Abstract
Glucose metabolism and insulin sensitivity of isolated rat epididymal fat cells and of their delipidated derivatives (“ghosts”) was studied as a function of cellular lipid content (fat cell size), cellular protein content, animal age, and state of nutrition in an effort to examine the relationship of adipose cell size to adipose tissue insulin sensitivity.
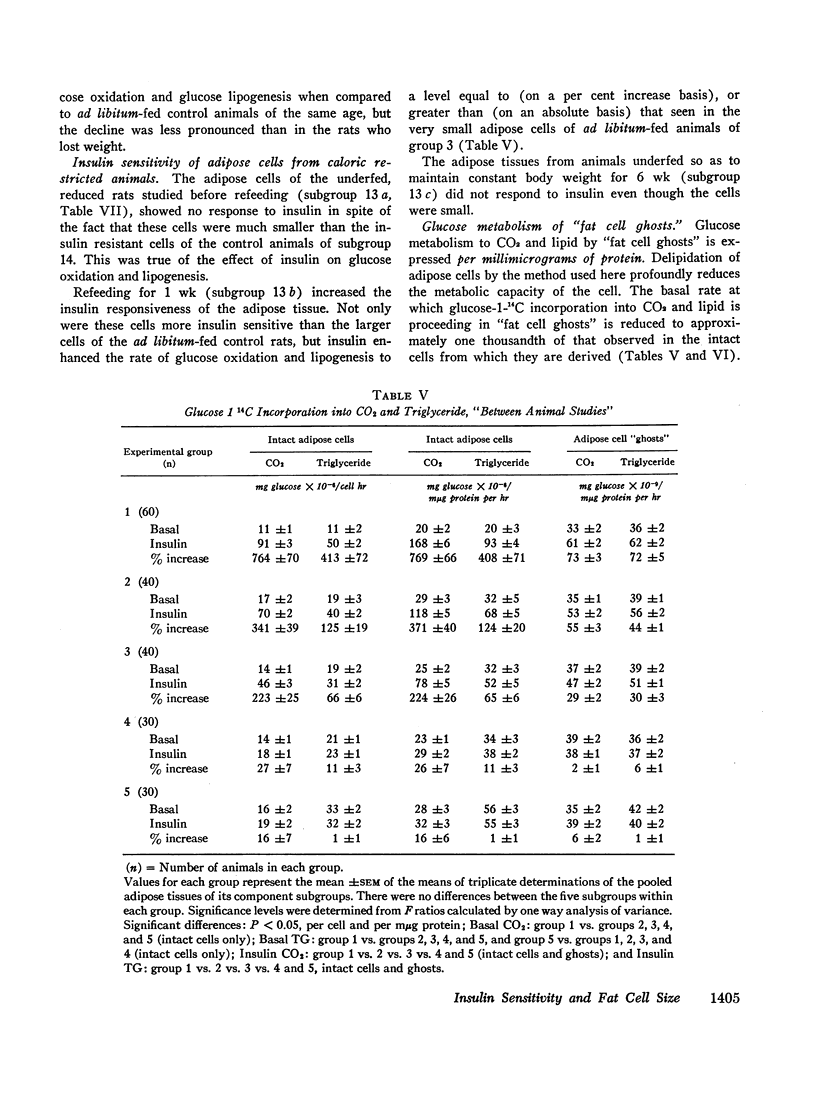
In ad libitum-fed rats, basal rates of glucose-1-14C incorporation into CO2 and triglyceride are similar over a wide range of adipose cell size. In contrast, the insulin sensitivity of intact fat cells from rats fed ad libitum is inversely related to their lipid content: the larger the cell, the less the response to insulin. This “resistance” of the enlarged adipose cell to the action of insulin was demonstrated by a reduction in the per cent rise above the basal rate as well as in the absolute rate of glucose oxidation and lipogenesis caused by insulin.
