Abstract
The utilization of circulating maltose was compared to that of glucose in six normal fasting subjects after intravenous injection of 25 g of either sugar. Blood samples were obtained over a 2 hr period and were assayed for free fatty acids (FFA), insulin, glucose, and total reducing substances. Urine was collected for 2 hr after maltose administration and assayed enzymatically for glucose and maltose. Blood glucose concentrations did not increase after maltose infusion, although a significant rise in total reducing substances was noted, indicating the presence of this disaccharide in the blood. Less than 3% of the administered maltose was excreted in the urine either as maltose or glucose. Initially, there was a fourfold increase in serum insulin concentration after glucose and a threefold increase after maltose infusion. Therefore, serum insulin concentrations gradually declined in a similar manner for both sugars. The plasma FFA at 15 min decreased 371 uEq/liter after glucose and 338 uEq/liter after maltose infusion.
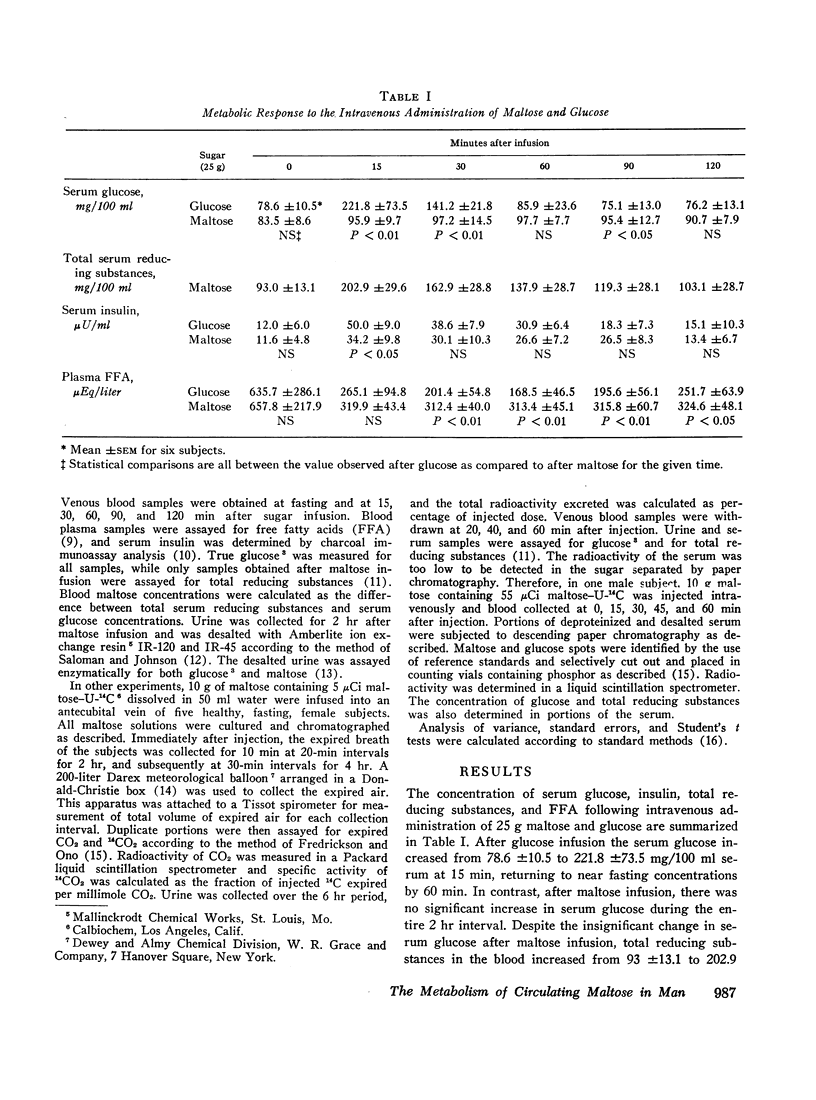
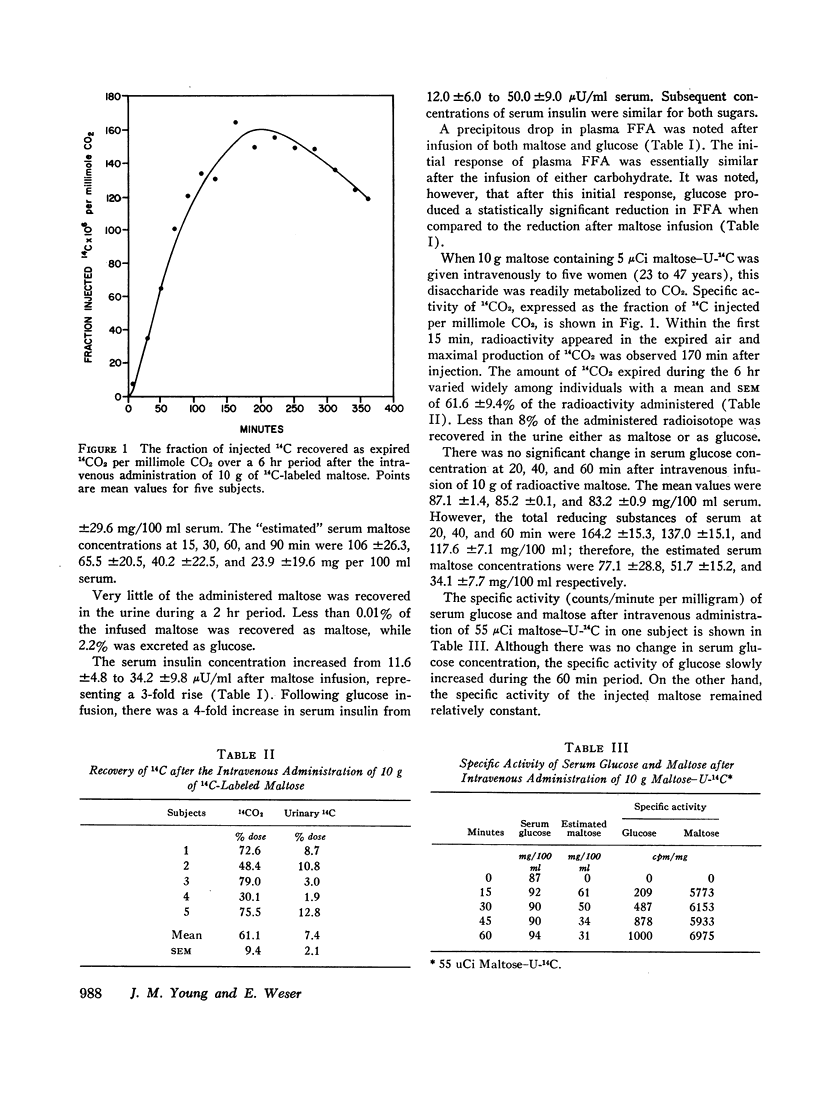
In other studies, 10 g maltose containing 5 μCi maltose-U-14C were injected into five human subjects and expired CO2 collected for 6 hr. Maximal 14CO2 specific activity was noted at 170 min and a mean of 61.1% of the injected radioactivity was recovered as 14CO2. Less than 8% of the injected 14C was excreted in the urine.
These results indicate that maltose administered intravenously has similar metabolic effects when compared to glucose, and may be efficiently utilized as a carbohydrate substrate. The oxidation of intravenously administered maltose-U14C to 14CO2 demonstrates that circulating maltose is readily metabolized. A solution of maltose could provide twice the mass of sugar (and of calories) per milliliter as an equimolar solution of glucose. Parenterally administered maltose may be of clinical value and should be further studied.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER N., SHREEVE W. W., SHIPLEY R. A., INCEFY G. E., MILLER M. C14 studies in carbohydrate metabolism. I. The oxidation of glucose in normal human subjects. J Biol Chem. 1954 Dec;211(2):575–592. [PubMed] [Google Scholar]
- Bressler R., Cordon M. V., Brendel K. Studies on the role of adenylcyclase in insulin secretion. Arch Intern Med. 1969 Mar;123(3):248–251. [PubMed] [Google Scholar]
- Coleman G. S. The metabolism of starch, maltose, glucose and some other sugars by the rumen ciliate Entodinium caudatum. J Gen Microbiol. 1969 Aug;57(3):303–332. doi: 10.1099/00221287-57-3-303. [DOI] [PubMed] [Google Scholar]
