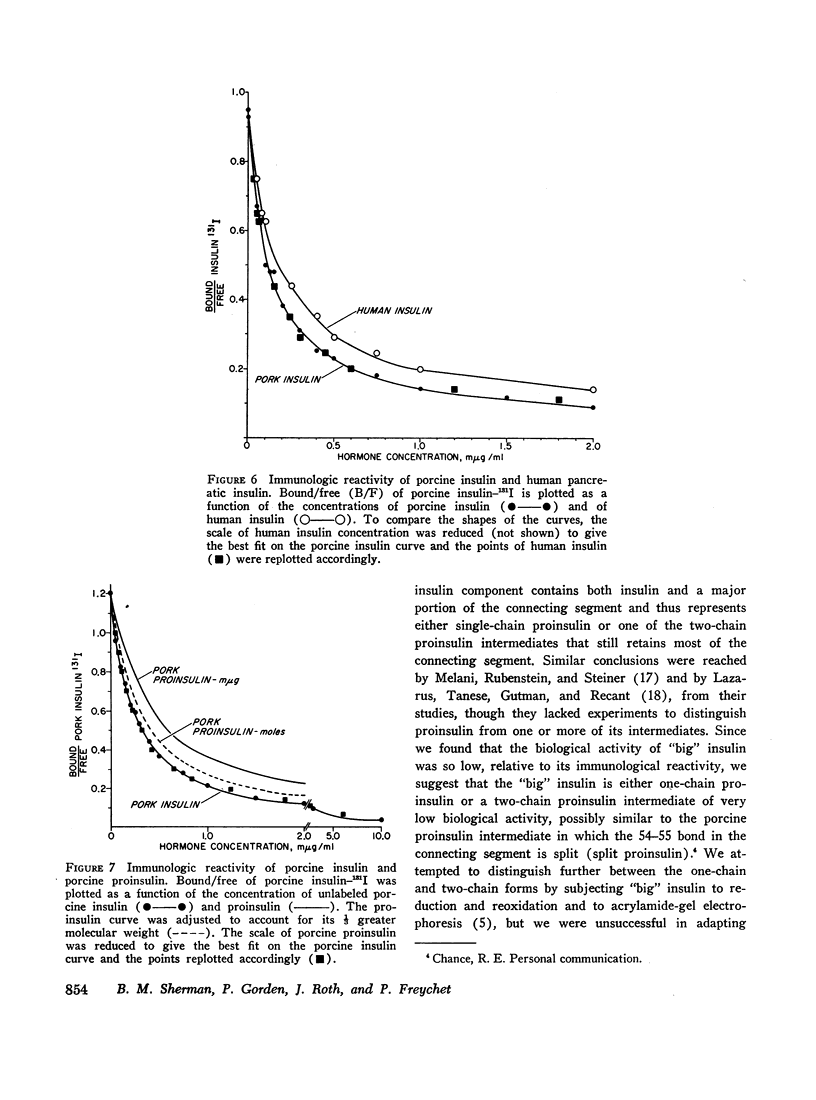
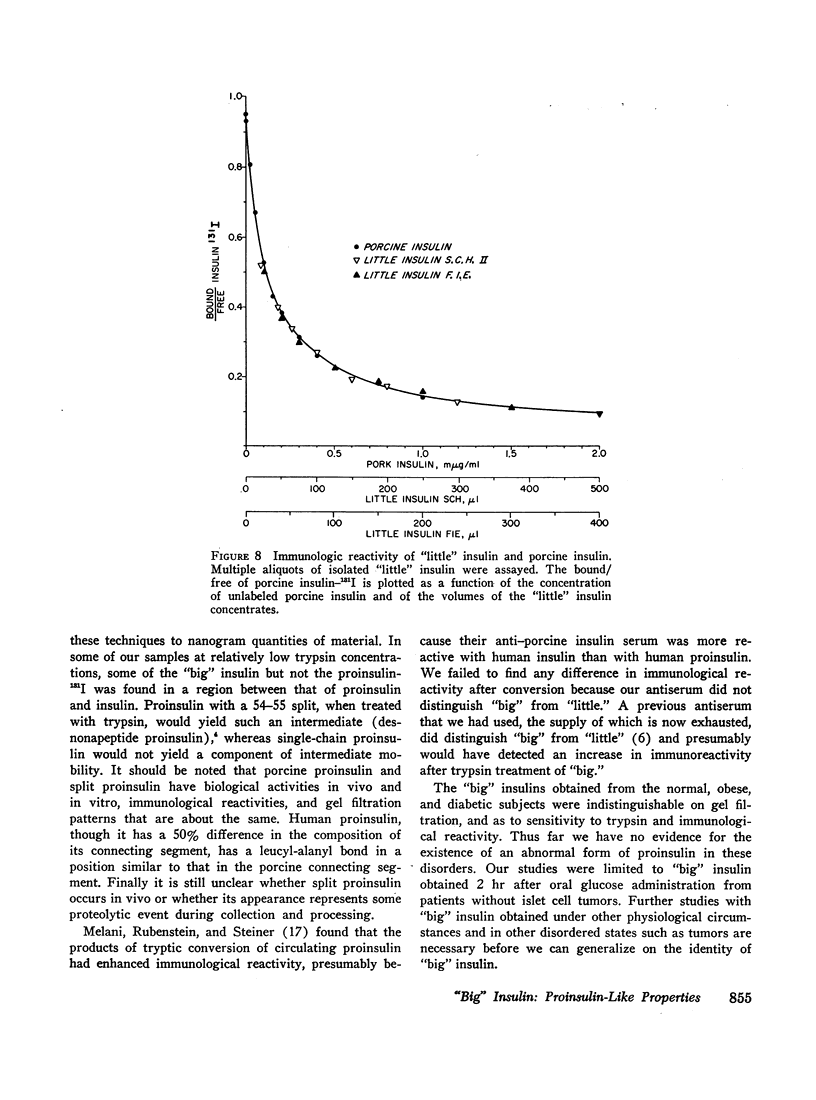
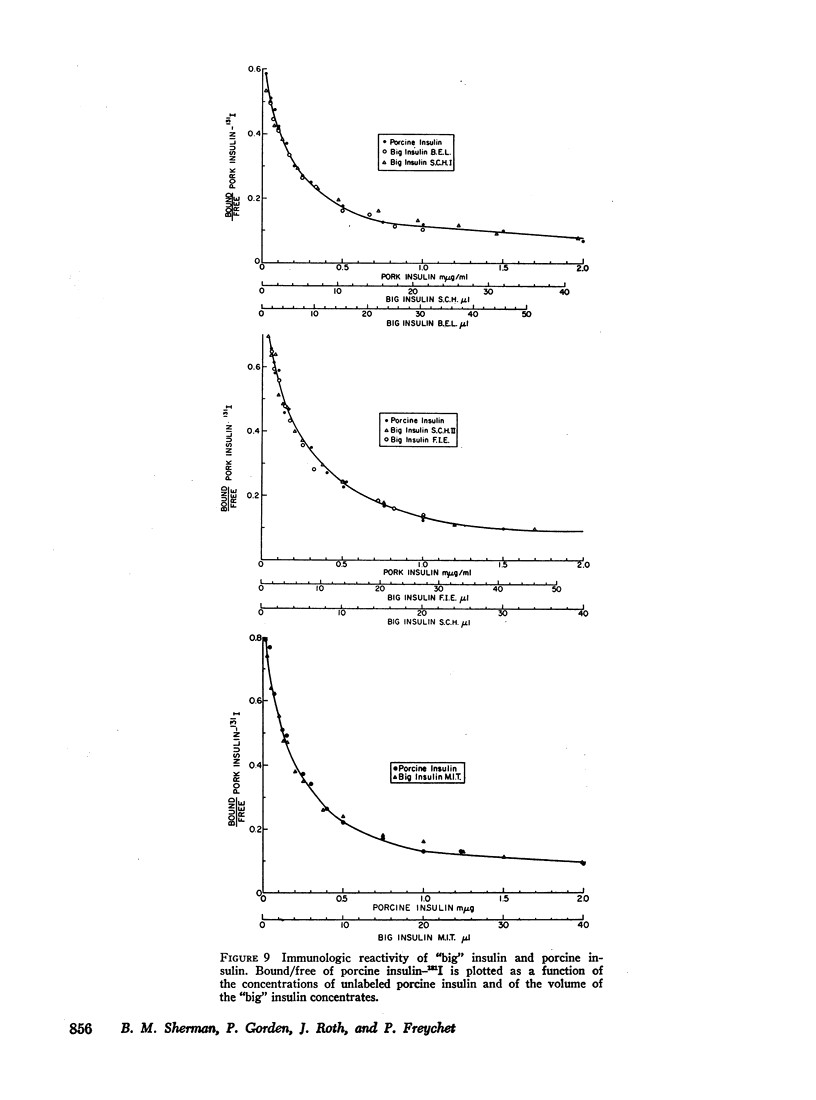
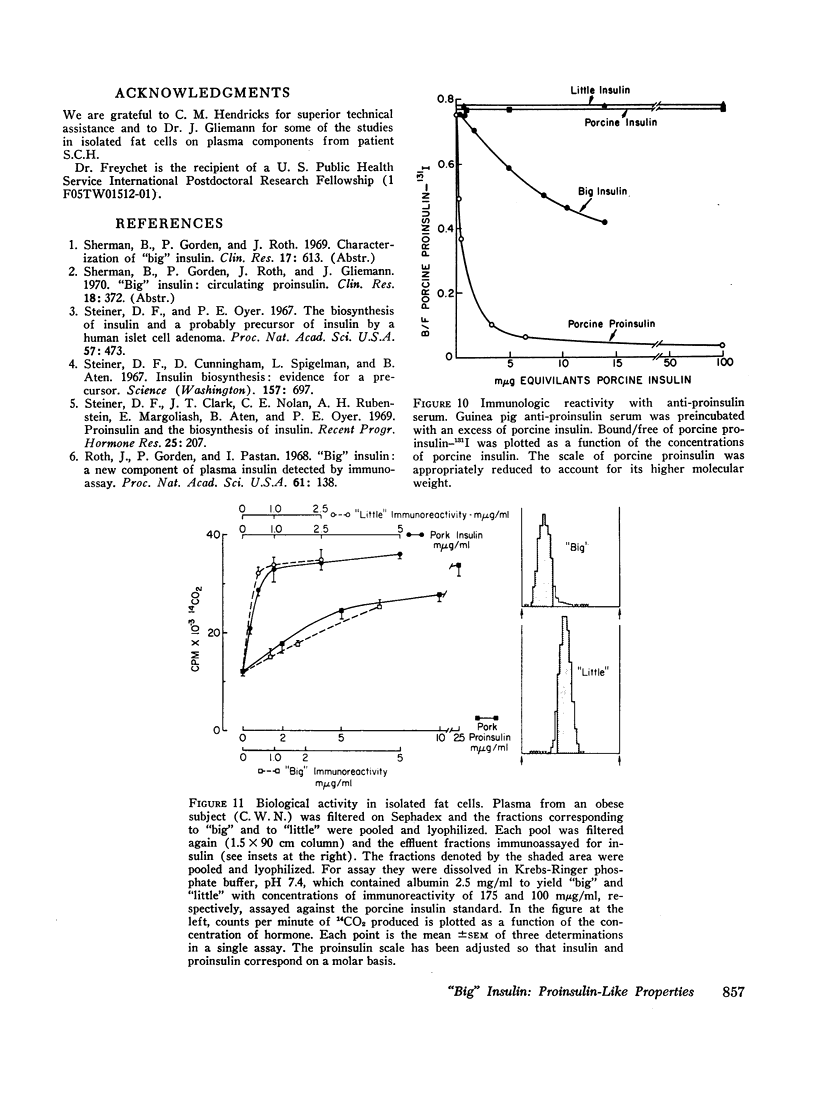
Abstract
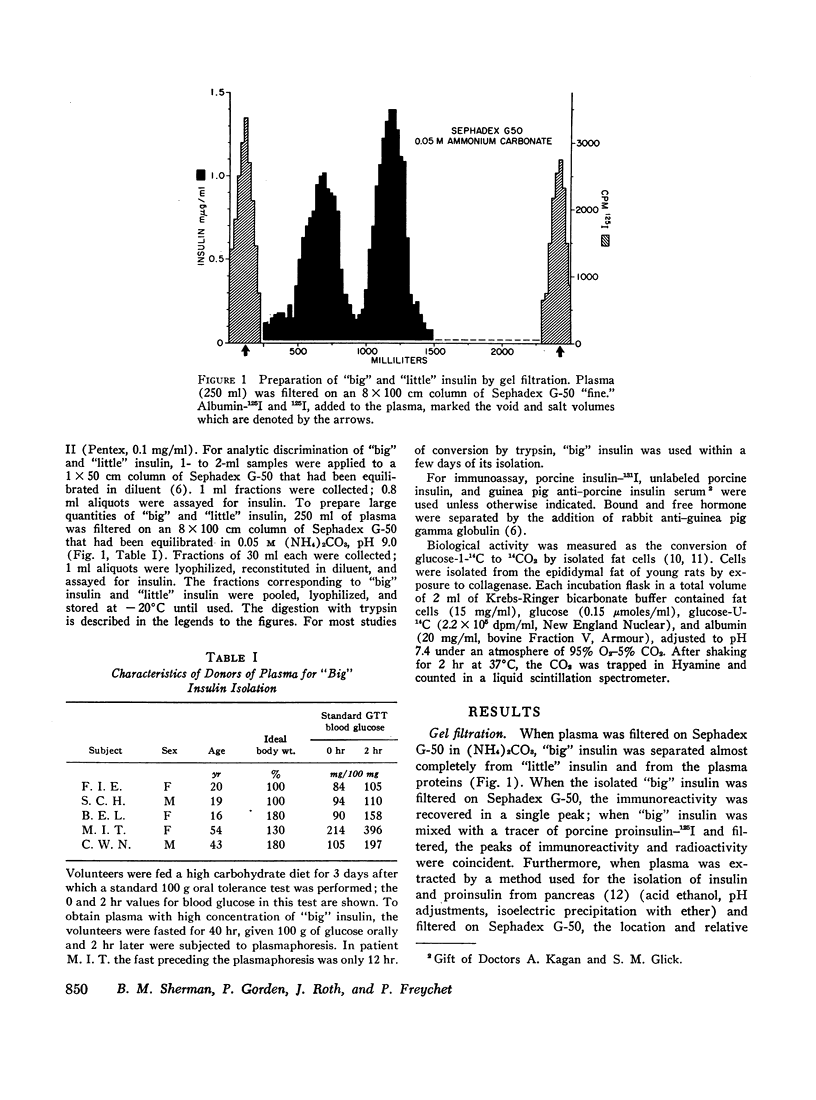
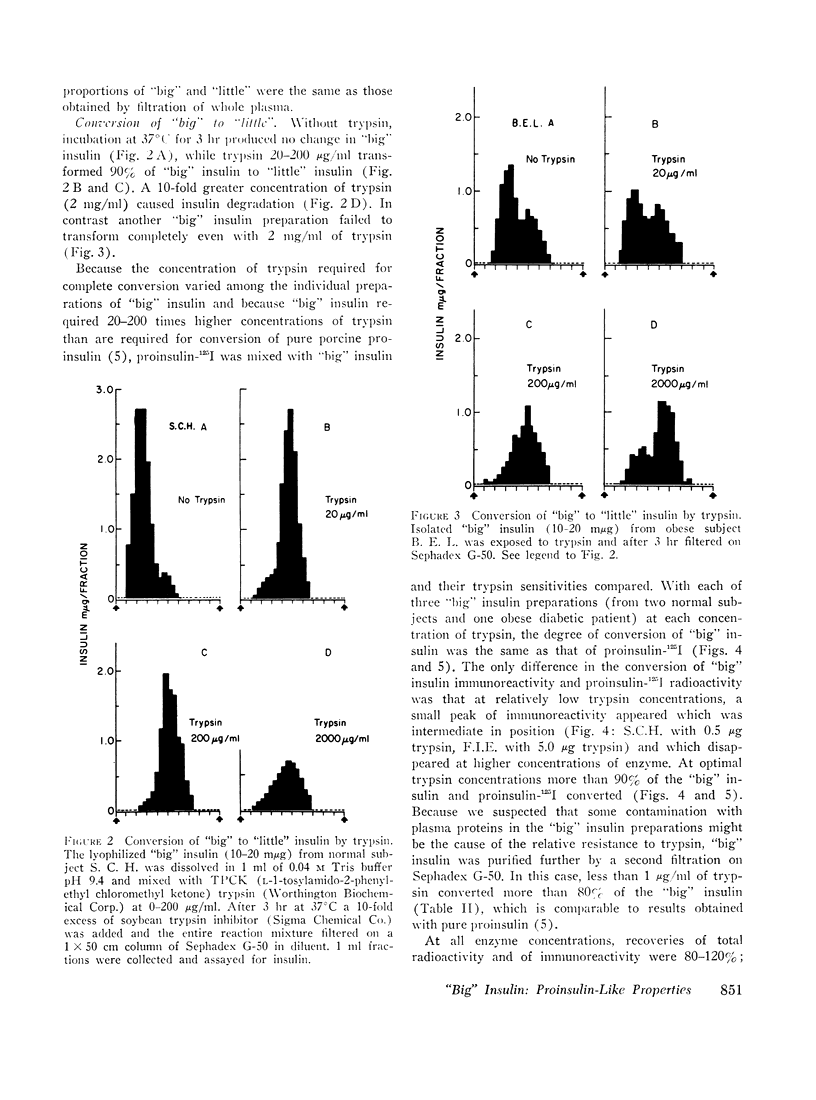
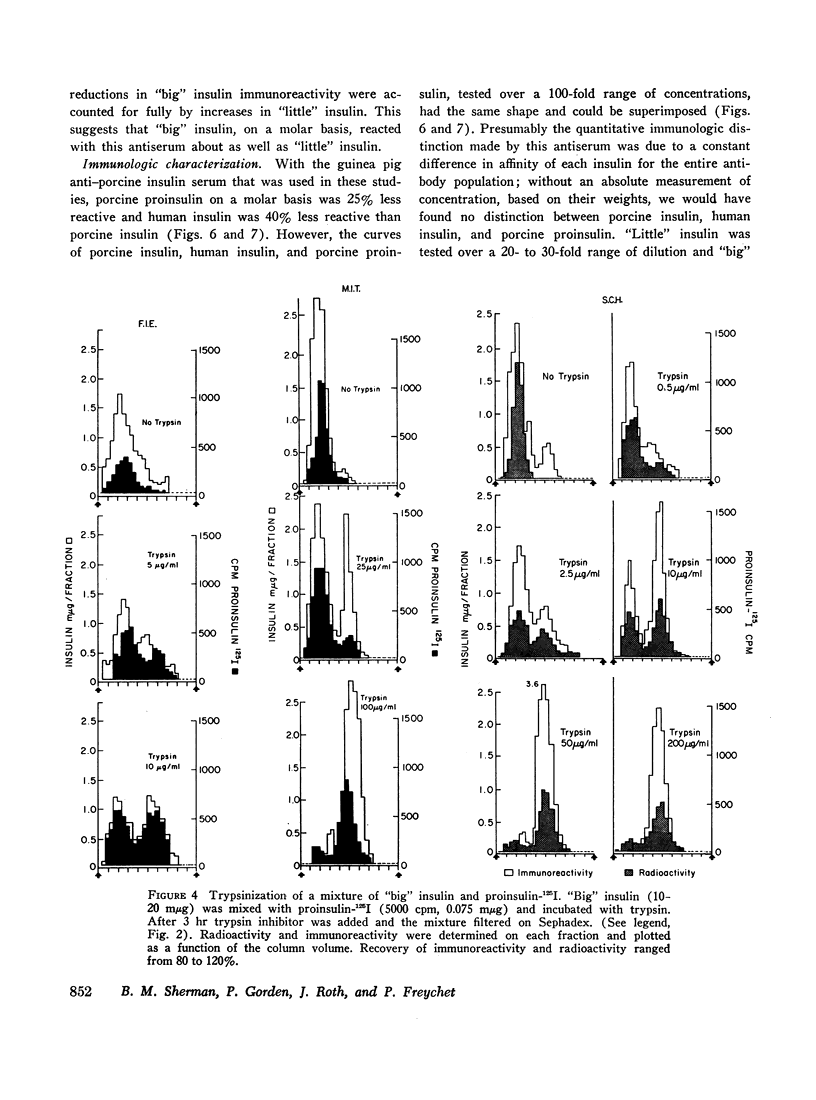
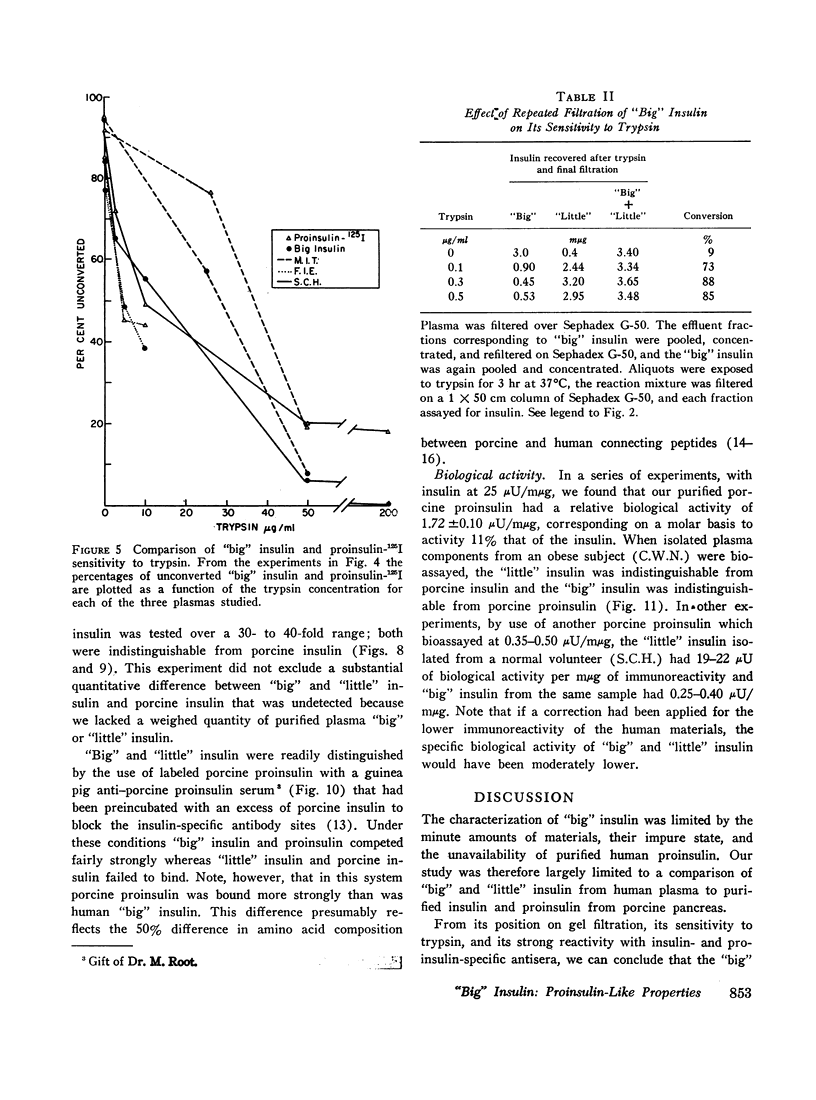
When plasma is filtered on Sephadex G-50, insulin immunoreactivity is recovered in two peaks. “Big” insulin, the higher molecular weight component, and “little” insulin, the lower molecular weight component, have elution volumes that correspond to