Abstract
Dihydrotestosterone metabolism was studied with a constant infusion technique in three men, three women, five hirsute women, and four estrogen-treated hirsute women. The mean dihydrotestosterone metabolic clearance rate was higher in men (336 liters/24 hr per m2 [range, 239-448]) than in women (153 liters/24 hr per m2 [range, 108-184]). The metabolic clearance rates in hirsute patients were intermediate between those men and women and were decreased by estrogen treatment. These observations demonstrate similarities in the metabolic rates of testosterone and dihydrotestosterone.
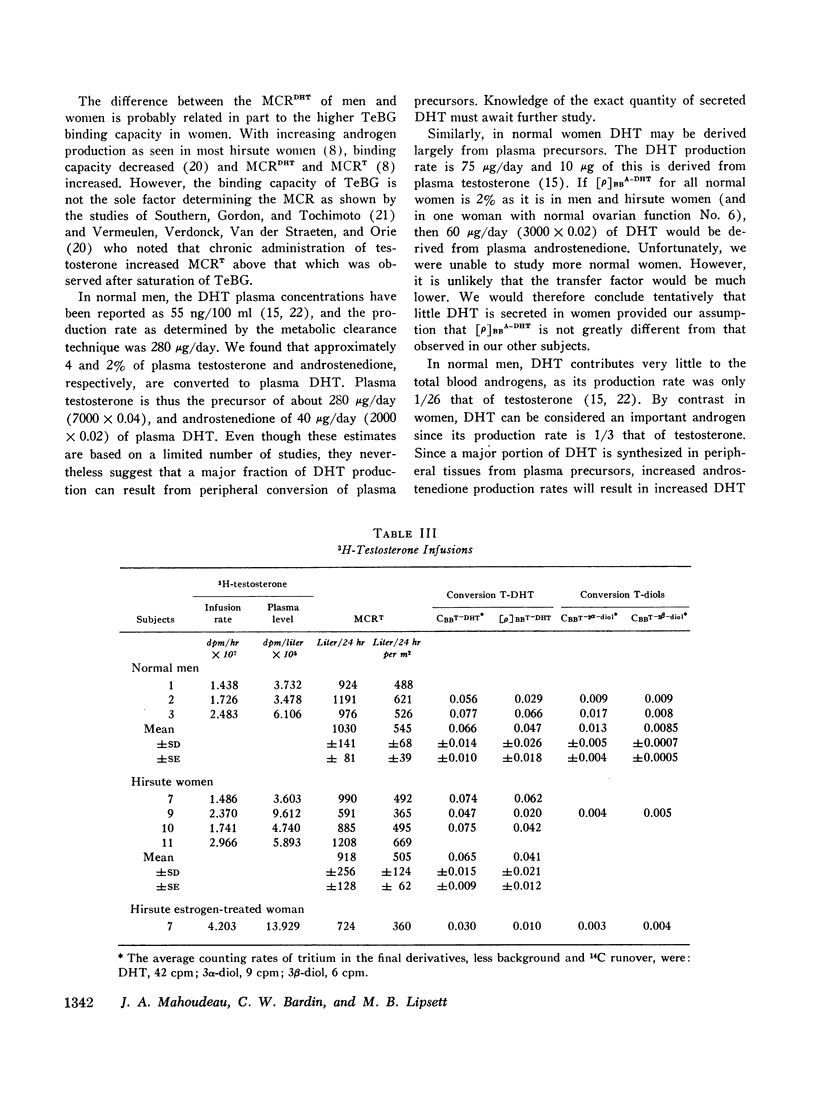
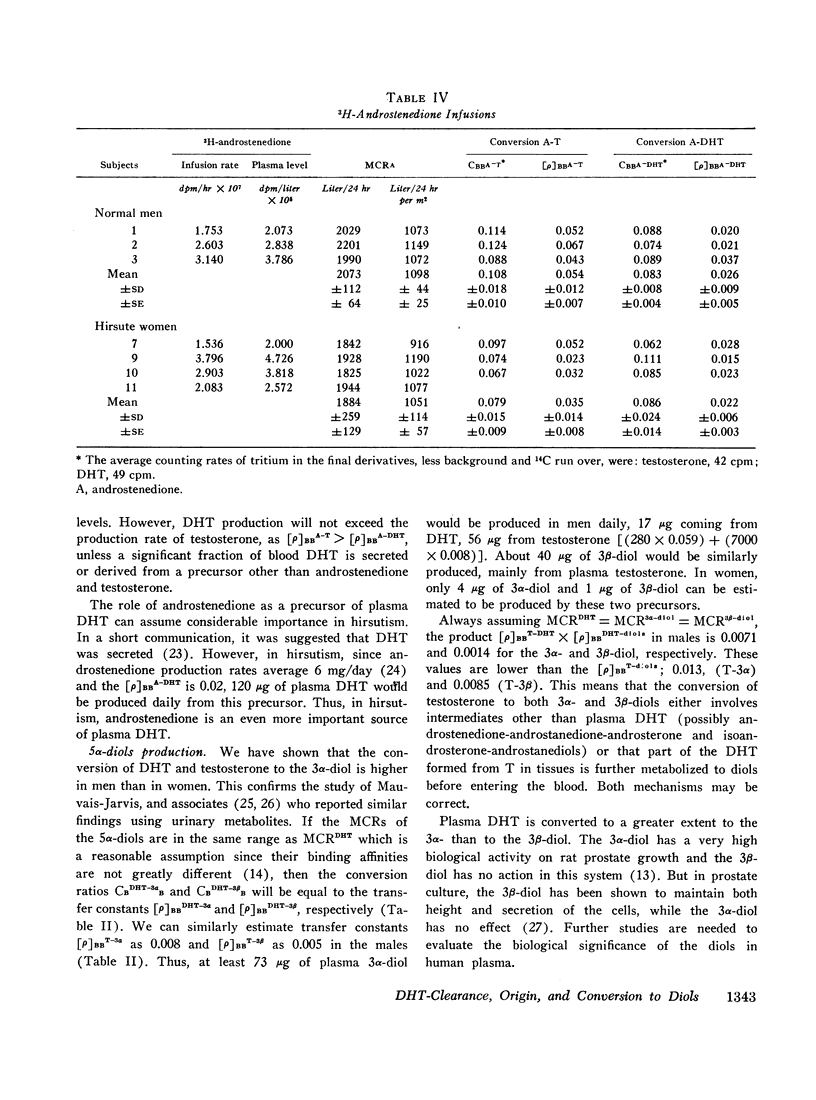
The conversion of plasma testosterone and androstenedione to dihydrotestosterone was studied in men and hirsute women. Approximately 4 and 2% of plasma testosterone and androstenedione, respectively, were converted to plasma dihydrotestosterone in both groups. From these observations it was determined that a major fraction of plasma dihydrotestosterone was derived from these plasma precursors rather than from glandular secretion.
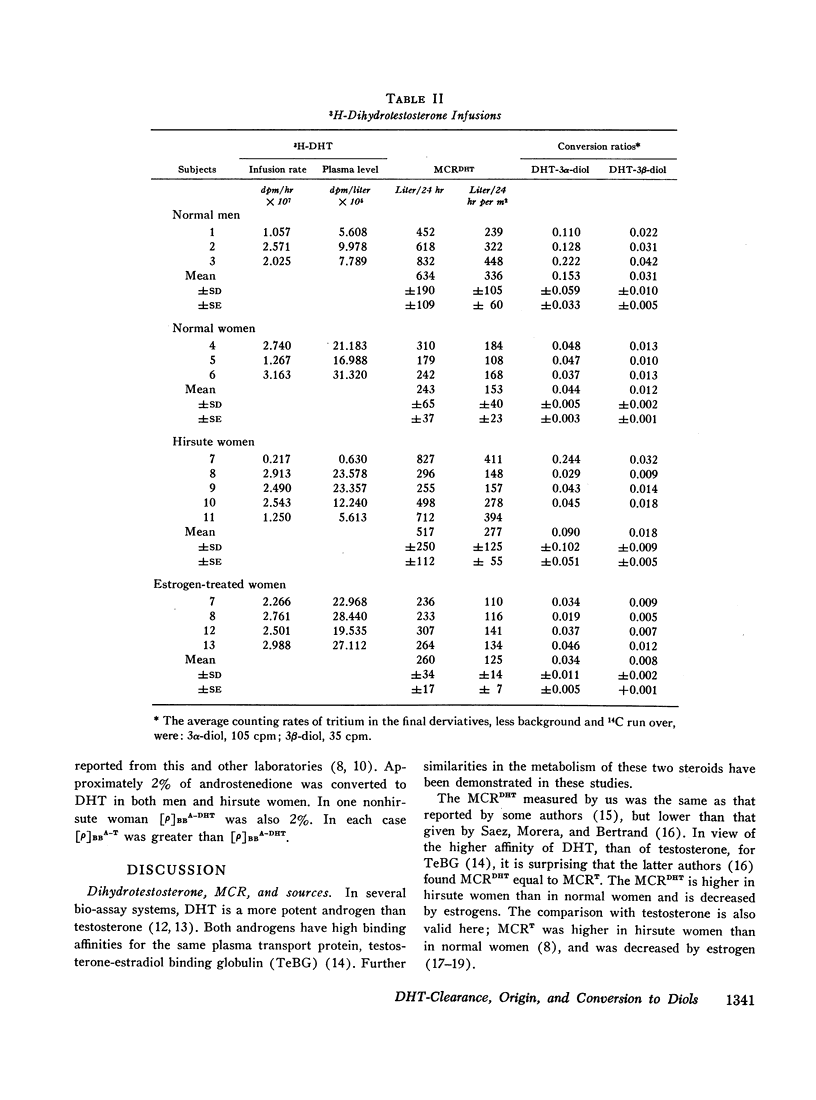