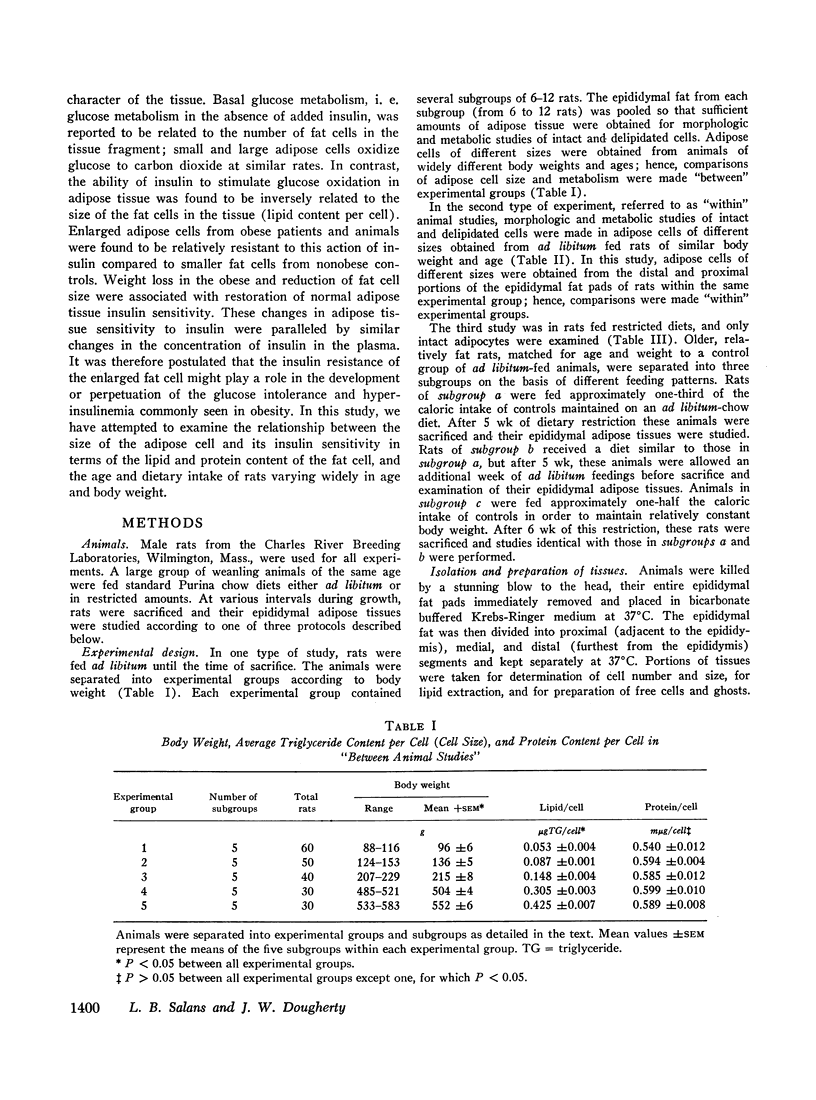
The protein content of fat cells was found to be relatively constant over a wide range of fat cell size. Thus, enlarged insulin “resistant” fat cells contained the same amount of protein as smaller insulin “sensitive” cells.
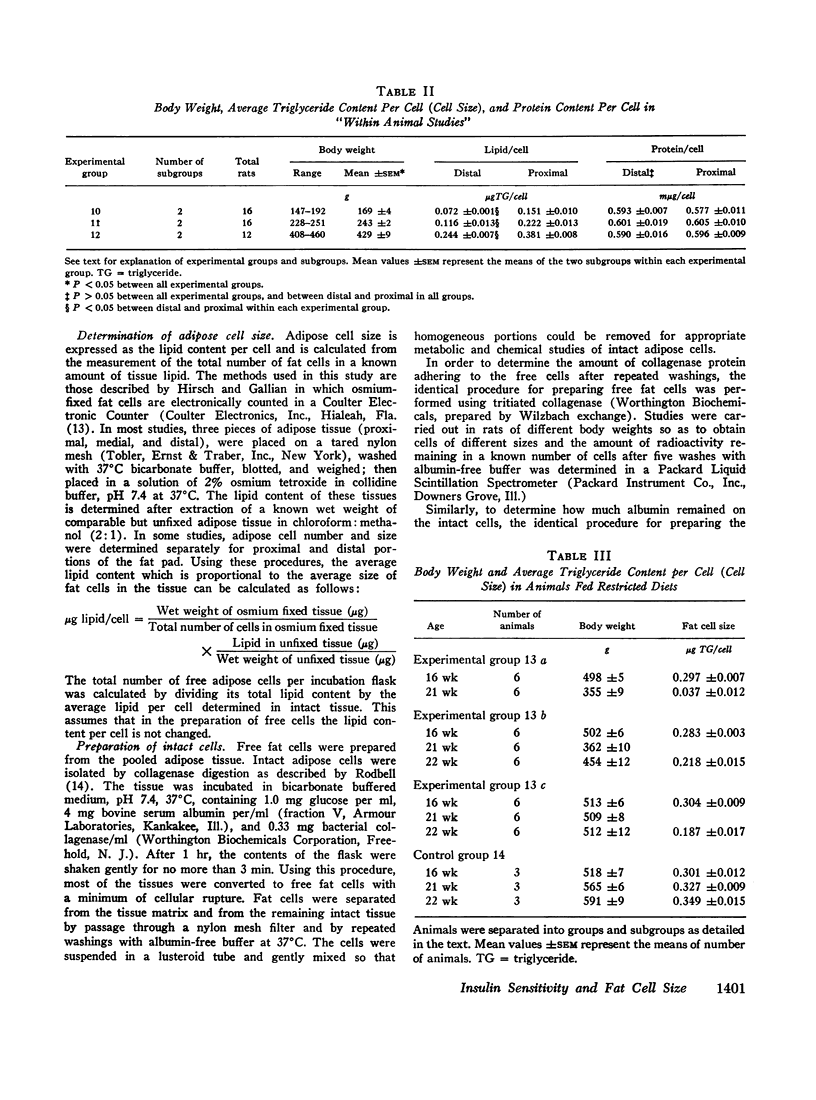
These relationships between insulin sensitivity and cellular lipid or protein content were true regardless of whether cells of different sizes were obtained from animals of different body weights and ages, or from different portions of the epididymal fat pads of animals of the same weight and age.