those of proinsulin-125I and insulin-125I respectively. When plasma was extracted with acid ethanol and filtered in 1.0 M acetic acid, the patterns and proportions of “big” and “little” insulin were indistinguishable from those obtained by filtration of whole plasma in neutral buffer. When “big” insulin was isolated from plasma and mixed with a tracer of porcine proinsulin-125I, trypsin converted the “big” insulin immunoreactivity to the gel filtration pattern of “little” insulin in the same way that it converted the proinsulin radioactivity. More than 90% of both “big” insulin and proinsulin were converted at optimal trypsin concentrations. Our present guinea pig anti-insulin serum failed to distinguish “big” from “little” but a porcine proinsulin anti-serum, under appropriate conditions of assay, reacted strongly with “big” insulin but not at all with “little.” When tested on isolated fat cells, “little” insulin had the same bioactivity as porcine insulin, whereas “big” insulin had the same low activity as porcine proinsulin. These studies suggest that “big” insulin represents either single-chain proinsulin and/or a proinsulin intermediate that has similar low bioactivity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chance R. E., Ellis R. M., Bromer W. W. Porcine proinsulin: characterization and amino acid sequence. Science. 1968 Jul 12;161(3837):165–167. doi: 10.1126/science.161.3837.165. [DOI] [PubMed] [Google Scholar]
- DAVOREN P. R. The isolation of insulin from a single cat pancreas. Biochim Biophys Acta. 1962 Sep 10;63:150–153. doi: 10.1016/0006-3002(62)90347-5. [DOI] [PubMed] [Google Scholar]
- Gliemann J. Assay of insulin-like activity by the isolated fat cell method. I. Factors influencing the response to crystalline insulin. Diabetologia. 