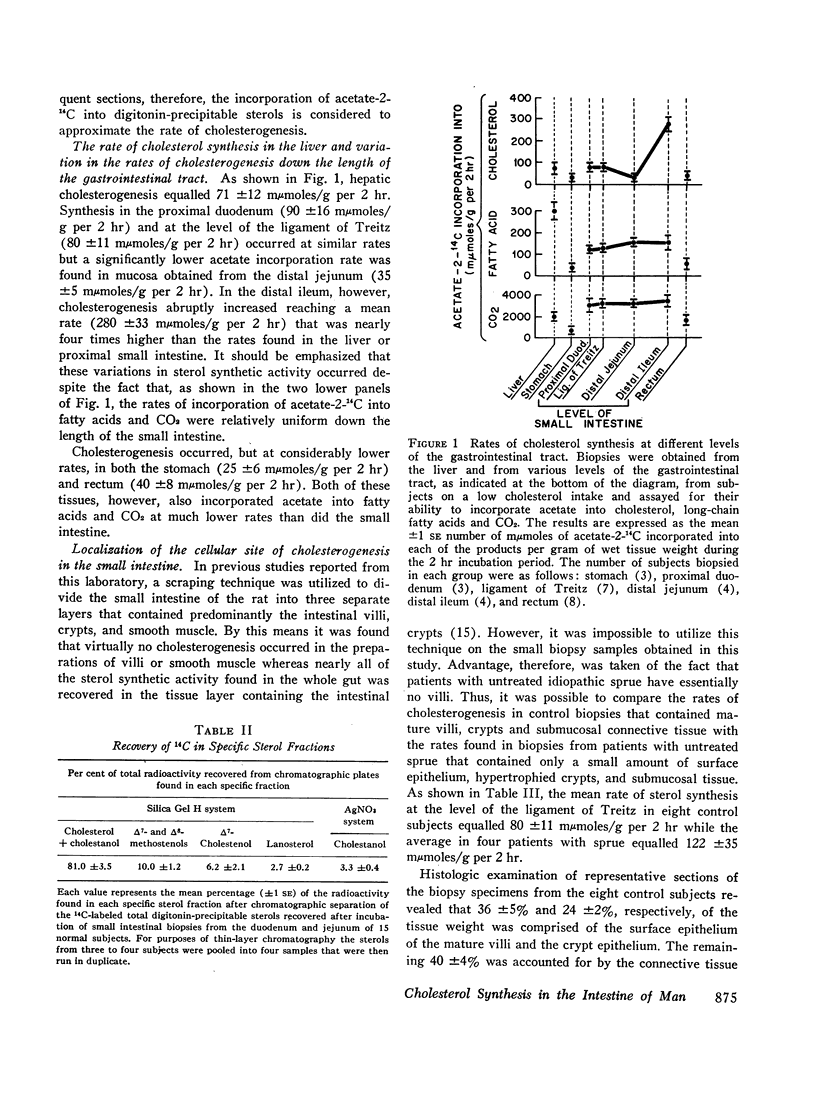
Abstract
Cholesterol in the circulating serum pool is derived either from absorption of dietary cholesterol or from endogenous synthesis principally in the liver and gastrointestinal tract. While the control of intestinal cholesterogenesis has been elucidated in several lower animal species, no data currently are available in the case of man. In the present study using tissue specimens obtained by suction biopsy in 29 normal subjects, we have shown the rate of cholesterogenesis is low in the stomach (25 ±6 mμmoles/g per 2 hr) and rectum (40 ±8 mμmoles/g per 2 hr); in the small bowel the rate progressively decreases in the proximal duodenum (90 ±16 mμmoles/g per 2 hr); distal duodenum (80 ±11 mμmoles/g per 2 hr); and distal jejunum (35 ±5 mμmoles/g per 2 hr); but abruptly increases in the distal ileum (280 ±33 mμmoles/g per 2 hr). Indirect evidence is provided that the intestinal crypt epithelium is the main site of this sterol synthesis. Fasting for 48 hr suppressed the rate of cholesterogenesis in the distal duodenum from a control value of 80 ±11 mμmoles/g per 2 hr to 40 ±8 mμmoles/g per 2 hr while cholesterol feeding for 7 days did not alter the rate of cholesterol synthesis (75 ±12 mμmoles/g per 2 hr). This resistance to cholesterol feeding also was present in the distal ileum where control and cholesterol-fed subjects had comparable rates of cholesterogenesis (280 and 261 mμmoles/g per 2 hr, respectively). Interruption of the enterohepatic circulation, in contrast, resulted in greatly enhanced sterol synthesis with a mean rate of 259 ±29 mμmoles/g per 2 hr being found in the duodenum of four patients with biliary obstruction as compared with the rate of 80 ±11 mμmoles/g per 2 hr in control subjects. These studies indicate that the mechanisms of control of cholesterol synthesis by the human intestine are similar to those described for the intestine of lower animals; this also appears to be true for the human liver. Thus, the marked differences in over-all cholesterol metabolism between various lower mammalian species and man cannot be explained by fundamental differences in control mechanisms; rather, these differences must reflect variations in some other parameter of cholesterol metabolism.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BHATTATHIRY E. P., SHIPERSTEIN M. D. FEEDBACK CONTROL OF CHOLESTEROL SYNTHESIS IN MAN. J Clin Invest. 1963 Oct;42:1613–1618. doi: 10.1172/JCI104846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCHER N. L., McGARRAHAN K., GOULD E., LOUD A. V. Cholesterol biosynthesis in preparations of liver from normal, fasting, x-irradiated, cholesterol-fed, triton, or delta 4-cholesten-3-one-treated rats. J Biol Chem. 1959 Feb;234(2):262–267. [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Cholesterol synthesis by the gastrointestinal tract: localization and mechanisms of control. J Clin Invest. 1965 Aug;44(8):1311–1327. doi: 10.1172/JCI105237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Effect of cholesterol feeding and fasting on sterol synthesis in seventeen tissues of the rat. J Lipid Res. 1967 Mar;8(2):97–104. [PubMed] [Google Scholar]
