Abstract
Previous work has suggested that intracellular proteolysis may play a role in lymphocyte stimulation. An inhibitor of proteolysis, epsilon amino caproic acid (EACA) was studied for its effect on the lymphocyte response to phytohemagglutinin (PHA). EACA was found to inhibit several parameters of lymphocyte stimulation (e.g. DNA, RNA, and protein synthesis as well as alterations in morphology) This inhibition was not due to diminished cellular viability and did not permanently impair the capacity of the lymphocyte to subsequently respond to PHA. Additionally, there was no evidence that this inhibition was due to other possible effects of EACA, such as alterations in Na+ — K+ transport, competitive amino acid deprivation or interference with PHA binding. Moreover, the inhibitors of proteolysis, tosyl arginine methyl ester (TAME), tosyl lysine chloromethyl ketone (TLCK), and tosyl phenyl-alanine chloromethyl ketone (TPCK), were also shown to inhibit lymphocyte stimulation.
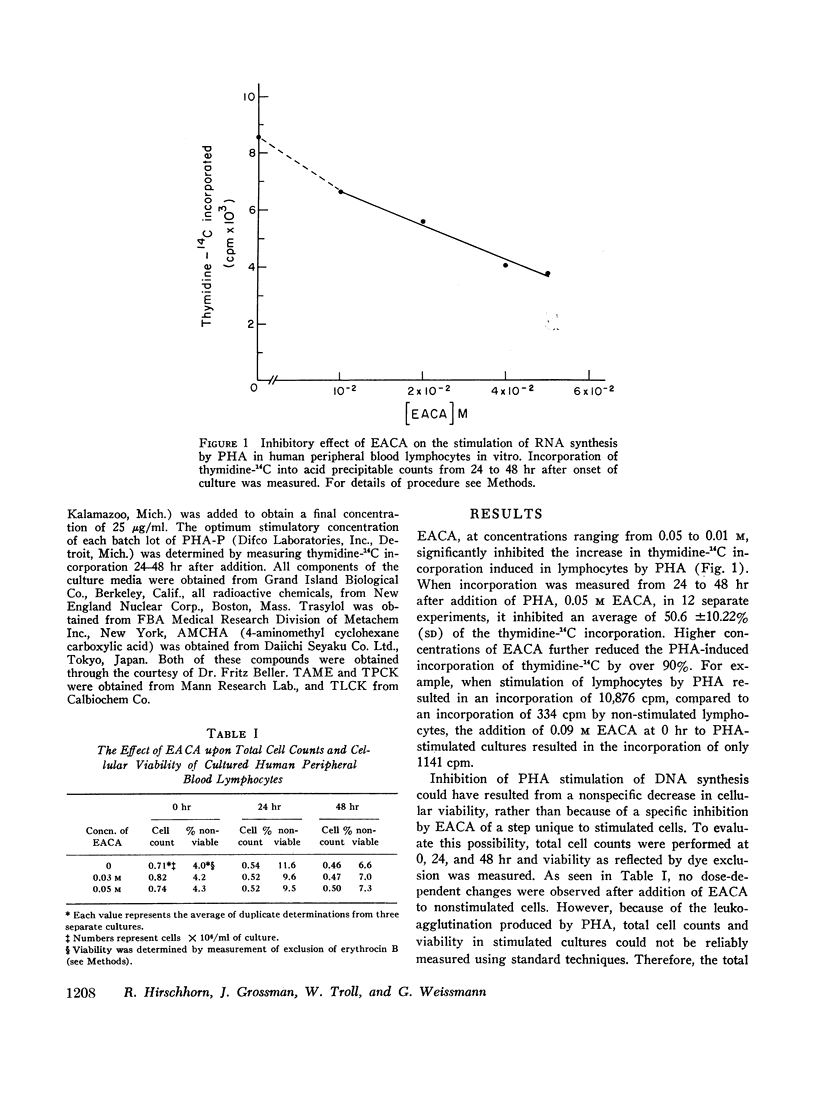
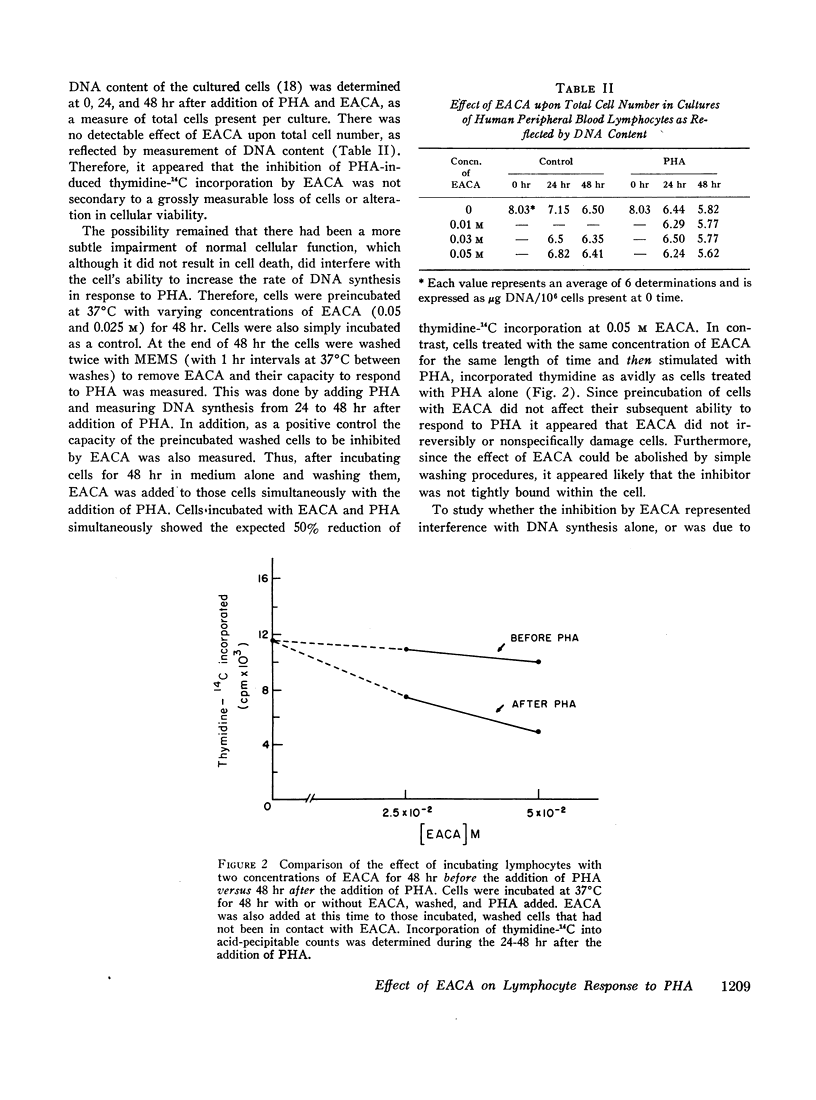
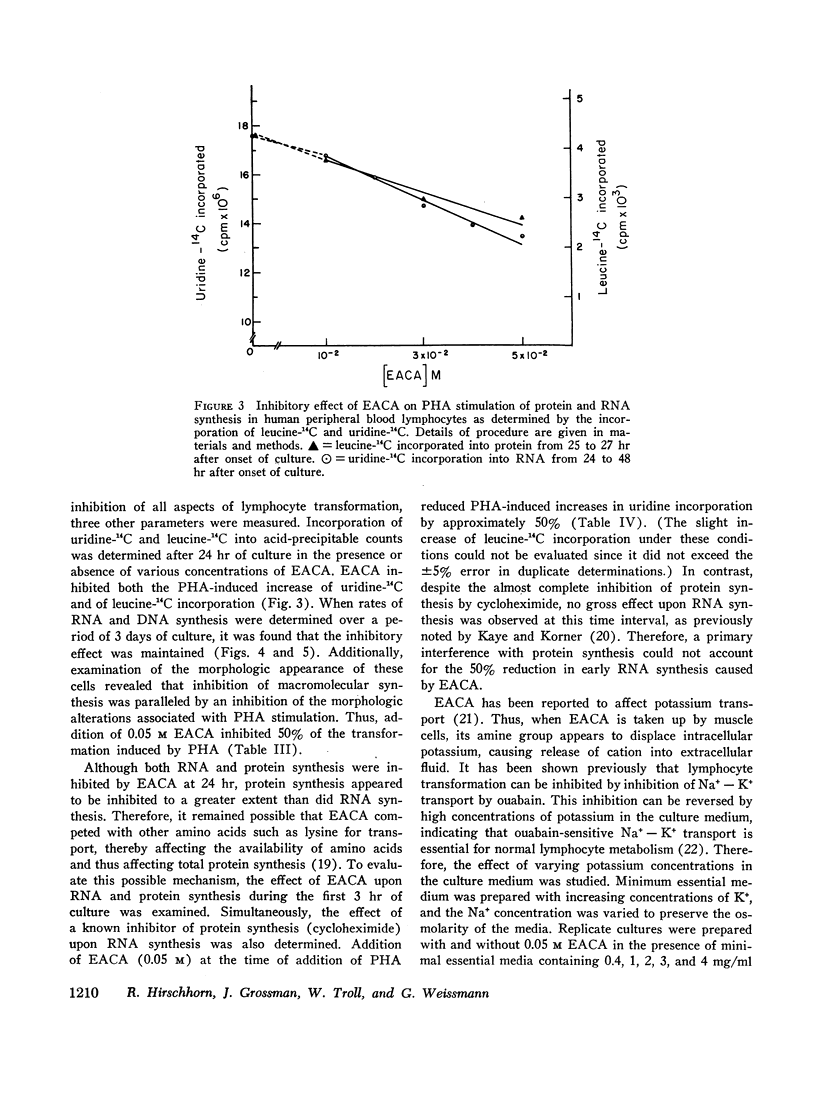