1967 Aug;3(4):382–388. doi: 10.1007/BF02342631. [DOI] [PubMed] [Google Scholar]
- Gorden P., Roth J. Circulating insulins. "Big" and "little". Arch Intern Med. 1969 Mar;123(3):237–247. [PubMed] [Google Scholar]
- Gorden P., Roth J. Plasma insulin: fluctuations in the "big" insulin component in man after glucose and other stimuli. J Clin Invest. 1969 Dec;48(12):2225–2234. doi: 10.1172/JCI106188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus N. R., Tanese T., Gutman R., Recant L. Synthesis and release of proinsulin and insulin by human insulinoma tissue. J Clin Endocrinol Metab. 1970 Mar;30(3):273–281. doi: 10.1210/jcem-30-3-273. [DOI] [PubMed] [Google Scholar]
- Melani F., Rubenstein A. H., Steiner D. F. Human serum proinsulin. J Clin Invest. 1970 Mar;49(3):497–507. doi: 10.1172/JCI106259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Roth J., Gorden P., Pastan I. "Big insulin": a new component of plasma insulin detected by immunoassay. Proc Natl Acad Sci U S A. 1968 Sep;61(1):138–145. doi: 10.1073/pnas.61.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L., Nolan C., Rubenstein A. H., Margoliash E., Aten B., Oyer P. E. Proinsulin and the biosynthesis of insulin. Recent Prog Horm Res. 1969;25:207–282. doi: 10.1016/b978-0-12-571125-8.50008-9. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Cunningham D., Spigelman L., Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967 Aug 11;157(3789):697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip C. C., Logothetopoulos J. A specific anti-proinsulin serum and the presence of proinsulin in calf serum. Proc Natl Acad Sci U S A. 1969 Feb;62(2):415–419. doi: 10.1073/pnas.62.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]



