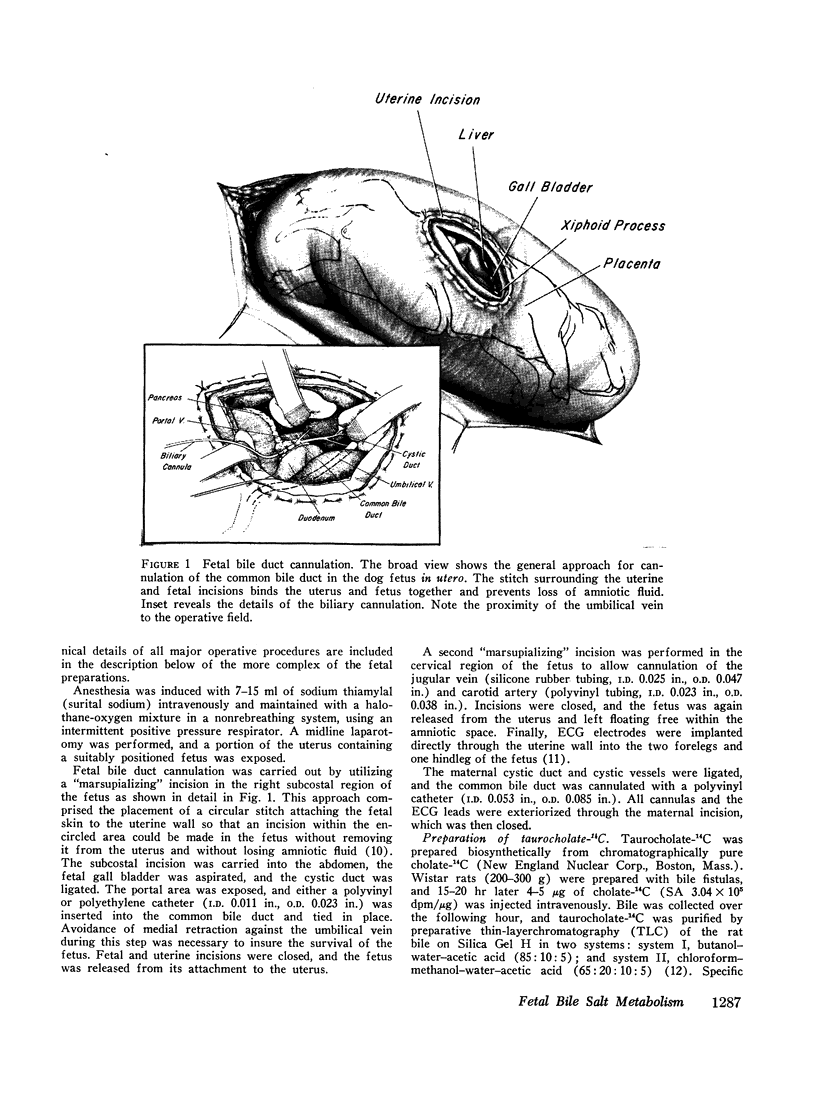
Abstract
Cholate metabolism was studied in fetal dogs 1 wk before term and was compared with cholate metabolism in adult dogs. Tracer amounts of sodium cholate-14C were administered to the fetus in utero by intravenous infusion over 6 hr. Fetal plasma disappearance, biliary excretion, tissue distribution, and placental transfer of cholate were measured over 10 hr.