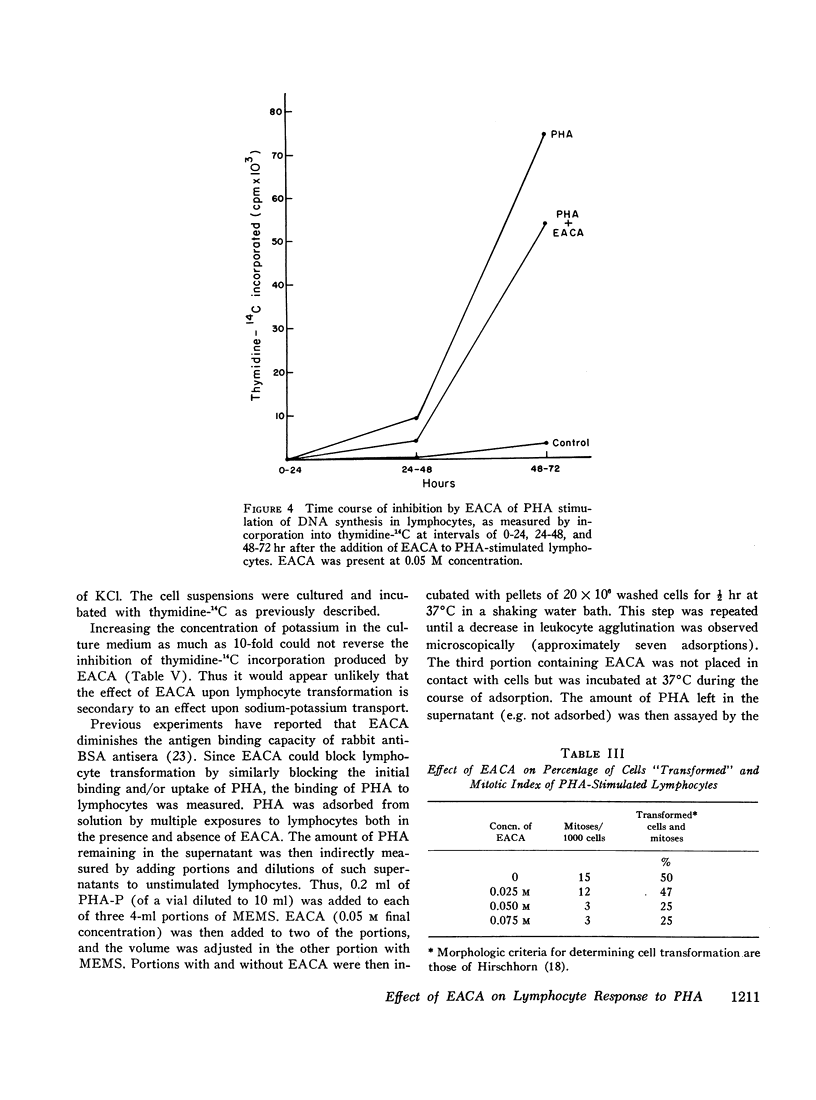
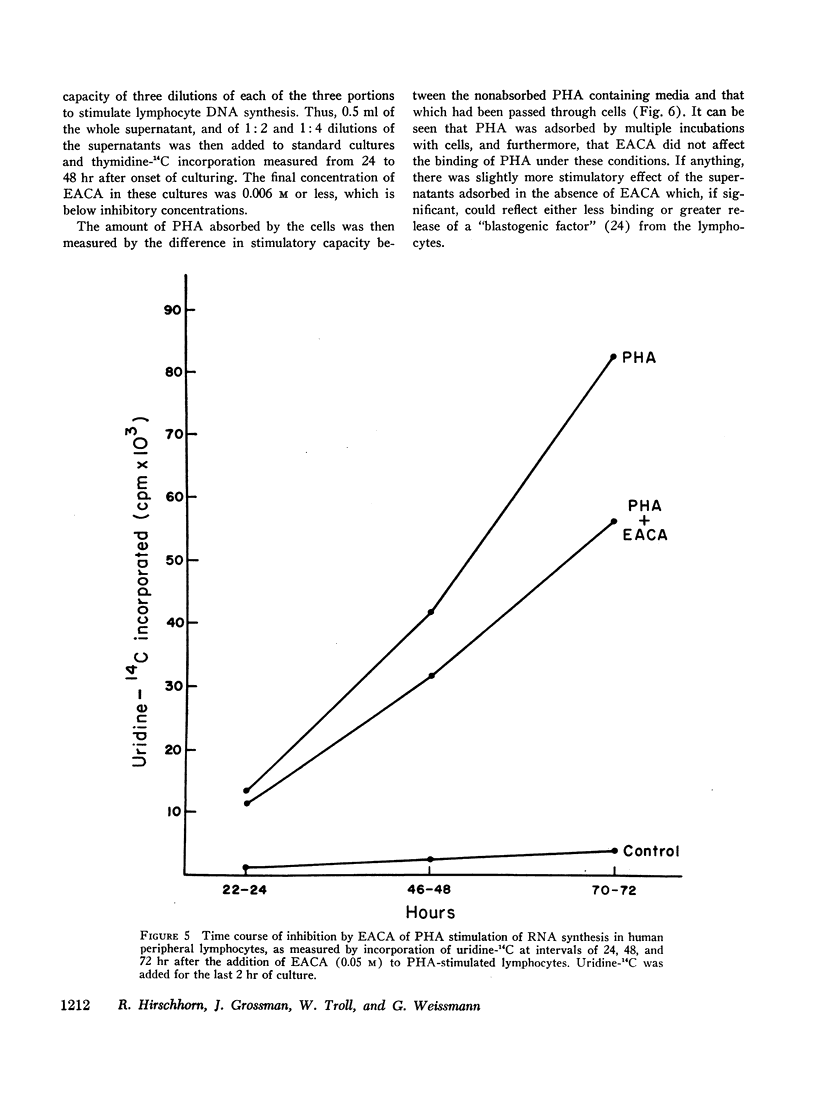
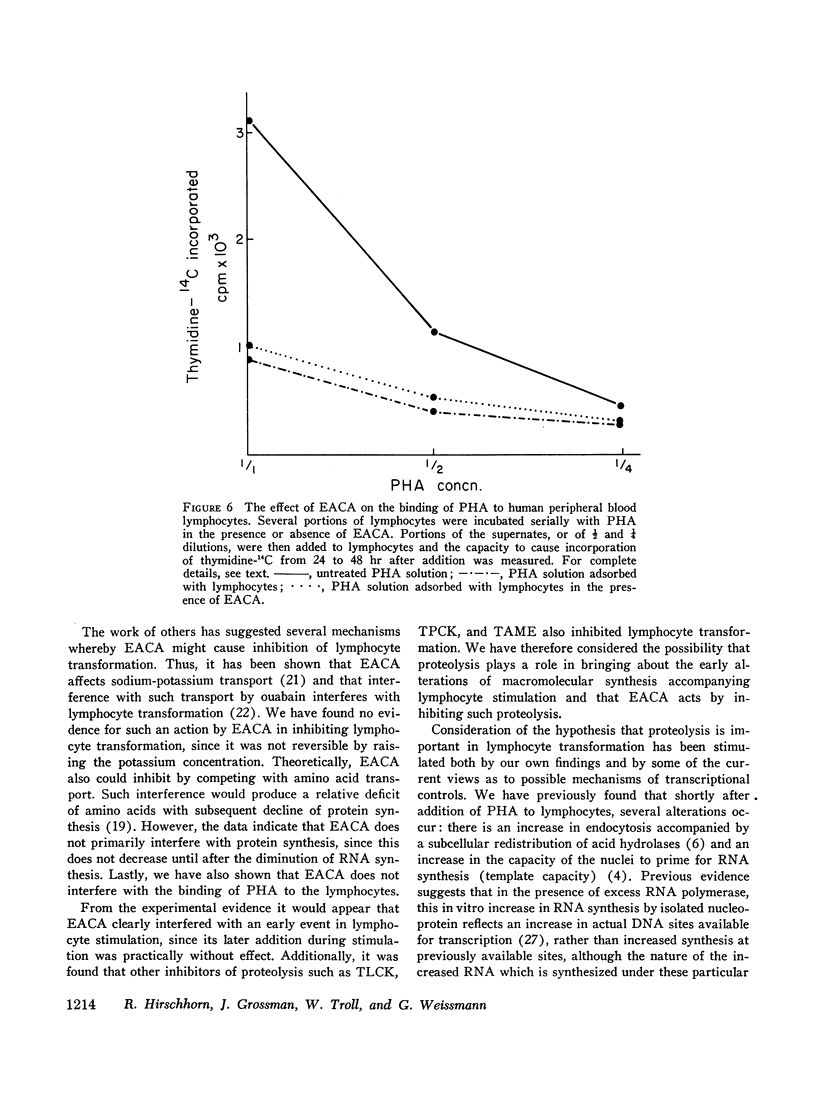
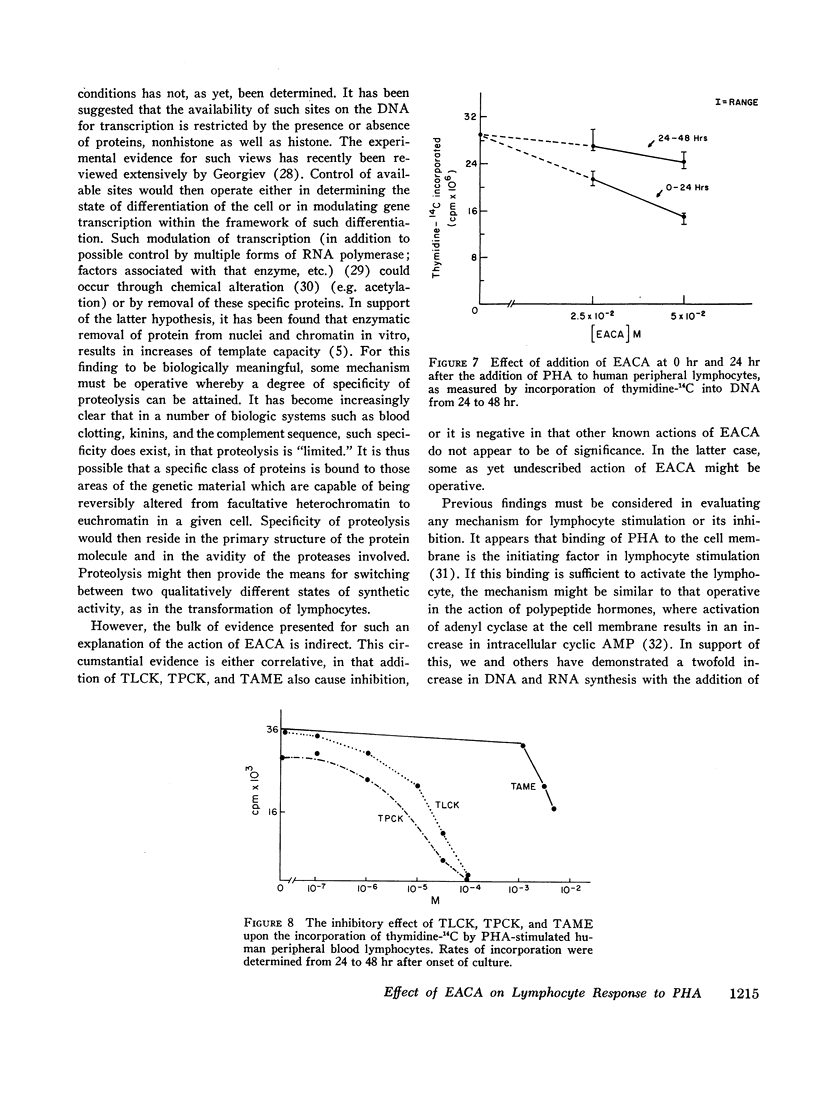
EACA was most effective when added during the first 24 hr of stimulation. Therefore, these experiments support the hypothesis that proteolysis is an essential step in the early phase of lymphocyte activation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALKJAERSIG N., FLETCHER A. P., SHERRY S. xi-Aminocaproic acid: an inhibitor of plasminogen activation. J Biol Chem. 1959 Apr;234(4):832–837. [PubMed] [Google Scholar]
- ALLFREY V. G., LITTAU V. C., MIRSKY A. E. On the role of of histones in regulation ribonucleic acid synthesis in the cell nucleus. Proc Natl Acad Sci U S A. 1963 Mar 15;49:414–421. doi: 10.1073/pnas.49.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON A. C., MALLUCCI L. LYSOSOMES IN DIVIDING CELLS, WITH SPECIAL REFERENCE TO LYMPHOCYTES. Lancet. 1964 Dec 26;2(7374):1371–1373. doi: 10.1016/s0140-6736(64)91162-6. [DOI] [PubMed] [Google Scholar]
