Abstract
The nature and magnitude of fluid and electrolyte loss into the small intestine were defined by the marker perfusion technique in patients with acute undifferentiated diarrhea (AUD) in the tropics. The patients were divided into two groups according to their small bowel bacteriologic findings, namely those with a predominant Escherichia coli flora and those with a mixed flora. 11 normal subjects served as controls. Net jejunal fluid secretion occurred into the lumen in four of seven patients with E. coli flora and three of seven with a mixed flora. The magnitude of secretion in the jejunum was greater in the E. coli flora patients than in those with a mixed flora. Four E. coli patients and one mixed flora patient had net fluid secretion in the ileum, although the magnitude of secretion in this area was less than in the jejunum. Intestinal fluid had higher bicarbonate concentration in the ileum than in the jejunum but was isotonic in both regions. It resembled in composition fluid from the same region of intestine in normal individuals. Recovery of normal fluid and electrolyte absorptive function was usually complete in both jejunum and ileum by 6-8 days after onset of the disease. Increase in unidirectional flux rates for H3O and 24Na occurred in acute E. coli flora diarrhea and returned to normal levels in recovery: increase in Jβ (plasma to lumen flux) primarily accounted for the increase in fluid loss. Intestinal biopsy revealed no alterations in villous architecture.
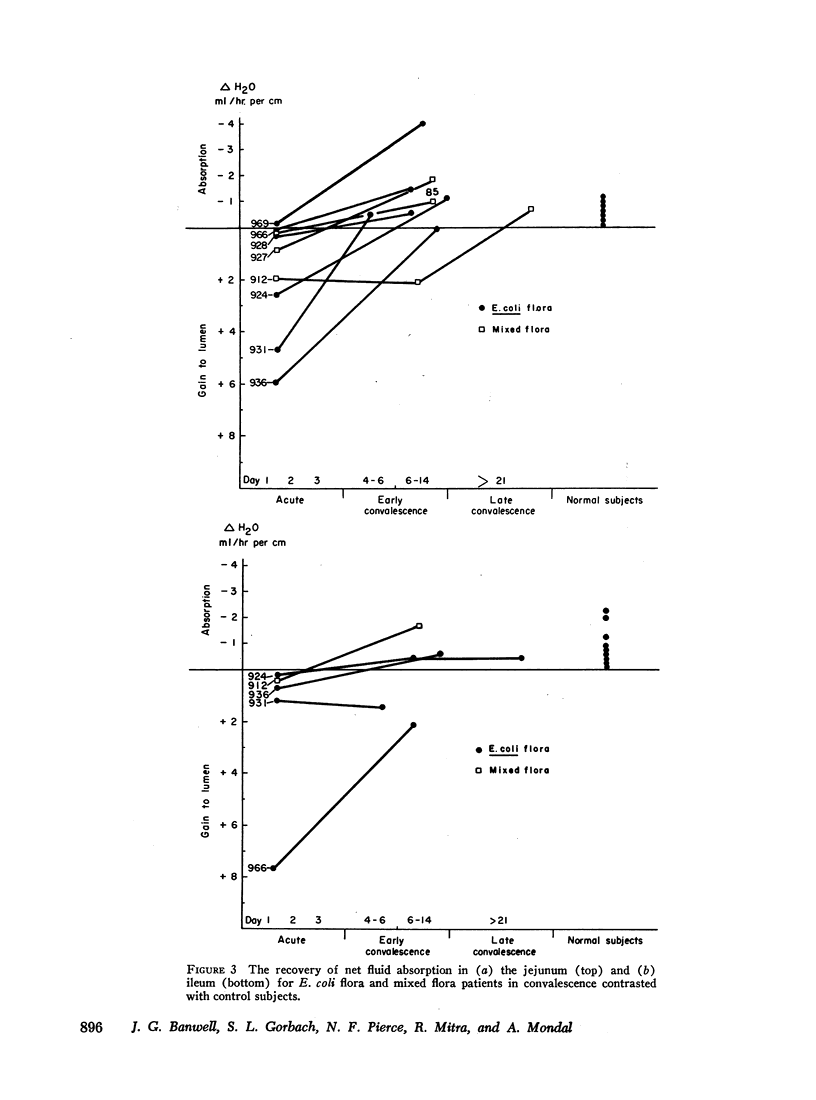
A relationship between small bowel fluid production and the presence of toxigenic strains of E. coli within the small bowel has been found for E. coli flora patients. In many respects this disease resembles acute cholera. The mixed flora group represents a less defined entity which requires further study.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banwell J. G., Pierce N. F., Mitra R. C., Brigham K. L., Caranasos G. J., Keimowitz R. I., Fedson D. S., Thomas J., Gorbach S. L., Sack R. B. Intestinal fluid and electrolyte transport in human cholera. J Clin Invest. 1970 Jan;49(1):183–195. doi: 10.1172/JCI106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banwell J. G., Pierce N. F., Mitra R., Caranasos G. J., Keimowitz R. I., Mondal A., Manji P. M. Preliminary results of a study of small intestinal water and solute movement in acute and convalescent human cholera. Indian J Med Res. 1968 May;56(5):633–639. [PubMed] [Google Scholar]
- Bayless T. M., Christopher N. L. Disaccharidase deficiency. Am J Clin Nutr. 1969 Feb;22(2):181–190. doi: 10.1093/ajcn/22.2.181. [DOI] [PubMed] [Google Scholar]
