Abstract
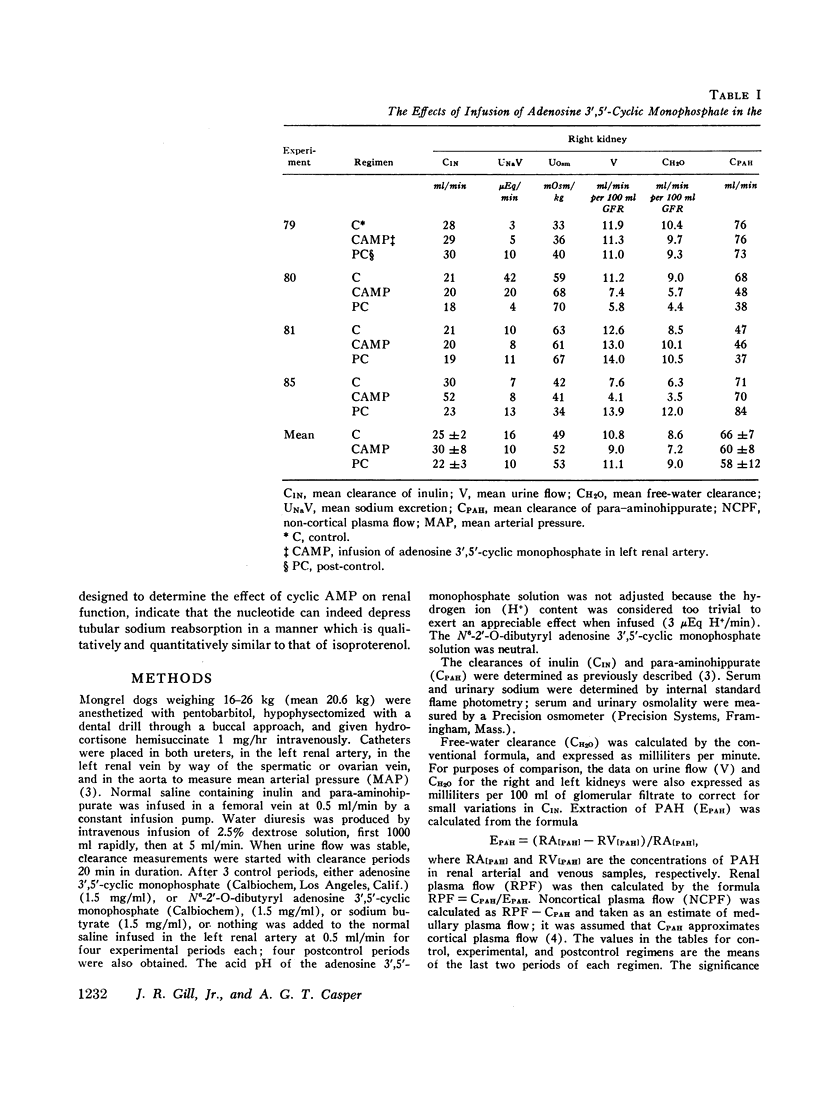
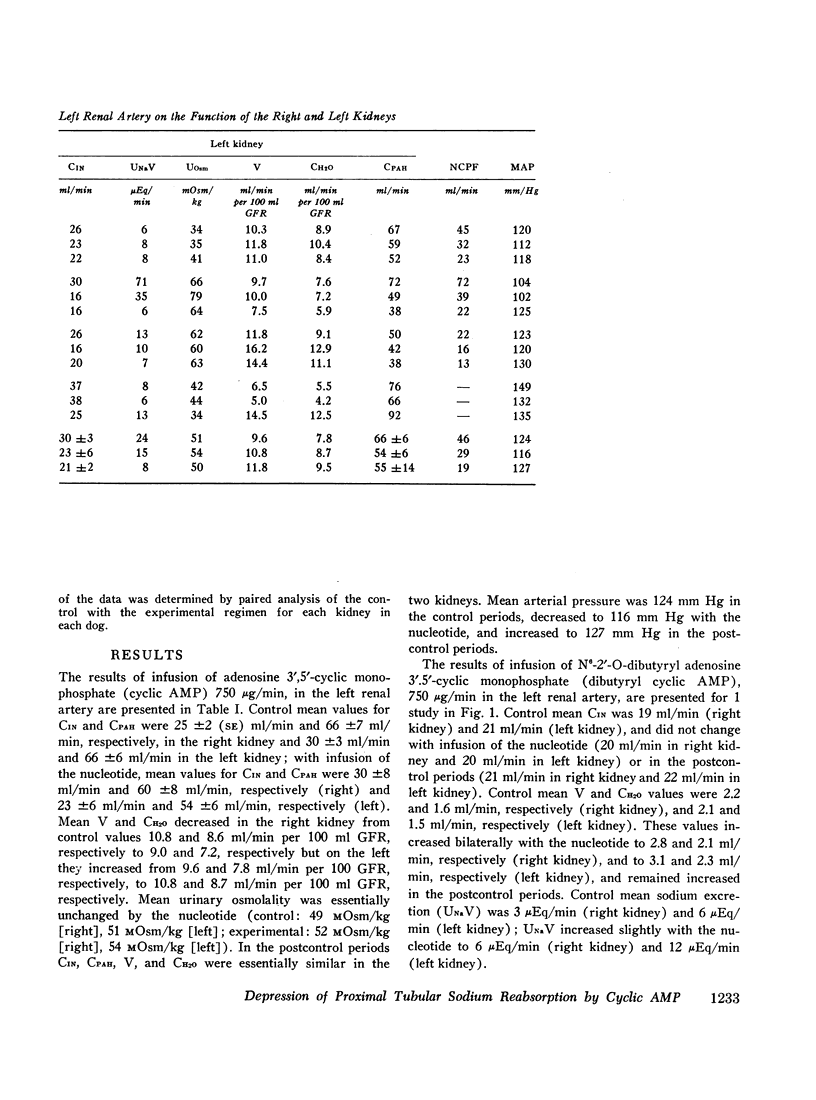
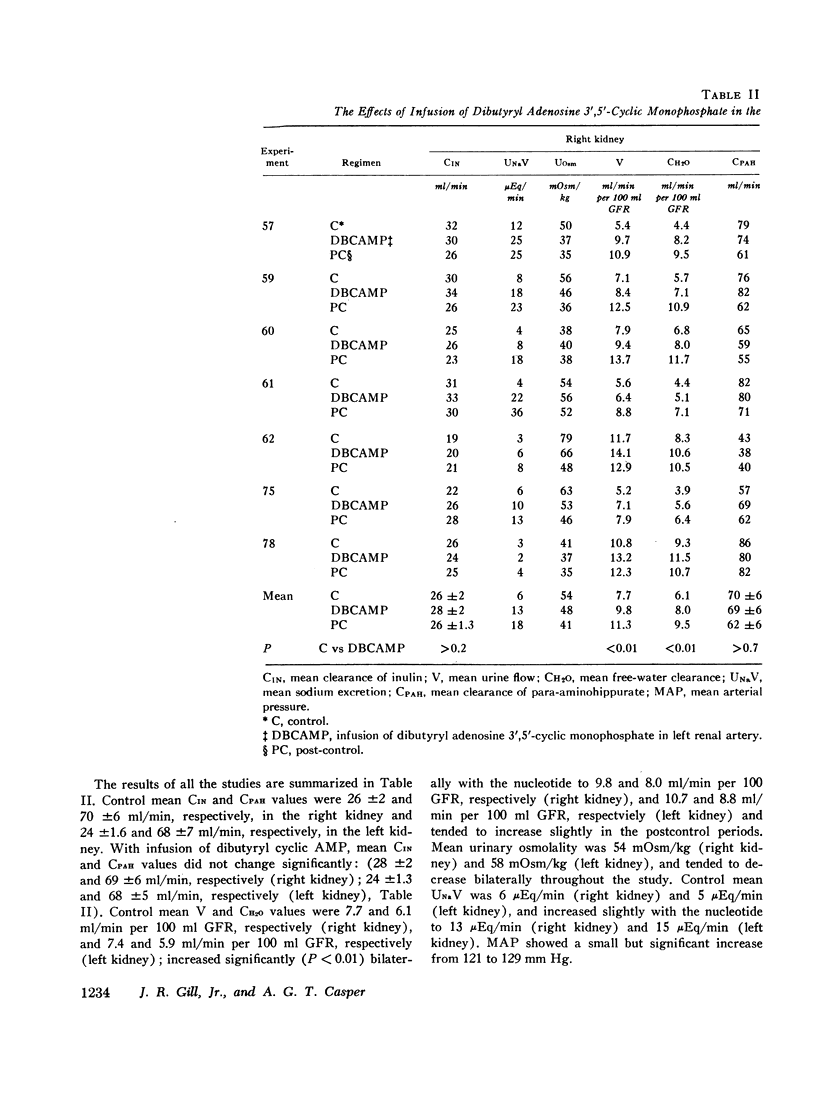
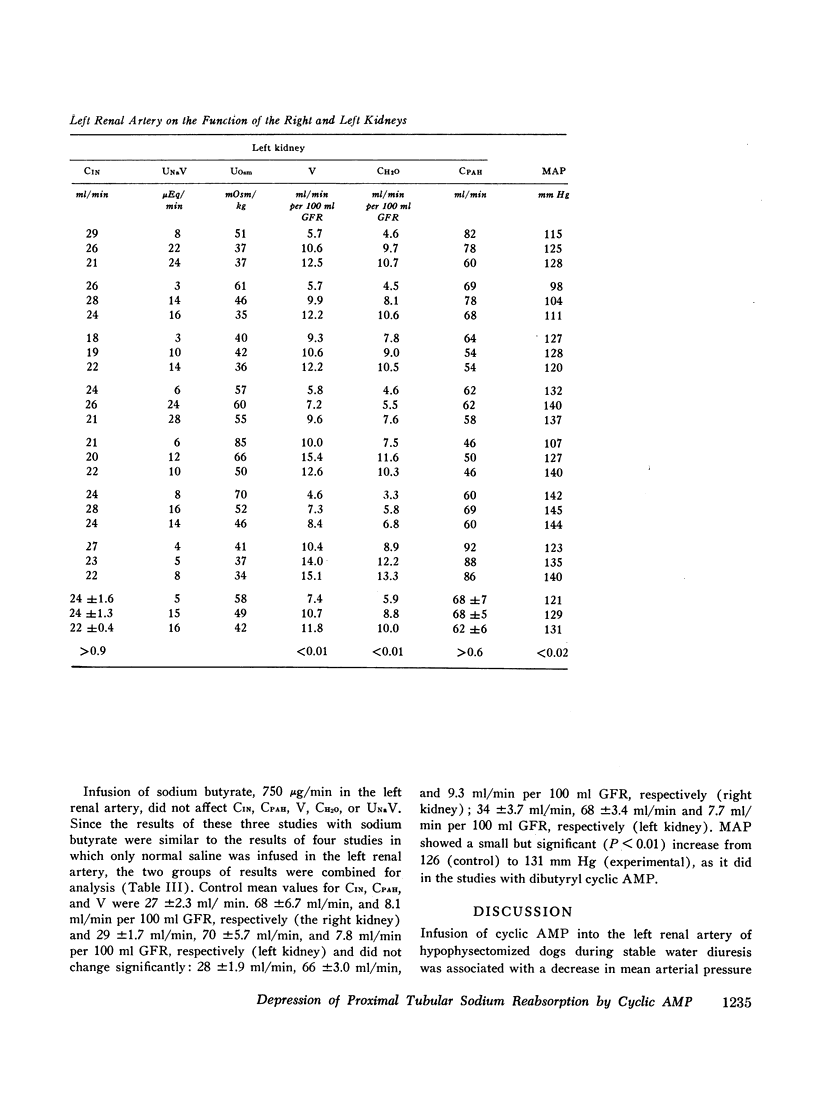
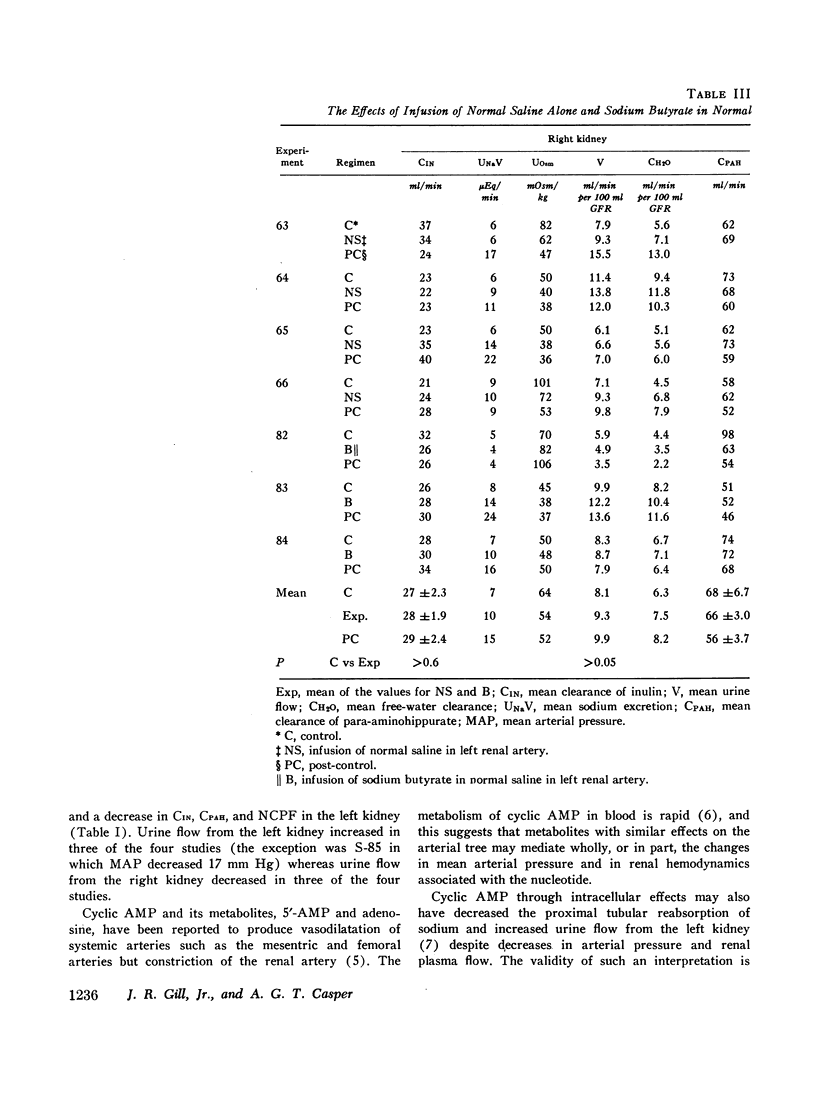
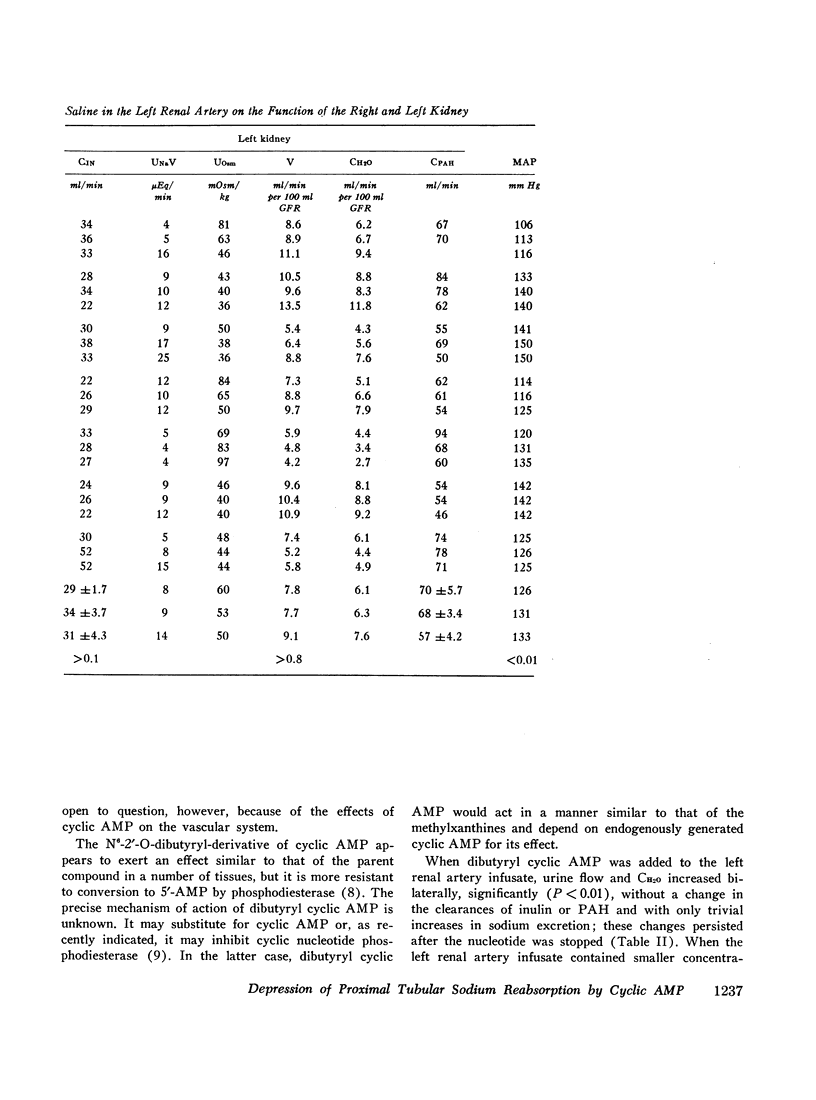
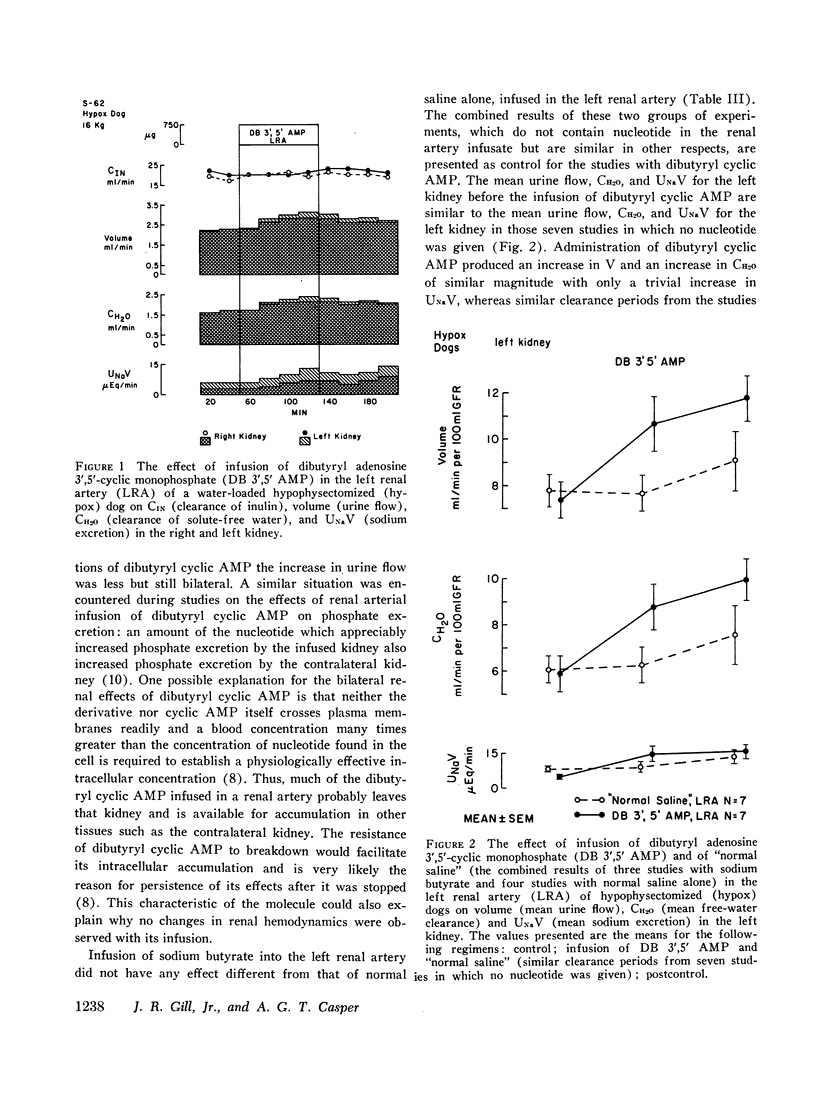
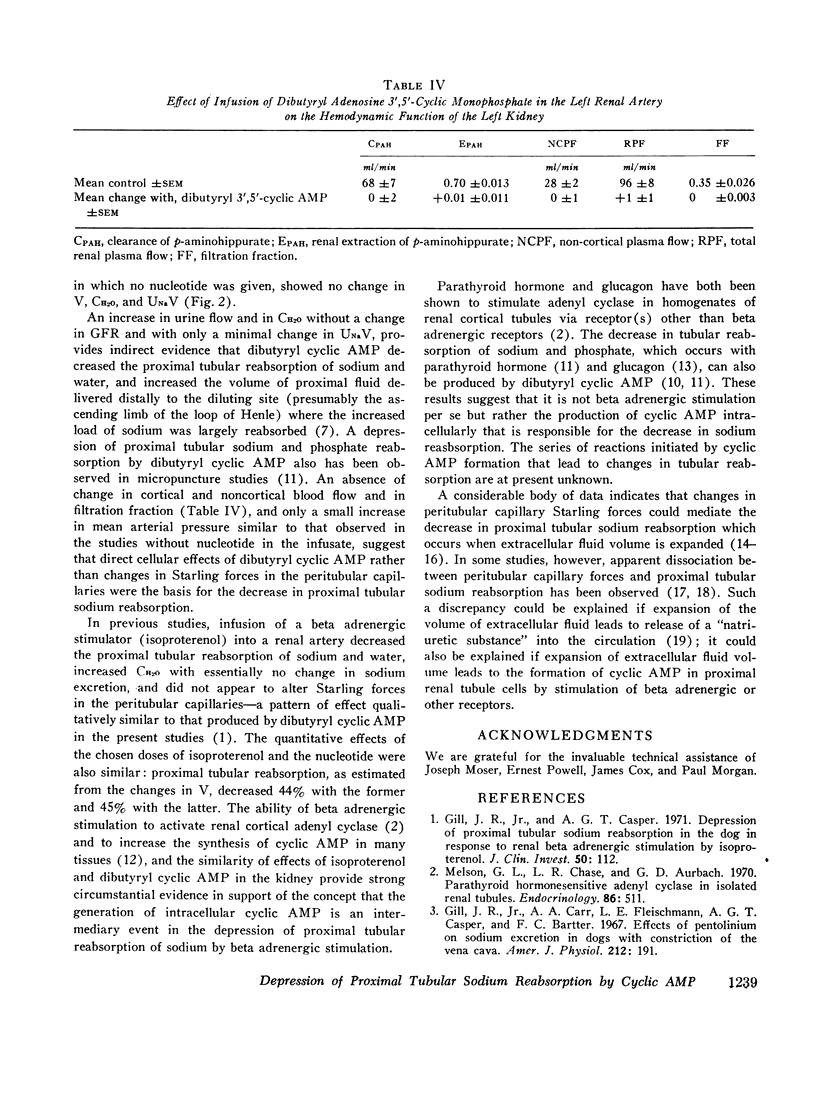