- Ali S. Y. The degradation of cartilage matrix by an intracellular protease. Biochem J. 1964 Dec;93(3):611–618. doi: 10.1042/bj0930611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allfrey V. G. Changes in chromosomal proteins at times of gene activation. Fed Proc. 1970 Jul-Aug;29(4):1447–1460. [PubMed] [Google Scholar]
- Ambrus C. M., Ambrus J. L., Lassman H. B., Mink I. B. Studies on the mechanism of action of inhibitors of the fibrinolysin system. Ann N Y Acad Sci. 1968 Jun 28;146(2):430–447. doi: 10.1111/j.1749-6632.1968.tb20304.x. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittinger G., Hirschhorn R., Douglas S. D., Weissmann G. Studies on lysosomes. XI. Characterization of a hydrolase-rich fraction from human lymphocytes. J Cell Biol. 1968 May;37(2):394–411. doi: 10.1083/jcb.37.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A. Escherichia coli RNA polymerase: purification, subunit structure, and factor requirements. Fed Proc. 1970 May-Jun;29(3):1164–1169. [PubMed] [Google Scholar]
- Carroll H. J., Tice D. A. The effects of epsilon amino-caproic acid upon potassium metabolism in the dog. Metabolism. 1966 May;15(5):449–457. doi: 10.1016/0026-0495(66)90087-4. [DOI] [PubMed] [Google Scholar]