Both 5α-androstan-3α,17β-diol (3α-diol) and 5α-androstan-3β,17β-diol (3β-diol) were identified in plasma during dihydrotestosterone and testosterone infusions. The conversion ratio of dihydrotestosterone to 3α-diol (CBBDHT-3α) was greater than the conversion ratio to the 3β-isomer (CBBDTH-3β) in all the patients studied. Both CBBDHT-3α and CBBDHT-3β were higher in men (mean values of 0.151 [range, 0.110-0.222] and 0..031 [range, 0.022-0.042]) than in women (means of 0.044 [range, 0.037-0.048] and 0.012 [range 0.010-0.013]). A smaller fraction of testosterone was converted to 3α-diol and 3β-diol.
Full text
PDF

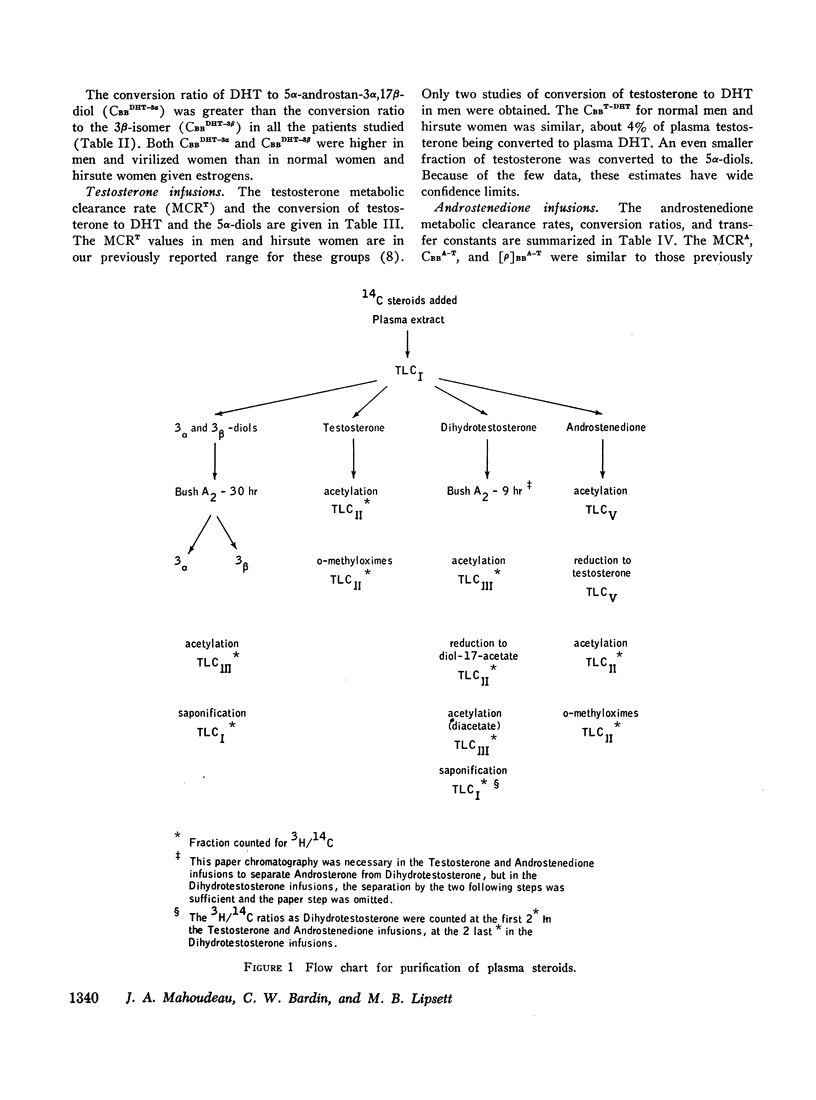

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardin C. W., Lipsett M. B. Testosterone and androstenedione blood production rates in normal women and women with idiopathic hirsutism or polycystic ovaries. J Clin Invest. 1967 May;46(5):891–902. doi: 10.1172/JCI105588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu E. E., Lasnizki I., Robel P. Metabolism of testosterone and action of metabolites on prostate glands grown in organ culture. Nature. 1968 Sep 14;219(5159):1155–1156. doi: 10.1038/2191155a0. [DOI] [PubMed] [Google Scholar]
- Bird C. E., Green R. N., Clark A. F. Effect of the administration of estrogen on the disappearance of 3H-testosterone in the plasma of human subjects. J Clin Endocrinol Metab. 1969 Jan;29(1):123–126. doi: 10.1210/jcem-29-1-123. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J. D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968 Apr 25;243(8):2012–2021. [PubMed] [Google Scholar]