- Dietschy J. M. The role of bile salts in controlling the rate of intestinal cholesterogenesis. J Clin Invest. 1968 Feb;47(2):286–300. doi: 10.1172/JCI105725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Cholesterol synthesis in the squirrel monkey: relative rates of synthesis in various tissues and mechanisms of control. J Clin Invest. 1968 Jan;47(1):166–174. doi: 10.1172/JCI105706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDRICKSON D. S., LOUD A. V., HINKELMAN B. T., SCHNEIDER H. S., FRANTZ I. D., Jr The effect of ligation of the common bile duct on cholesterol synthesis in the rat. J Exp Med. 1954 Jan 1;99(1):43–53. doi: 10.1084/jem.99.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN M., BYERS S. O., MICHAELIS F. Production and excretion of cholesterol in mammals. Iv. Role of liver in restoration of plasma cholesterol after experimentally induced hypocholesteremia. Am J Physiol. 1951 Mar;164(3):789–791. doi: 10.1152/ajplegacy.1951.164.3.789. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Hirono H., Arakawa T. Idiopathic hypercholesterolemia: demonstration of an impaired feedback control of cholesterol synthesis in vivo. Tohoku J Exp Med. 1965 Nov 25;87(2):155–167. doi: 10.1620/tjem.87.155. [DOI] [PubMed] [Google Scholar]
- GOULD R. G. Lipid metabolism and atherosclerosis. Am J Med. 1951 Aug;11(2):209–227. doi: 10.1016/0002-9343(51)90107-6. [DOI] [PubMed] [Google Scholar]
- GOULD R. G., TAYLOR C. B., HAGERMAN J. S., WARNER I., CAMPBELL D. J. Cholesterol metabolism. I. Effect of dietary cholesterol on the synthesis of cholesterol in dog tissue in vitro. J Biol Chem. 1953 Apr;201(2):519–528. [PubMed] [Google Scholar]
- HOTTA S., CHAIKOFF I. L. The role of the liver in the turnover of plasma cholesterol. Arch Biochem Biophys. 1955 May;56(1):28–37. doi: 10.1016/0003-9861(55)90330-1. [DOI] [PubMed] [Google Scholar]
- LINDSEY C. A., Jr, WILSON J. D. EVIDENCE FOR A CONTRIBUTION BY THE INTESTINAL WALL TO THE SERUM CHOLESTEROL OF THE RAT. J Lipid Res. 1965 Apr;6:173–181. [PubMed] [Google Scholar]
- MORRIS M. D., CHAIKOFF I. L., FELTS J. M., ABRAHAM S., FANSAH N. O. The origin of serum cholesterol in the rat; diet versus synthesis. J Biol Chem. 1957 Feb;224(2):1039–1045. [PubMed] [Google Scholar]
- SIPERSTEIN M. D., GUEST M. J. Studies on the site of the feedback control of cholesterol synthesis. J Clin Invest. 1960 Apr;39:642–652. doi: 10.1172/JCI104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siperstein M. D., Fagan V. M. Feedback control of mevalonate synthesis by dietary cholesterol. J Biol Chem. 1966 Feb 10;241(3):602–609. [PubMed] [Google Scholar]
- TAYLOR C. B., PATTON D., YOGI N., COX G. E. Diet as source of serum cholesterol in man. Proc Soc Exp Biol Med. 1960 Apr;103:768–772. doi: 10.3181/00379727-103-25664. [DOI] [PubMed] [Google Scholar]
- TOMKINS G. M., CHAIKOFF I. L. Cholesterol synthesis by liver. I. Influence of fasting and of diet. J Biol Chem. 1952 May;196(2):569–573. [PubMed] [Google Scholar]
- Weis H. J., Dietschy J. M. Failure of bile acids to control hepatic cholesterogenesis: evidence for endogenous cholesterol feedback. J Clin Invest. 1969 Dec;48(12):2398–2408. doi: 10.1172/JCI106206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D. Biosynthetic origin of serum cholesterol in the squirrel monkey: evidence for a contribution by the intestinal wall. J Clin Invest. 1968 Jan;47(1):175–187. doi: 10.1172/JCI105707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Lindsey C. A., Jr Studies on the influence of dietary cholesterol on cholesterol metabolism in the isotopic steady state in man. J Clin Invest. 1965 Nov;44(11):1805–1814. doi: 10.1172/JCI105288. [DOI] [PMC free article] [PubMed] [Google Scholar]


