Abstract
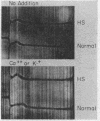
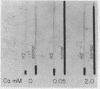
We present evidence that the hereditable hemolytic disease, hereditary spherocytosis (HS), involves an abnormality in protein of the red cell membrane. Unlike that from normal red cells, lipid-free proteins extracted from HS red cell membranes fail to increase in sedimentation rate when treated with cations; such treatment of normal membrane proteins has been shown by others to cause the formation of microfilaments. That microfilament formation might be defective in HS red cell membranes is supported by observations with vinblastine. This compound, a potent precipitant of filamentous, structure proteins throughout phylogeny, precipitates significantly less HS membrane protein than normal. The resistance of HS membrane protein to changes in conformation by cations is observable at the cellular level as well. That is, both normal and HS red cells agglutinate after repeated washing and suspension in electrolyte-free media. Tiny concentrations of Ca++ (5 × 10-5 M) changes the surfaces of normal cells in such a way as to cause disagglutination; HS red cells resist this change and remain agglutinated unless Ca++ concentrations are increased many-fold.
We conclude that membrane (“structure”) proteins of HS red cells are genetically altered in such a way as to interfere with their proper conformation, perhaps into fibrils. Potentially many mutations in membrane proteins might preclude this alignment, with the result that normal erythrocyte biconcavity and plasticity is prevented and the clinical syndrome of hereditary spherocytosis is manifest.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Borisy G. G., Shelanski M. L., Weisenberg R. C., Taylor E. W. Cytoplasmic filaments and tubules. Fed Proc. 1968 Sep-Oct;27(5):1186–1193. [PubMed] [Google Scholar]
- BERTLES J. F. Sodium transport across the surface membrane of red blood cells in hereditary spherocytosis. J Clin Invest. 1957 Jun;36(6 Pt 1):816–824. doi: 10.1172/JCI103487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. G. Red cell aldolase deficiency in hereditary spherocytosis. Br J Haematol. 1969 Jan-Feb;16(1):145–156. doi: 10.1111/j.1365-2141.1969.tb00386.x. [DOI] [PubMed] [Google Scholar]
- Dutton G. R., Barondes S. Microtubular protein: synthesis and metabolism in developing brain. Science. 1969 Dec 26;166(3913):1637–1638. doi: 10.1126/science.166.3913.1637. [DOI] [PubMed] [Google Scholar]
- Gershman L. C., Dreizen P. Relationship of structure to function in myosin. I. Subunit dissociation in concentrated salt solutions. Biochemistry. 1970 Apr 14;9(8):1677–1687. doi: 10.1021/bi00810a005. [DOI] [PubMed] [Google Scholar]
- HUGGINS C. E. Reversible agglomeraton used to remove dimethylsulfoxide from large volumes of frozen blood. Science. 1963 Feb 8;139(3554):504–505. doi: 10.1126/science.139.3554.504. [DOI] [PubMed] [Google Scholar]
- Hanes T. E., Patterson J., van Eys J. Red cell aldolase and other enzyme activities in hereditary spherocytosis. J Lab Clin Med. 1970 Apr;75(4):654–658. [PubMed] [Google Scholar]
- Hatano S., Tazawa M. Isolation, purification and characterization of byosin B from myxomycete plasmodium. Biochim Biophys Acta. 1968 Apr 9;154(3):507–519. doi: 10.1016/0005-2795(68)90011-1. [DOI] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. I. Mechanism of hemolysis. J Clin Invest. 1962 Apr;41:779–792. doi: 10.1172/JCI104536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. II. Studies in vivo. J Clin Invest. 1962 Jul;41:1514–1523. doi: 10.1172/JCI104607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. INCREASED CELL MEMBRANE PERMEABILITY IN THE PATHOGENESIS OF HEREDITARY SPHEROCYTOSIS. J Clin Invest. 