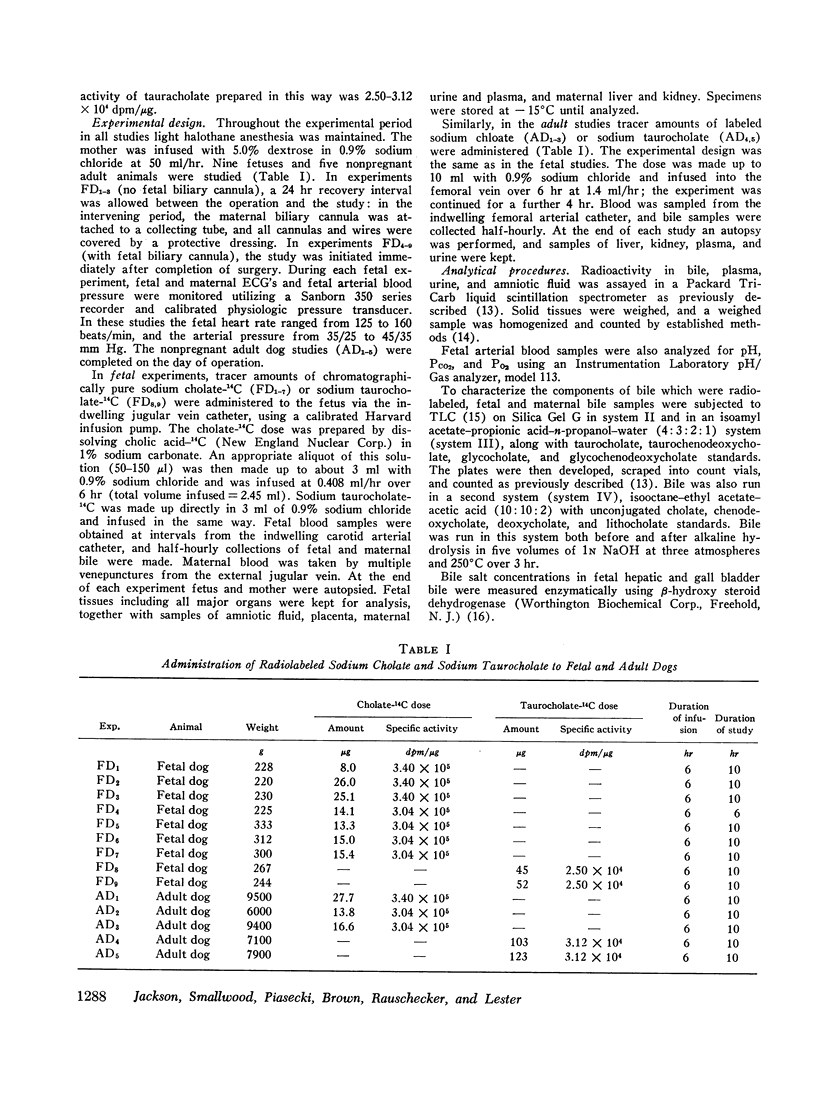
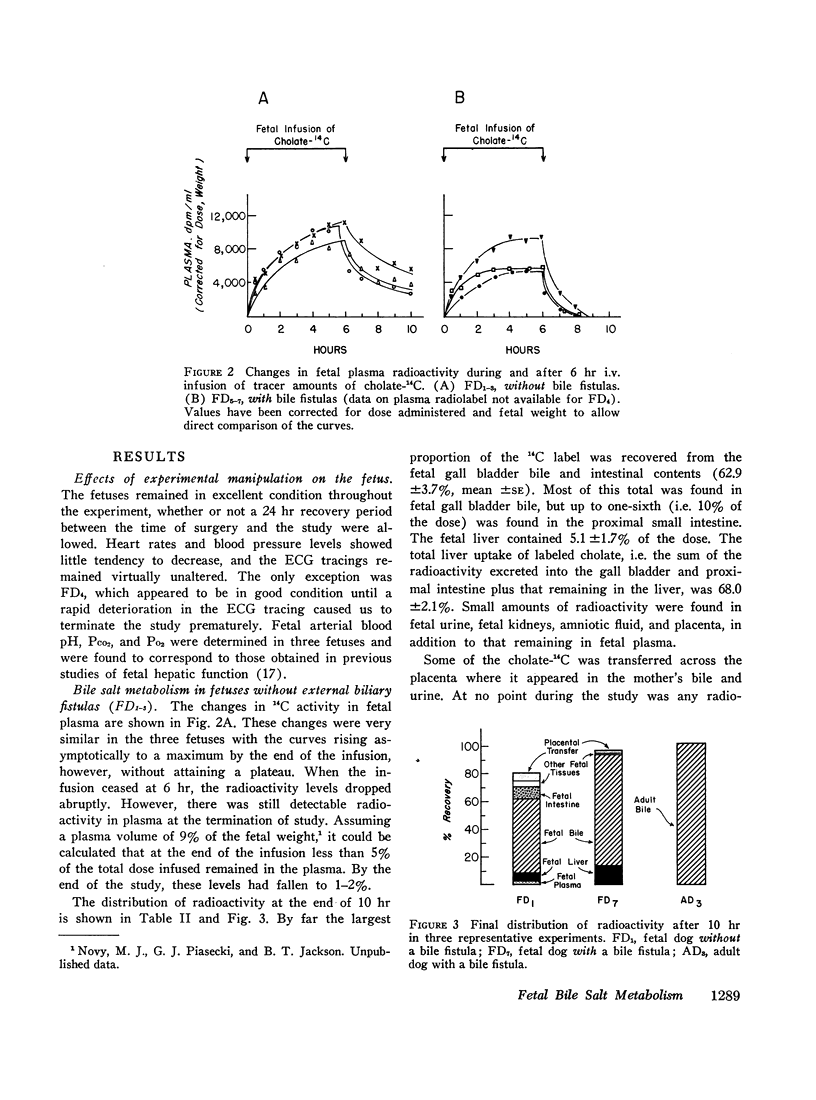
Infused cholate-14C was cleared rapidly from fetal plasma principally by the fetal liver and to a minor extent by placental transfer to the mother. The taurine conjugate was formed in the fetal liver and was excreted into the proximal small intestine via the biliary tree. Indirect evidence for the functioning enterohepatic circulation of bile salt in the fetus was obtained.
Comparison with the results of similar experiments in adult dogs showed that the fetal liver was almost as efficient as the adult liver in the uptake, conjugation, and excretion of tracer amounts of cholate-14C. The maximal rate of excretion of radiolabel attained by the fetus was somewhat slower than in the adult (82.8 ±1.4% and 96.1 ±4.0% [mean ±SE] of the infusion rate, respectively), and the proportion of the total dose excreted by the fetal liver during 10 hr was smaller (81.4 ±1.3% vs. 96.6 ±4.4%). This difference could be only partly accounted for by placental transfer (2.8 ±0.6% of the fetal dose).