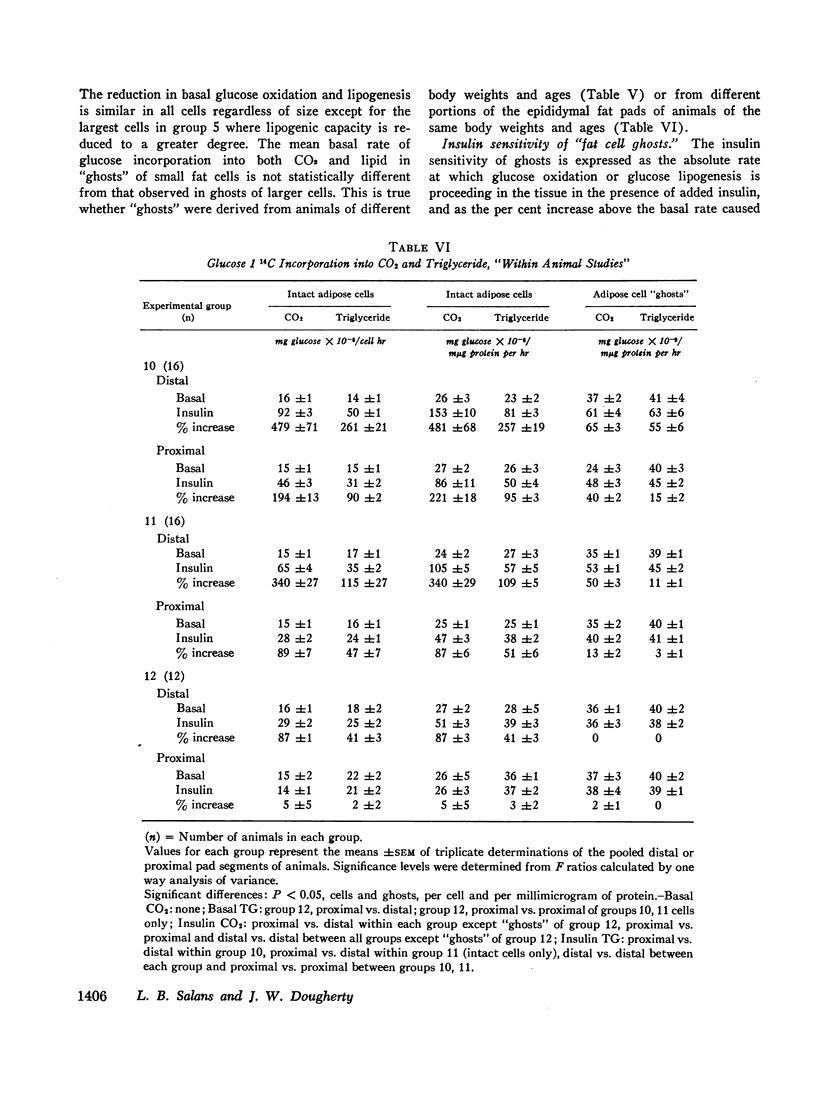
Acute delipidation of intact fat cells did not appear to alter these relationships between basal glucose metabolism, insulin sensitivity, and cell size. “Ghosts” prepared from fat cells of widely different sizes metabolized glucose to CO2 and triglyceride at similar rates. The insulin sensitivity of the fat cell “ghost” appeared to be inversely related to the size of the intact cell from which it was derived: the larger the intact cell the less insulin sensitive its “ghost.”