- Cooper H. L. Ribsomal ribonucleic acid production and growth regulation in human lymphocytes. J Biol Chem. 1969 Apr 10;244(7):1946–1952. [PubMed] [Google Scholar]
- Fritz H., Trautschold I., Haendle H., Werle E. Chemistry and biochemistry of proteinase inhibitors from mammalian tissues. Ann N Y Acad Sci. 1968 Jun 28;146(2):400–413. doi: 10.1111/j.1749-6632.1968.tb20301.x. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Brittinger G., Hirschhorn K., Weissmann G. Studies on lysosomes. XII. Redistribution of acid hydrolases in human lymphocytes stimulated by phytohemagglutinin. J Cell Biol. 1968 May;37(2):412–423. doi: 10.1083/jcb.37.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Grossman J., Weissmann G. Effect of cyclic 3',5'-adenosine monophosphate and theophylline on lymphocyte transformation. Proc Soc Exp Biol Med. 1970 Apr;133(4):1361–1365. doi: 10.3181/00379727-133-34690. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Troll W., Brittinger G., Weissmann G. Template activity of nuclei from stimulated lymphocytes. Nature. 1969 Jun 28;222(5200):1247–1250. doi: 10.1038/2221247a0. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Korner A. Effect of cycloheximide on protein and ribonucleic acid synthesis in cultured human lymphocytes. Biochem J. 1966 Sep;100(3):815–822. doi: 10.1042/bj1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killander D., Rigler R., Jr Initial changes of deoxyribonucleoprotein and synthesis of nucleic acid in phytohemagglutinine-stimulated human leucocytes in vitro. Exp Cell Res. 1965 Sep;39(2):701–704. doi: 10.1016/0014-4827(65)90075-3. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphorylation of nuclear protein early in the course of gene activation in lymphocytes. Science. 1966 Nov 11;154(3750):780–781. doi: 10.1126/science.154.3750.780. [DOI] [PubMed] [Google Scholar]
- Lindahl-Kiessling K., Peterson R. D. The mechanism of phytohemagglutinin (PHA) action. 3. Stimulation of lymphocytes by allogeneic lymphocytes and phytohemagglutinin. Exp Cell Res. 1969 Apr;55(1):85–87. doi: 10.1016/0014-4827(69)90460-1. [DOI] [PubMed] [Google Scholar]