Labeled cholate and taurocholate were excreted by the fetus at similar rates, which suggests that, under the conditions of study, conjugation had little influence on the rate of transfer of cholate across the liver cell.
It is concluded that the fetal dog, 1 wk before birth, has a remarkably mature and efficient mechanism for the uptake and excretion of cholate.
Full text
PDF
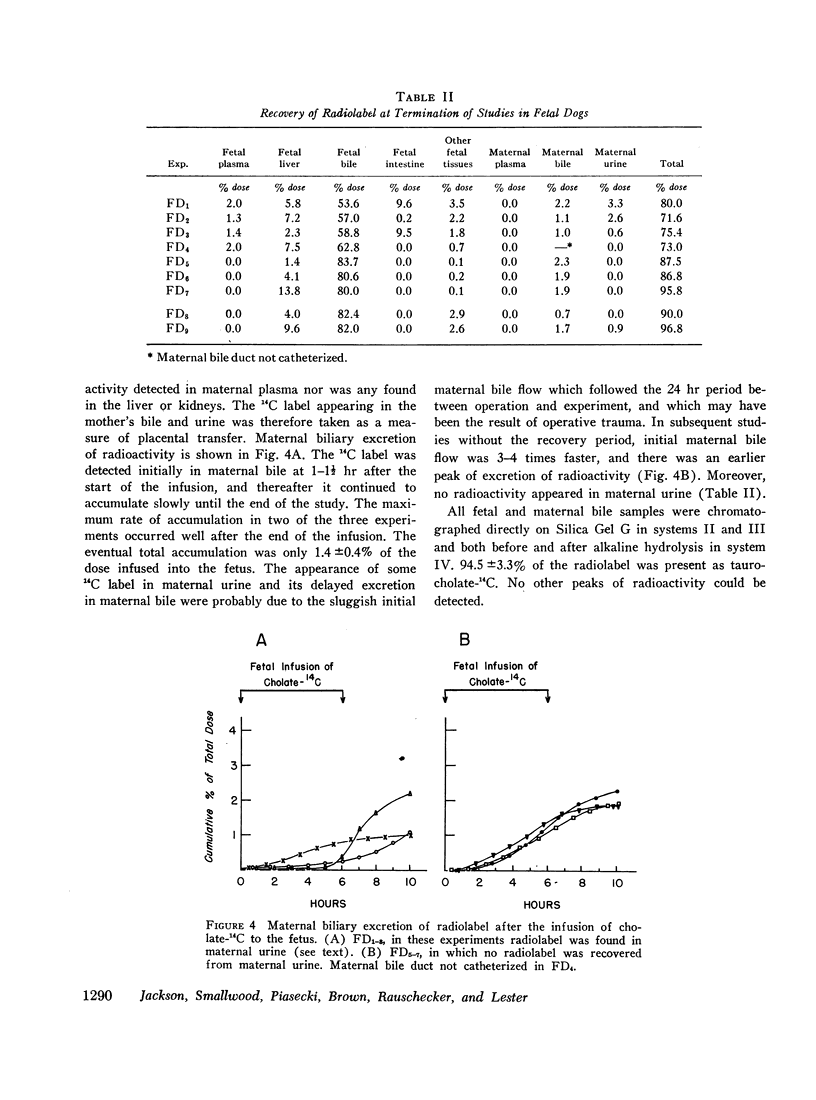
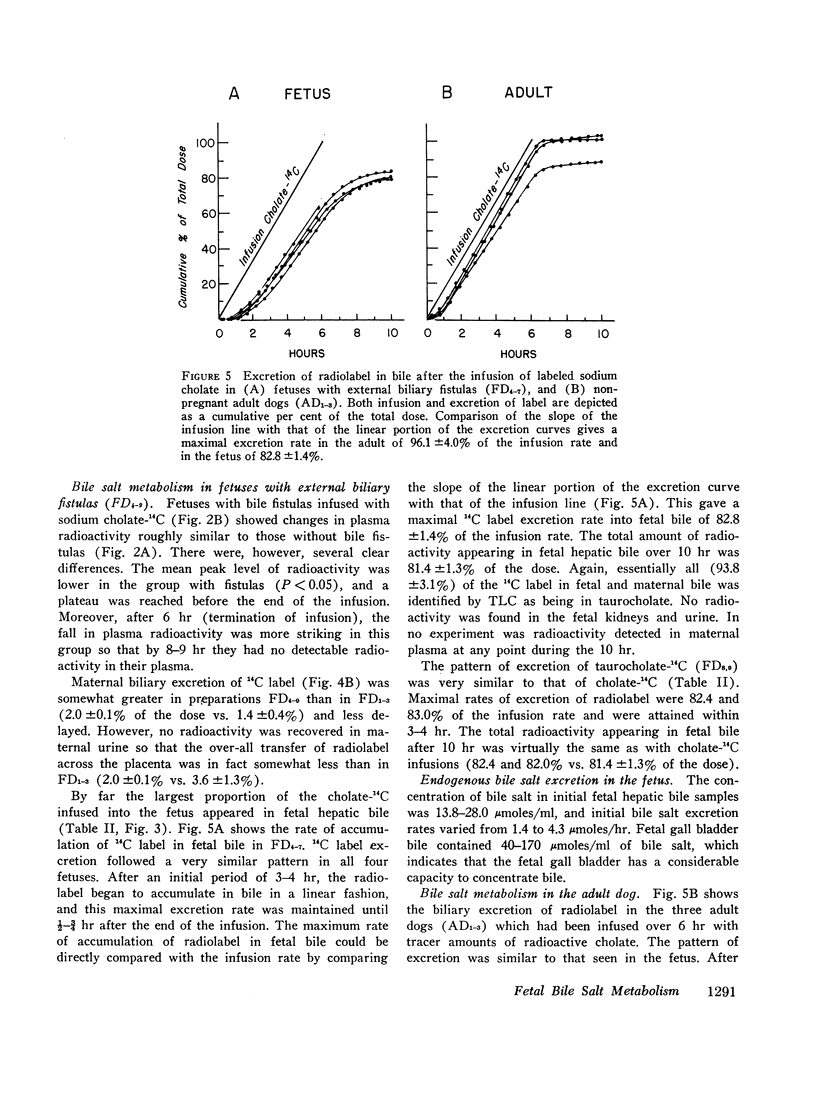



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONGIOVANNI A. M. BILE ACID CONTENT OF GALLBLADDER OF INFANTS, CHILDREN AND ADULTS. J Clin Endocrinol Metab. 1965 May;25:678–685. doi: 10.1210/jcem-25-5-678. [DOI] [PubMed] [Google Scholar]
- Bernstein R. B., Novy M. J., Piasecki G. J., Lester R., Jackson B. T. Bilirubin metabolism in the fetus. J Clin Invest. 1969 Sep;48(9):1678–1688. doi: 10.1172/JCI106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCRANTZ J. C., SJOVALL J. On the bile acids in duodenal contents of infants and children. Bile acids and steroids 72. Clin Chim Acta. 1959 Nov;4:793–799. doi: 10.1016/0009-8981(59)90030-0. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F. A physicochemical approach to the intraluminal phase of fat absorption. Gastroenterology. 1966 Jan;50(1):56–64. [PubMed] [Google Scholar]
- Hofmann A. F., Small D. M. Detergent properties of bile salts: correlation with physiological function. Annu Rev Med. 1967;18:333–376. doi: 10.1146/annurev.me.18.020167.002001. [DOI] [PubMed] [Google Scholar]
- JACKSON B. T., CLARKE J. P., EGDAHL R. H. Direct lead fetal electrocardiography with undisturbed fetal-maternal relationships. Surg Gynecol Obstet. 1960 Jun;110:687–692. [PubMed] [Google Scholar]
- JACKSON B. T., EGDAHL R. H. The performance of complex fetal operations in utero without amniotic fluid loss or other disturbances of fetal-maternal relationships. Surgery. 1960 Sep;48:564–570. [PubMed] [Google Scholar]
- LESTER R., BEHRMAN R. E., LUCEY J. F. TRANSFER OF BILIRUBIN-C14 ACROSS MONKEY PLACENTA. Pediatrics. 1963 Sep;32:416–419. [PubMed] [Google Scholar]
- LESTER R., SCHMID R. Intestinal absorption of bile pigments. I. The enterohepatic circulation of bilirubin in the rat. J Clin Invest. 1963 May;42:736–746. doi: 10.1172/JCI104766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OBRINSKY W., DENLEY M. L., BRAUER R. W. Sulfobromophthalein sodium excretion test as a measure of liver function in premature infants. Pediatrics. 1952 Apr;9(4):421–438. [PubMed] [Google Scholar]
- OLIVERIO V. T., DENHAM C., DAVIDSON J. D. Oxygen flask combustion in determination of C-14 and H3 in biological materials. Anal Biochem. 1962 Aug;4:188–189. doi: 10.1016/0003-2697(62)90035-0. [DOI] [PubMed] [Google Scholar]
- POLEY J. R., DOWER J. C., OWEN C. A., Jr, STICKLER G. B. BILE ACIDS IN INFANTS AND CHILDREN. J Lab Clin Med. 1964 May;63:838–846. [PubMed] [Google Scholar]
- Peric-Golia L., Socic H. Biliary bile acids and cholesterol in developing sheep. Am J Physiol. 1968 Nov;215(5):1284–1287. doi: 10.1152/ajplegacy.1968.215.5.1284. [DOI] [PubMed] [Google Scholar]