Stable water diuresis was produced in anesthetized, hydrocortisone-treated hypophysectomized dogs by infusion of 2.5% dextrose. Infusion of adenosine 3′,5′-cyclic monophosphate (cyclic AMP) in the left renal artery decreased ipsilaterally glomerular filtration rate (GFR), cortical and non-cortical renal plasma flow, and tended to increase urine flow (V) and free-water clearance (CH2O) despite a decrease in mean arterial pressure. Infusion of dibutyryl adenosine 3′,5′-cyclic monophosphate (dibutyryl cyclic AMP) in the left renal artery increased V and CH2O significantly (P<0.01) bilaterally with essentially no change in GFR, in total renal plasma flow or its cortical and non-cortical components. For each kidney the magnitude of the change in V was similar to the magnitude of the change in CH2O and the change in sodium excretion was trivial. Cyclic AMP probably produced its effects on renal hemodynamics and mean arterial pressure wholly or in part through the action of metabolites such as 5′-AMP and adenosine on the renal and systemic vasculature. The absence of an effect of dibutyryl cyclic AMP on renal hemodynamics and its bilateral effect may be explained by the resistance of this nucleotide derivative to metabolism.
Dibutyryl cyclic AMP appears to decrease by direct cellular effect(s) proximal tubular sodium reabsorption but does not prevent virtually complete reabsorption of the increased load of sodium in the distal nephron. This effect on the kidney is qualitatively and quantitatively similar to the effect of renal arterial infusion of isoproterenol.