1964 Aug;43:1704–1720. doi: 10.1172/JCI105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S., Karnovsky M. L. Concomitant alterations of sodium flux and membrane phospholipid metabolism in red blood cells: studies in hereditary spherocytosis. J Clin Invest. 1967 Feb;46(2):173–185. doi: 10.1172/JCI105520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S. Membrane lipid depletion in hyperpermeable red blood cells: its role in the genesis of spherocytes in hereditary spherocytosis. J Clin Invest. 1967 Dec;46(12):2083–2094. doi: 10.1172/JCI105695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S. The defective red blood cell in hereditary spherocytosis. Annu Rev Med. 1969;20:41–46. doi: 10.1146/annurev.me.20.020169.000353. [DOI] [PubMed] [Google Scholar]
- Limber G. K., Davis R. F., Bakerman S. Acrylamide gel electrophoresis studies of human erythrocyte membrane. Blood. 1970 Jul;36(1):111–118. [PubMed] [Google Scholar]
- Marantz R., Ventilla M., Shelanski M. Vinblastine-induced precipitation of microtubule protein. Science. 1969 Aug 1;165(3892):498–499. doi: 10.1126/science.165.3892.498. [DOI] [PubMed] [Google Scholar]
- Marchesi S. L., Steers E., Marchesi V. T., Tillack T. W. Physical and chemical properties of a protein isolated from red cell membranes. Biochemistry. 1970 Jan 6;9(1):50–57. doi: 10.1021/bi00803a007. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968 Jan 12;159(3811):203–204. doi: 10.1126/science.159.3811.203. [DOI] [PubMed] [Google Scholar]
- Mazia D., Ruby A. Dissolution of erythrocyte membranes in water and comparison of the membrane protein with other structural proteins. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1005–1012. doi: 10.1073/pnas.61.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puszkin S., Berl S. Actin-like properties of colchicine binding protein isolated from brain. Nature. 1970 Feb 7;225(5232):558–559. doi: 10.1038/225558a0. [DOI] [PubMed] [Google Scholar]
- SCHIFFMAN G., KABAT E. A., THOMPSON W. IMMUNOCHEMICAL STUDIES ON BLOOD GROUPS. XXX. CLEAVAGE OF A, B, AND H BLOOD-GROUP SUBSTANCES BY ALKALI. Biochemistry. 1964 Jan;3:113–120. doi: 10.1021/bi00889a018. [DOI] [PubMed] [Google Scholar]
- Salsbury A. J., Clarke J. A. New method for detecting changes in the surface appearance of human red blood cells. J Clin Pathol. 1967 Jul;20(4):603–610. doi: 10.1136/jcp.20.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitel P., Marcu I., Xenakis A. Erythrocyte microrheology: its dependence on the reduced sulfhydryl groups and hemoglobin integrity. Folia Haematol Int Mag Klin Morphol Blutforsch. 1968;90(2):281–295. [PubMed] [Google Scholar]
- Weed R. I., LaCelle P. L., Merrill E. W. Metabolic dependence of red cell deformability. J Clin Invest. 1969 May;48(5):795–809. doi: 10.1172/JCI106038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I., Reed C. F. Membrane alterations leading to red cell destruction. Am J Med. 1966 Nov;41(5):681–698. doi: 10.1016/0002-9343(66)90030-1. [DOI] [PubMed] [Google Scholar]
- White J. G., Krivit W. An ultrastructural basis for the shape changes induced in platelets by chilling. Blood. 1967 Nov;30(5):625–635. [PubMed] [Google Scholar]
- Wilson L., Bryan J., Ruby A., Mazia D. Precipitation of proteins by vinblastine and calcium ions. Proc Natl Acad Sci U S A. 1970 Jul;66(3):807–814. doi: 10.1073/pnas.66.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zail S. S., Joubert S. M. Starch gel electrophoresis of erythrocyte membranes in hereditary spherocytosis. Br J Haematol. 1968 Jan;14(1):57–60. doi: 10.1111/j.1365-2141.1968.tb01472.x. [DOI] [PubMed] [Google Scholar]