- Curry D. L. Factors affecting insulin secretion in vitro. Am J Clin Nutr. 1970 Mar;23(3):305–310. doi: 10.1093/ajcn/23.3.305. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A., THOMSON D. L. THE DIGESTION AND ABSORPTION OF LACTOSE BY THE INTACT RAT. Acta Physiol Scand. 1964 May-Jun;61:20–33. doi: 10.1111/j.1748-1716.1964.tb02939.x. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A., THOMSON D. L. THE DIGESTION AND ABSORPTION OF MALTOSE AND TREHALOSE BY THE INTACT RAT. Acta Physiol Scand. 1963 Sep-Oct;59:111–125. doi: 10.1111/j.1748-1716.1963.tb02728.x. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A., THOMSON D. L. The digestion and absorption of sucrose by the intact rat. J Physiol. 1963 Jul;167:193–209. doi: 10.1113/jphysiol.1963.sp007141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEANE N., SCHREINER G. E., ROBERTSON J. S. The velocity of distribution of sucrose between plasma and interstitial fluid, with reference to the use of sucrose for the measurement or extracellular fluid in man. J Clin Invest. 1951 Dec;30(122):1463–1468. doi: 10.1172/JCI102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEANE N., SMITH H. W. Fate of inulin and sucrose in normal subjects as determined by a urine reinfusion technique. J Clin Invest. 1955 May;34(5):681–684. doi: 10.1172/JCI103118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P. The significance of nonesterified fatty acids in plasma. AMA Arch Intern Med. 1958 May;101(5):1005–1008. doi: 10.1001/archinte.1958.00260170161017. [DOI] [PubMed] [Google Scholar]
- ELRICK H., STIMMLER L., HLAD C. J., Jr, ARAI Y. PLASMA INSULIN RESPONSE TO ORAL AND INTRAVENOUS GLUCOSE ADMINISTRATION. J Clin Endocrinol Metab. 1964 Oct;24:1076–1082. doi: 10.1210/jcem-24-10-1076. [DOI] [PubMed] [Google Scholar]
- FITTING C., SCHERP H. W. Observations on a strain of Neisseria meningitides in the presence of glucose and maltose. II. Studies with washed cells1. J Bacteriol. 1952 Apr;63(4):545–560. doi: 10.1128/jb.63.4.545-560.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITTING C., SCHERP H. W. Observations on a strain of Neisseria meningitidis in the presence of glucose and maltose. III. Cell-free extracts and the phosphorolysis of maltose. J Bacteriol. 1952 Sep;64(3):287–298. doi: 10.1128/jb.64.3.287-298.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITTING C., SCHERP H. W. Observations on a strain of Neisseria meningitidis in the presence of glucose and maltose. J Bacteriol. 1951 Feb;61(2):203–214. doi: 10.1128/jb.61.2.203-214.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDRICKSON D. S., GORDON R. S., Jr The metabolism of albumin-bound C14-labeled unesterified fatty acids in normal human subjects. J Clin Invest. 1958 Nov;37(11):1504–1515. doi: 10.1172/JCI103742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDRICKSON D. S., GORDON R. S., Jr Transport of fatty acids. Physiol Rev. 1958 Oct;38(4):585–630. doi: 10.1152/physrev.1958.38.4.585. [DOI] [PubMed] [Google Scholar]