- MCNICOL G. P., DOUGLAS A. S. EPSILON-AMINOCAPROIC ACID AND OTHER INHIBITORS OF FIBRINOLYSIS. Br Med Bull. 1964 Sep;20:233–239. doi: 10.1093/oxfordjournals.bmb.a070338. [DOI] [PubMed] [Google Scholar]
- Maden B. E. Effects of lysine or valine starvation on ribosome subunit balance in HeLa cells. Nature. 1969 Dec 20;224(5225):1203–1205. doi: 10.1038/2241203a0. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Oshiba S., Mihara H., Okamoto U. Synthetic inhibitors of fibrinolysis: in vitro and in vivo mode of action. Ann N Y Acad Sci. 1968 Jun 28;146(2):414–429. doi: 10.1111/j.1749-6632.1968.tb20303.x. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Allfrey V. G., Mirsky A. E. RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc Natl Acad Sci U S A. 1966 Apr;55(4):805–812. doi: 10.1073/pnas.55.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel M. R., Kaplan J. G. Inhibition by ouabain of human lymphocyte transformation induced by phytohaemagglutinin in vitro. Nature. 1968 Jul 13;219(5150):198–200. doi: 10.1038/219198a0. [DOI] [PubMed] [Google Scholar]
- Rigler R., Killander D. Activation of deoxyribonucleoprotein in human leucocytes stimulated by phytohemagglutinin. II. Structural changes of deoxyribonucleoprotein and synthesis of RNA. Exp Cell Res. 1969 Feb;54(2):171–180. doi: 10.1016/0014-4827(69)90229-8. [DOI] [PubMed] [Google Scholar]
- Ringertz N. R., Darzynkiewicz Z., Bolund L. Actinomycin binding properties of stimulated human lymphocytes. Exp Cell Res. 1969 Aug;56(2):411–417. doi: 10.1016/0014-4827(69)90032-9. [DOI] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Tedesco T. A., Mellman W. J. Desoxyribonucleic acid assay as a measure of cell number in preparations from monolayer cell cultures and blood leucocytes. Exp Cell Res. 1967 Jan;45(1):230–232. doi: 10.1016/0014-4827(67)90126-7. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Spilberg I. Breakdown of cartilage proteinpolysaccharide by lysosomes. Arthritis Rheum. 1968 Apr;11(2):162–169. doi: 10.1002/art.1780110206. [DOI] [PubMed] [Google Scholar]
- Wold R. T., Reid R. T., Farr R. S. The effect of epsilon-aminocaproic acid on antigen-antibody reactions. J Immunol. 1967 Oct;99(4):797–802. [PubMed] [Google Scholar]


