reticulitermes (Isoptera Rhinotermitidae): A review of the evidence from behavioral, ecological, and genetic studies. Ethol Ecol Evol. 1999;11:149–169. [Google Scholar]
- 12.Vargo EL, Husseneder C. Biology of subterranean termites: Insights from molecular studies of Reticulitermes and Coptotermes. Annu Rev Entomol. 2009;54:379–403. doi: 10.1146/annurev.ento.54.110807.090443. [DOI] [PubMed] [Google Scholar]
- 13.Matsuura K, et al. Queen succession through asexual reproduction in termites. Science. 2009;323:1687. doi: 10.1126/science.1169702. [DOI] [PubMed] [Google Scholar]
- 14.Karlson P, Butenandt A. Pheromones (ectohormones) in insects. Annu Rev Entomol. 1959;4:39–58. [Google Scholar]
- 15.Pickens AL. Intraspecific problems in the taxonomy of insect caste. Insectes Soc. 1954;1:71–74. [Google Scholar]
- 16.Endler A, et al. Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc Natl Acad Sci USA. 2004;101:2945–2950. doi: 10.1073/pnas.0308447101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuura K, Tamura T, Kobayashi N, Yashiro T, Tatsumi S. The antibacterial protein lysozyme identified as the termite egg recognition pheromone. PLoS ONE. 2007;2:e813. doi: 10.1371/journal.pone.0000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuura K, Tanaka C, Nishida T. Symbiosis of a termite and a sclerotium-forming fungus: Sclerotia mimic termite eggs. Ecol Res. 2000;15:405–414. [Google Scholar]
- 19.Matsuura K. Termite-egg mimicry by a sclerotium-forming fungus. Proc Biol Sci. 2006;273:1203–1209. doi: 10.1098/rspb.2005.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuura K, Yashiro T, Shimizu K, Tatsumi S, Tamura T. Cuckoo fungus mimics termite eggs by producing the cellulose-digesting enzyme beta-glucosidase. Curr Biol. 2009;19:30–36. doi: 10.1016/j.cub.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 21.West Eberhard MJ. In: Natural Selection and Social Behaviour. Alexander RD, Tinkle DW, editors. New York: Chiron Press; 1988. pp. 3–17. [Google Scholar]
- 22.Ratnieks FLW. Reproductive harmony via mutual policing by workers in eusocial Hymenoptera. Am Nat. 1988;132:217–236. [Google Scholar]
- 23.Vargo EL, Passera L. Pheromonal and behavioral queen control over the production of gynes in the Argentine ant, Iridomyrmex humilis (Mayr) Behav Ecol Sociobiol. 1991;28:161–169. [Google Scholar]
- 24.Tsunoda K, Matsuoka H, Yoshimura T, Tokoro M. Foraging populations and territories of Reticulitermes speratus (Isoptera: Rhinotermitidae) J Econ Entomol. 1999;92:604–609. [Google Scholar]
- 25.Camazine S, et al. Self-Organization in Biological Systems. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 26.Miura T, et al. Soldier caste-specific gene expression in the mandibular glands of Hodotermopsis japonica (Isoptera: termopsidae) Proc Natl Acad Sci USA. 1999;96:13874–13879. doi: 10.1073/pnas.96.24.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Oi FM, Scharf ME. Social exploitation of hexamerin: RNAi reveals a major caste-regulatory factor in termites. Proc Natl Acad Sci USA. 2006;103:4499–4504. doi: 10.1073/pnas.0508866103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korb J, Weil T, Hoffmann K, Foster KR, Rehli M. A gene necessary for reproductive suppression in termites. Science. 2009;324:758. doi: 10.1126/science.1170660. [DOI] [PubMed] [Google Scholar]
- 29.Weil T, Korb J, Rehli M. Comparison of queen-specific gene expression in related lower termite species. Mol Biol Evol. 2009;26:1841–1850. doi: 10.1093/molbev/msp095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.