- FREDRICKSON D. S., ONO K. An improved technique for assay of C14O2 in expired air using the liquid scintillation counter. J Lab Clin Med. 1958 Jan;51(1):147–151. [PubMed] [Google Scholar]
- GORDON E. S., GOLDBERG M. STUDIES OF ENERGY METABOLISM IN HUMAN SUBJECTS USING CARBON 14-LABELED COMPOUNDS. I. EFFECT OF SEX, STATE OF NUTRITION AND BODY WEIGHT. Metabolism. 1964 Sep;13:775–790. doi: 10.1016/0026-0495(64)90044-7. [DOI] [PubMed] [Google Scholar]
- GORDON R. S., Jr, CHERKES A. Unesterified fatty acid in human blood plasma. J Clin Invest. 1956 Feb;35(2):206–212. doi: 10.1172/JCI103265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS G., THOMPSON C. C. The uptake of nutrients by yeasts. III. The maltose permease of a brewing yeast. Biochim Biophys Acta. 1961 Sep 2;52:176–183. doi: 10.1016/0006-3002(61)90915-5. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- JACOBS G., REICHARD G., GOODMAN E. H., Jr, FRIEDMANN B., WEINHOUSE S. Action of insulin and tolbutamide on blood glucose entry and removal. Diabetes. 1958 Sep-Oct;7(5):358–364. doi: 10.2337/diab.7.5.358. [DOI] [PubMed] [Google Scholar]
- KALANT N., CSORBA T. R., HELLER N. EFFECT OF INSULIN ON GLUCOSE PRODUCTION AND UTILIZATION IN DIABETES. Metabolism. 1963 Dec;12:1100–1111. [PubMed] [Google Scholar]
- Karam J. H., Grasso S. G., Wegienka L. C., Grodsky G. M., Forsham P. H. Effect of selected hexoses, of epinephrine and of glucagon on insulin secretion in man. Diabetes. 1966 Aug;15(8):571–578. doi: 10.2337/diab.15.8.571. [DOI] [PubMed] [Google Scholar]
- LAURELL S. Turnover rate of unesterified fatty acids in human plasma. Acta Physiol Scand. 1957 Dec 7;41(2-3):158–167. doi: 10.1111/j.1748-1716.1957.tb01517.x. [DOI] [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., Wright P. H. A new method for the measurement in vitro of pancreatic insulin secretion. Endocrinology. 1967 Jan;80(1):99–108. doi: 10.1210/endo-80-1-99. [DOI] [PubMed] [Google Scholar]
- McIntyre N., Holdsworth C. D., Turner D. S. Intestinal factors in the control of insulin secretion. J Clin Endocrinol Metab. 1965 Oct;25(10):1317–1324. doi: 10.1210/jcem-25-10-1317. [DOI] [PubMed] [Google Scholar]
- Perley M., Kipnis D. M. Plasma insulin responses to glucose and tolbutamide of normal weight and obese diabetic and nondiabetic subjects. Diabetes. 1966 Dec;15(12):867–874. doi: 10.2337/diab.15.12.867. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J. THE INTERRELATIONSHIPS OF HORMONES, FATTY ACID AND GLUCOSE IN THE PROVISION OF ENERGY. Postgrad Med J. 1964 Aug;40:457–463. doi: 10.1136/pgmj.40.466.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICHARD G. A., JACOBS A. G., KIMBEL P., HOCHELLA N. J., WEINHOUSE S. Effects of insulin on blood glucose entry and removal rates in man. Diabetes. 1960 Nov-Dec;9:447–453. doi: 10.2337/diab.9.6.447. [DOI] [PubMed] [Google Scholar]