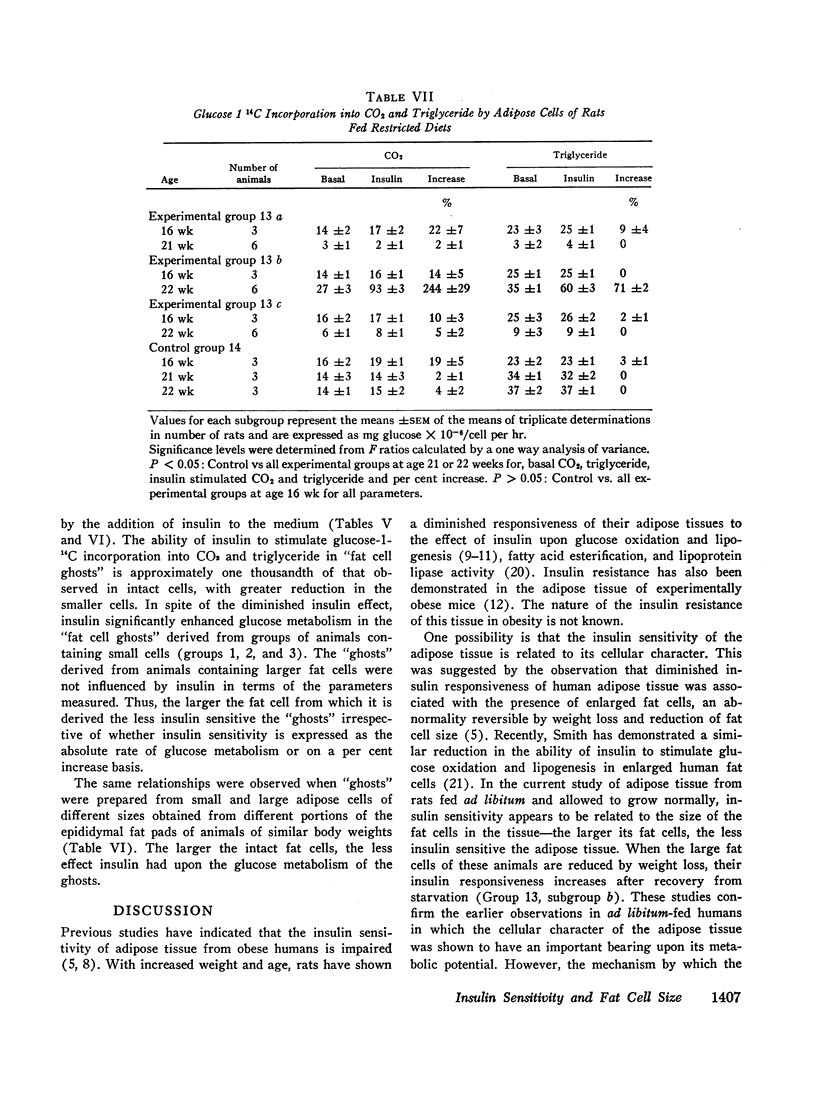
Although the insulin “resistance” of adipose tissue was reversed by weight loss and reduction of fat cell size, these studies also demonstrate that the insulin sensitivity of adipose cells of similar sizes can vary widely depending upon the state of nutrition and growth of the animal. Thus, factors other than cell size can also influence the insulin sensitivity of the adipose tissue.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdade J. D., Bierman E. L., Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967 Oct;46(10):1549–1557. doi: 10.1172/JCI105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G. A. Effect of diet and triiodothyronine on the activity of sn-glycerol-3-phosphate dehydrogenase and on the metabolism of glucose and pyruvate by adipose tissue of obese patients. J Clin Invest. 1969 Aug;48(8):1413–1422. doi: 10.1172/JCI106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Rizack M. A. Structure-function relationships in the adipose cell. 3. Effects of bovine serum albumin on the metabolism of glucose and the release of nonesterified fatty acids and glycerol by the isolated adipose cell. J Cell Biol. 1970 Aug;46(2):354–361. doi: 10.1083/jcb.46.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo M., Rudman D. Variations in glucose metabolism and sensitivity to insulin of the rat's adipose tissue, in relation to age and body weight. Endocrinology. 1968 Jun;82(6):1133–1141. doi: 10.1210/endo-82-6-1133. [DOI] [PubMed] [Google Scholar]
- Gliemann J. Insulin-like activity of dilute human serum assayed by an isolated adipose cell method. Diabetes. 1965 Oct;14(10):643–649. doi: 10.2337/diab.14.10.643. [DOI] [PubMed] [Google Scholar]
- Greenwood M. R., Johnson P. R., Hirsch J. Relationship of age and cellularity to metabolic activity in C57B mice. Proc Soc Exp Biol Med. 1970 Mar;133(3):944–947. doi: 10.3181/00379727-133-34600. [DOI] [PubMed] [Google Scholar]
- Grey N. J., Goldring S., Kipnis D. M. The effect of fasting, diet, and actinomycin D on insulin secretion in the rat. J Clin Invest. 1970 May;49(5):881–889. doi: 10.1172/JCI106307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gries F. A., Steinke J. Comparative effects of insulin on adipose tissue segments and isolated fat cells of rat and man. J Clin Invest. 1967 Sep;46(9):1413–1421. doi: 10.1172/JCI105633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBER A. M., GERSHOFF S. N., ANTONIADES H. N. THE EFFECT OF FASTING AND REFEEDING ON ISOLATED RAT ADIPOSE TISSUE ACTIVITY IN THE PRESENCE OF CRYSTALLINE AND "BOUND" INSULIN. Metabolism. 