- CURRAN P. F., SOLOMON A. K. Ion and water fluxes in the ileum of rats. J Gen Physiol. 1957 Sep 20;41(1):143–168. doi: 10.1085/jgp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. C., Barua D., Wallace C. K., Sack R. B., Mitra P. P., Werner A. S., Duffy T. P., Oleinick A., Khanra S. R., Lewis G. W. Clinical and physiological observations during an epidemic outbreak of non-vibrio cholera-like disease in Calcutta. Bull World Health Organ. 1965;33(5):665–671. [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. C., Sack R. B., Feeley J. C., Steenberg R. W. Site and characteristics of electrolyte loss and effect of intraluminal glucose in experimental canine cholera. J Clin Invest. 1968 May;47(5):1210–1220. doi: 10.1172/JCI105810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H., Levitan R., Fordtran J. S., Ingelfinger F. J. A method for studying absorption of water and solute from the human small intestine. Gastroenterology. 1966 Jan;50(1):1–7. [PubMed] [Google Scholar]
- FORDTRAN J. S., LEVITAN R., BIKERMAN V., BURROWS B. A., INGELFINGER F. J. The kinetics of water absorption in the human intestine. Trans Assoc Am Physicians. 1961;74:195–206. [PubMed] [Google Scholar]
- Fordtran J. S. Marker perfusion techniques for measuring intestinal absorption in man. Gastroenterology. 1966 Dec;51(6):1089–1093. [PubMed] [Google Scholar]
- Gorbach S. L., Banwell J. G., Chatterjee B. D., Jacobs B., Sack R. B. Acute undifferentiated human diarrhea in the tropics. I. Alterations in intestinal micrflora. J Clin Invest. 1971 Apr;50(4):881–889. doi: 10.1172/JCI106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyles C. L., Barnum D. A. A heat-labile enterotoxin from strains of Eschericha coli enteropathogenic for pigs. J Infect Dis. 1969 Oct;120(4):419–426. doi: 10.1093/infdis/120.4.419. [DOI] [PubMed] [Google Scholar]
- Hakim A. A., Lifson N. Effects of pressure on water and solute transport by dog intestinal mucosa in vitro. Am J Physiol. 1969 Feb;216(2):276–284. doi: 10.1152/ajplegacy.1969.216.2.276. [DOI] [PubMed] [Google Scholar]
- Hirschhorn N., Molla A., Molla A. M. Reversible jejunal disaccharidase deficiency in cholera and other acute diarrheal diseases. Johns Hopkins Med J. 1969 Dec;125(6):291–300. [PubMed] [Google Scholar]
- Iber F. L., McGonagle T., Serebro H. A., Luebbers E., Bayless T. M., Hendrix T. R. Unidirectional sodium flux in small intestine in experimental canine cholera. Am J Med Sci. 1969 Nov;258(5):340–350. doi: 10.1097/00000441-196911000-00005. [DOI] [PubMed] [Google Scholar]
- KOYA G., KOSAKAI N., FUKASAWA Y. Supplementary studies on the multiplication of Escherichia coli O-111 B4 in the intestinal tract of adult volunteers and its relation to manifestation of coli enteritis. Jpn J Med Sci Biol. 1954 Dec;7(6):655–661. doi: 10.7883/yoken1952.7.655. [DOI] [PubMed] [Google Scholar]
- Kent T. H., Lindenbaum J. Correlation of jejunal function and morphology in patients with acute and chronic diarrhea in East Pakistan. Gastroenterology. 1967 Jun;52(6):972–984. [PubMed] [Google Scholar]