- Chamberlain J., Jagarinec N., Ofner P. Catabolism of [4-14C]testosterone by subcellular fractions of human prostate. Biochem J. 1966 Jun;99(3):610–616. doi: 10.1042/bj0990610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARNSWORTH W. E., BROWN J. R. TESTOSTERONE METABOLISM IN THE PROSTATE. Natl Cancer Inst Monogr. 1963 Oct;12:323–329. [PubMed] [Google Scholar]
- Horton R., Tait J. F. Androstenedione production and interconversion rates measured in peripheral blood and studies on the possible site of its conversion to testosterone. J Clin Invest. 1966 Mar;45(3):301–313. doi: 10.1172/JCI105344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe R. B. Testosterone metabolism in target tissues. Hypothalamic and pituitary tissues of the adult rat and human fetus, and the immature rat epiphysis. Steroids. 1969 Nov;14(5):483–498. doi: 10.1016/s0039-128x(69)80043-7. [DOI] [PubMed] [Google Scholar]
- Kato T., Horton R. Studies of testosterone binding globulin. J Clin Endocrinol Metab. 1968 Aug;28(8):1160–1168. doi: 10.1210/jcem-28-8-1160. [DOI] [PubMed] [Google Scholar]
- Kirschner M. A., Bardin C. W., Hembree W. C., Ross G. T. Effect of estrogen administration on androgen production and plasma luteinizing hormone in hirsute women. J Clin Endocrinol Metab. 1970 Jun;30(6):727–732. doi: 10.1210/jcem-30-6-727. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis P., Floch H. H., Bercovici J. P. Studies on testosterone metabolism in human subjects with normal and pathological sexual differentiation. J Clin Endocrinol Metab. 1968 Apr;28(4):460–471. doi: 10.1210/jcem-28-4-460. [DOI] [PubMed] [Google Scholar]
- Migeon C. J., Rivarola M. A., Forest M. G. Studies of androgens in transsexual subjects. Effects of estrogen therapy. Johns Hopkins Med J. 1968 Sep;123(3):128–133. [PubMed] [Google Scholar]
- Southren A. L., Gordon G. G., Tochimoto S. Further study of factors affecting the metabolic clearance rate of testosterone in man. J Clin Endocrinol Metab. 1968 Aug;28(8):1105–1112. doi: 10.1210/jcem-28-8-1105. [DOI] [PubMed] [Google Scholar]
- Stern J. M., Eisenfeld A. J. Androgen accumulation andbinding to macromolecules in seminal vesicles: inhibition cyproterone. Science. 1969 Oct 10;166(3902):233–235. doi: 10.1126/science.166.3902.233. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Walker J. D. The conversion of testosterone to 5 alpha-androstan-17 beta-ol-3-one (dihydrotestosterone) by skin slices of man. J Clin Invest. 1969 Feb;48(2):371–379. doi: 10.1172/JCI105994. [DOI] [PMC free article] [PubMed] [Google Scholar]