1965 May;14:619–624. doi: 10.1016/s0026-0495(65)80024-5. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- Hirsch J., Han P. W. Cellularity of rat adipose tissue: effects of growth, starvation, and obesity. J Lipid Res. 1969 Jan;10(1):77–82. [PubMed] [Google Scholar]
- KARAM J. H., GRODSKY G. M., FORSHAM P. H. Excessive insulin response to glucose in obese subjects as measured by immunochemical assay. Diabetes. 1963 May-Jun;12:197–204. doi: 10.2337/diab.12.3.197. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nestel P. J., Austin W., Foxman C. Lipoprotn lipase content and triglyceride fatty acid uptake in adipose tissue of rats of differing body weights. J Lipid Res. 1969 Jul;10(4):383–387. [PubMed] [Google Scholar]
- Nestel P. J., Whyte H. M. Plasma free fatty acid and triglyceride turnover in obesity. Metabolism. 1968 Dec;17(12):1122–1128. doi: 10.1016/0026-0495(68)90092-9. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ D., ZIERLER K. L. Forearm metabolism in obesity and its response to intra-arterial insulin. Characterization of insulin resistance and evidence for adaptive hyperinsulinism. J Clin Invest. 1962 Dec;41:2173–2181. doi: 10.1172/JCI104676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPPORT M. M., ALONZO N. Photometric determination of fatty acid ester groups in phospholipides. J Biol Chem. 1955 Nov;217(1):193–198. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rabinowitz D. Some endocrine and metabolic aspects of obesity. Annu Rev Med. 1970;21:241–258. doi: 10.1146/annurev.me.21.020170.001325. [DOI] [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. V. Preparation of "ghosts" and their properties; adenyl cyclase and other enzymes. J Biol Chem. 1967 Dec 25;242(24):5744–5750. [PubMed] [Google Scholar]
- Salans L. B., Knittle J. L., Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest. 1968 Jan;47(1):153–165. doi: 10.1172/JCI105705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salans L. B., Reaven G. M. Effect of insulin pretreatment on glucose and lipid metabolism of liver slices from normal rats. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1208–1213. doi: 10.3181/00379727-122-31362. [DOI] [PubMed] [Google Scholar]
- Smith U. Effect of cell size on lipid synthesis by human adipose tissue in vitro. J Lipid Res. 1971 Jan;12(1):65–70. [PubMed] [Google Scholar]