The results suggest that synthesis of cyclic AMP in proximal renal tubule cells in response to stimulation of beta adrenergic or other receptors can mediate a decrease in the proximal tubular reabsorption of sodium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadus A. E., Kaminsky N. I., Hardman J. G., Sutherland E. W., Liddle G. W. Kinetic parameters and renal clearances of plasma adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in man. J Clin Invest. 1970 Dec;49(12):2222–2236. doi: 10.1172/JCI106441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckalew V. M., Jr, Martinez F. J., Green W. E. The effect of dialysates and ultrafiltrates of plasma of saline-loaded dogs on toad bladder sodium transport. J Clin Invest. 1970 May;49(5):926–935. doi: 10.1172/JCI106312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eknoyan G., Suki W. N., Rector F. C., Jr, Seldin D. W. Functional characteristics of the diluting segment of the dog nephron and the effect of extracellular volume expansion on its reabsorptive capacity. J Clin Invest. 1967 Jul;46(7):1178–1188. doi: 10.1172/JCI105611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J. R., Jr, Carr A. A., Fleischmann L. E., Casper A. G., Bartter F. C. Effects of pentolinium on sodium excretion in dogs with constriction of the vena cava. Am J Physiol. 1967 Jan;212(1):191–196. doi: 10.1152/ajplegacy.1967.212.1.191. [DOI] [PubMed] [Google Scholar]
- Gill J. R., Jr, Casper A. G. Depression of proximal tubular sodium reabsorption in the dog in response to renal beta adrenergic stimulation by isoproterenol. J Clin Invest. 1971 Jan;50(1):112–118. doi: 10.1172/JCI106464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henion W. F., Sutherland E. W., Posternak T. Effects of derivatives of adenosine 3',5'-phosphate on liver slices and intact animals. Biochim Biophys Acta. 1967 Oct 9;148(1):106–113. doi: 10.1016/0304-4165(67)90284-x. [DOI] [PubMed] [Google Scholar]
- Howards S. S., Davis B. B., Knox F. G., Wright F. S., Berliner R. W. Depression of fractional sodium reabsorption by the proximal tubule of the dog without sodium diuresis. J Clin Invest. 1968 Jul;47(7):1561–1572. doi: 10.1172/JCI105848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D. C., Berg G. R. Pineal gland: stimulation of melatonin production by norepinephrine involves cyclic AMP-mediated stimulation of N-acetyltransferase. Adv Biochem Psychopharmacol. 1970;3:241–263. [PubMed] [Google Scholar]
- Knox F. G., Howards S. S., Wright F. S., Davis B. B., Berliner R. W. Effect of dilution and expansion of blood volume on proximal sodium reabsorption. Am J Physiol. 1968 Nov;215(5):1041–1048. doi: 10.1152/ajplegacy.1968.215.5.1041. [DOI] [PubMed] [Google Scholar]
- Lewy J. E., Windhager E. E. Peritubular control of proximal tubular fluid reabsorption in the rat kidney. Am J Physiol. 1968 May;214(5):943–954. doi: 10.1152/ajplegacy.1968.214.5.943. [DOI] [PubMed] [Google Scholar]
- Martino J. A., Earley L. E. Demonstraton of a role of physical factors as determinants of the natriuretic response to volume expansion. J Clin Invest. 1967 Dec;46(12):1963–1978. doi: 10.1172/JCI105686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melson G. L., Chase L. R., Aurbach G. D. Parathyroid hormone-sensitive adenyl cyclase in isolated renal tubules. Endocrinology. 1970 Mar;86(3):511–518. doi: 10.1210/endo-86-3-511. [DOI] [PubMed] [Google Scholar]
- PILKINGTON L. A., BINDER R., DEHAAS J. C., PITTS R. F. INTRARENAL DISTRIBUTION OF BLOOD FLOW. Am J Physiol. 1965 Jun;208:1107–1113. doi: 10.1152/ajplegacy.1965.208.6.1107. [DOI] [PubMed] [Google Scholar]
- Pullman T. N., Lavender A. R., Aho I. Direct effects of glucagon on renal hemodynamics and excretion of inorganic ions. Metabolism. 1967 Apr;16(4):358–373. doi: 10.1016/0026-0495(67)90047-9. [DOI] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]