- LEVITAN R., FORDTRAN J. S., BURROWS B. A., INGELFINGER F. J. Water and salt absorption in the human colon. J Clin Invest. 1962 Sep;41:1754–1759. doi: 10.1172/JCI104634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDENBAUM J., GREENOUGH W. B., 3rd, BENENSON A. S., OSEASOHN R., RIZVI S., SAAD A. NON-VIBRIO CHOLERA. Lancet. 1965 May 22;1(7395):1081–1083. doi: 10.1016/s0140-6736(65)92671-1. [DOI] [PubMed] [Google Scholar]
- LINDENBAUM J. MALABSORPTION DURING AND AFTER RECOVERY FROM ACUTE INTESTINAL INFECTION. Br Med J. 1965 Aug 7;2(5457):326–329. doi: 10.1136/bmj.2.5457.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love A. H. Water and sodium absorption by the intestine in cholera. Gut. 1969 Jan;10(1):63–67. doi: 10.1136/gut.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Banwell J. G., Pierce N. F., Mondal A. Studies of intestinal absorption using a marker perfusion technique in cholera and acute tropical gastroenteritis. J Assoc Physicians India. 1968 Jun;16(6):339–342. [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Mitra R. C., Banwell J. G., Brigham K. L., Fedson D. S., Mondal A. Replacement of water and electrolyte losses in cholera by an oral glucose-electrolyte solution. Ann Intern Med. 1969 Jun;70(6):1173–1181. doi: 10.7326/0003-4819-70-6-1173. [DOI] [PubMed] [Google Scholar]
- Sachar D. B., Taylor J. O., Saha J. R., Phillips R. A. Intestinal transmural electric potential and its response to glucose in acute and convalescent cholera. Gastroenterology. 1969 Mar;56(3):512–521. [PubMed] [Google Scholar]
- Soergel K. H., Whalen G. E., Harris J. A. Passive movement of water and sodium across the human small intestinal mucosa. J Appl Physiol. 1968 Jan;24(1):40–48. doi: 10.1152/jappl.1968.24.1.40. [DOI] [PubMed] [Google Scholar]
- TAYLOR J., WILKINS M. P., PAYNE J. M. Relation of rabbit gut reaction to enteropathogenic Escherichia coli. Br J Exp Pathol. 1961 Feb;42:43–52. [PMC free article] [PubMed] [Google Scholar]
- THOMSON S. The role of certain varieties of Bacterium coli in gastro-enteritis of babies. J Hyg (Lond) 1955 Sep;53(3):357–367. doi: 10.1017/s002217240000084x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. B. William Appleton Cuthbert 1912-1966. J Appl Bacteriol. 1966 Dec;29(3 Suppl):–2. [PubMed] [Google Scholar]
- Torres-Pinedo R., Rivera C. L., Fernández S. Studies on infant diarrhea. II. Absorption of glucose and net fluxes of water and sodium chloride in a segment of the jejunum. J Clin Invest. 1966 Dec;45(12):1916–1922. doi: 10.1172/JCI105496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATTEN R. H., MORGAN F. M., YACHAI NA SONGKHLA, VANIKIATI B., PHILLIPS R. A. Water and electrolyte studies in cholera. J Clin Invest. 1959 Nov;38:1879–1889. doi: 10.1172/JCI103965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen G. E., Harris J. A., Geenen J. E., Soergel K. H. Sodium and water absorption from the human small intestine. The accuracy of the perfusion method. Gastroenterology. 1966 Dec;51(6):975–984. [PubMed] [Google Scholar]