- Rhoads J. E., Steiger E., Dudrick S. J., Long J. M. Intravenous hyperalimentation. Med Clin North Am. 1970 May;54(3):577–589. [PubMed] [Google Scholar]
- Ruderman N. B., Toews C. J., Shafrir E. Role of free fatty acids in glucose homeostasis. Arch Intern Med. 1969 Mar;123(3):299–313. [PubMed] [Google Scholar]
- SEGAL S., BERMAN M., BLAIR A. The metabolism of variously C14-labeled glucose in man and an estimation of the extent of glucose metabolism by the hexose monophosphate pathway. J Clin Invest. 1961 Jul;40:1263–1279. doi: 10.1172/JCI104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELTZER H. S., SMITH W. L. Plasma insulin activity after glucose: an index of insulogenic reserve in normal and diabetic man. Diabetes. 1959 Nov-Dec;8:417–424. doi: 10.2337/diab.8.6.417. [DOI] [PubMed] [Google Scholar]
- Seltzer H. S., Allen E. W., Herron A. L., Jr, Brennan M. T. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967 Mar;46(3):323–335. doi: 10.1172/JCI105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROUT D. L., ESTES E. H., Jr, FRIEDBERG S. J. Titration of free fatty acids of plasma: a study of current methods and a new modification. J Lipid Res. 1960 Apr;1:199–202. [PubMed] [Google Scholar]
- Van Handel E. Trehalase and maltase in the serum of vertebrates. Comp Biochem Physiol. 1968 Aug;26(2):561–566. doi: 10.1016/0010-406x(68)90649-x. [DOI] [PubMed] [Google Scholar]
- WIESMEYER H., COHN M. The characterization of the pathway of maltose utilization by Escherichia coli. III. Adescription of the concentrating mechanism. Biochim Biophys Acta. 1960 Apr 22;39:440–447. doi: 10.1016/0006-3002(60)90196-7. [DOI] [PubMed] [Google Scholar]
- Weser E., Sleisenger M. H. Metabolism of circulating disaccharides in man and the rat. J Clin Invest. 1967 Apr;46(4):499–505. doi: 10.1172/JCI105552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. J., McDonald I. J. Permeability of a micrococcal cell to maltose and some related sugars. Can J Microbiol. 1966 Dec;12(6):1213–1223. doi: 10.1139/m66-163. [DOI] [PubMed] [Google Scholar]
- Young D. R., Pelligra R., Shapira J., Adachi R. R., Skrettingland K. Glucose oxidation and replacement during prolonged exercise in man. J Appl Physiol. 1967 Nov;23(5):734–741. doi: 10.1152/jappl.1967.23.5.734. [DOI] [PubMed] [Google Scholar]
- Young J. M., Weser E. Effect of insulin on the metabolism of circulating maltose. Endocrinology. 1970 Feb;86(2):426–429. doi: 10.1210/endo-86-2-426. [DOI] [PubMed] [Google Scholar]


