Abstract
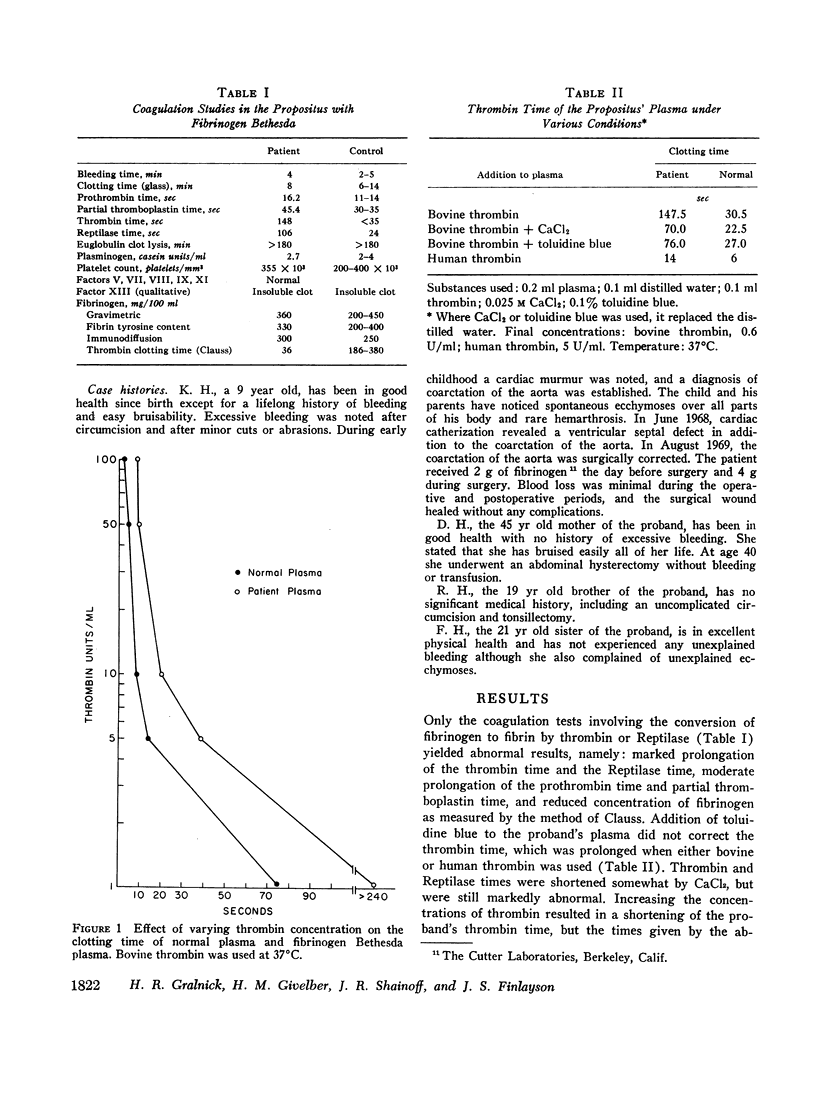
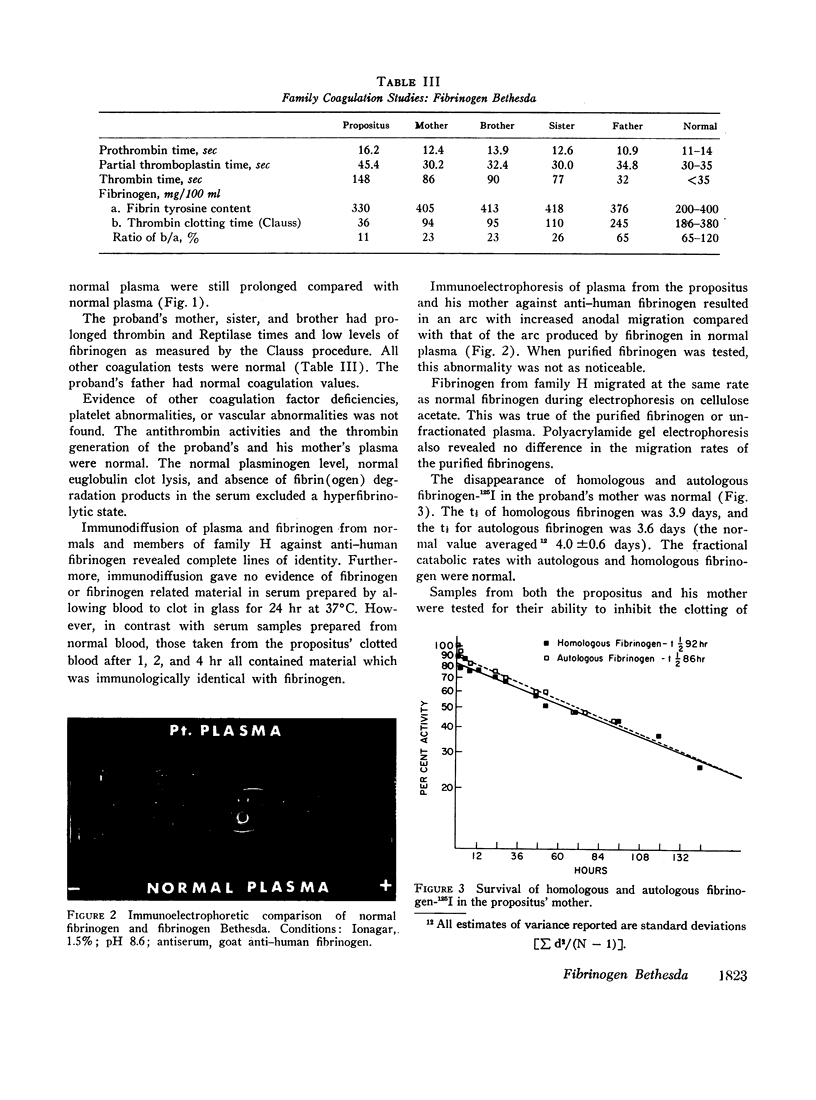
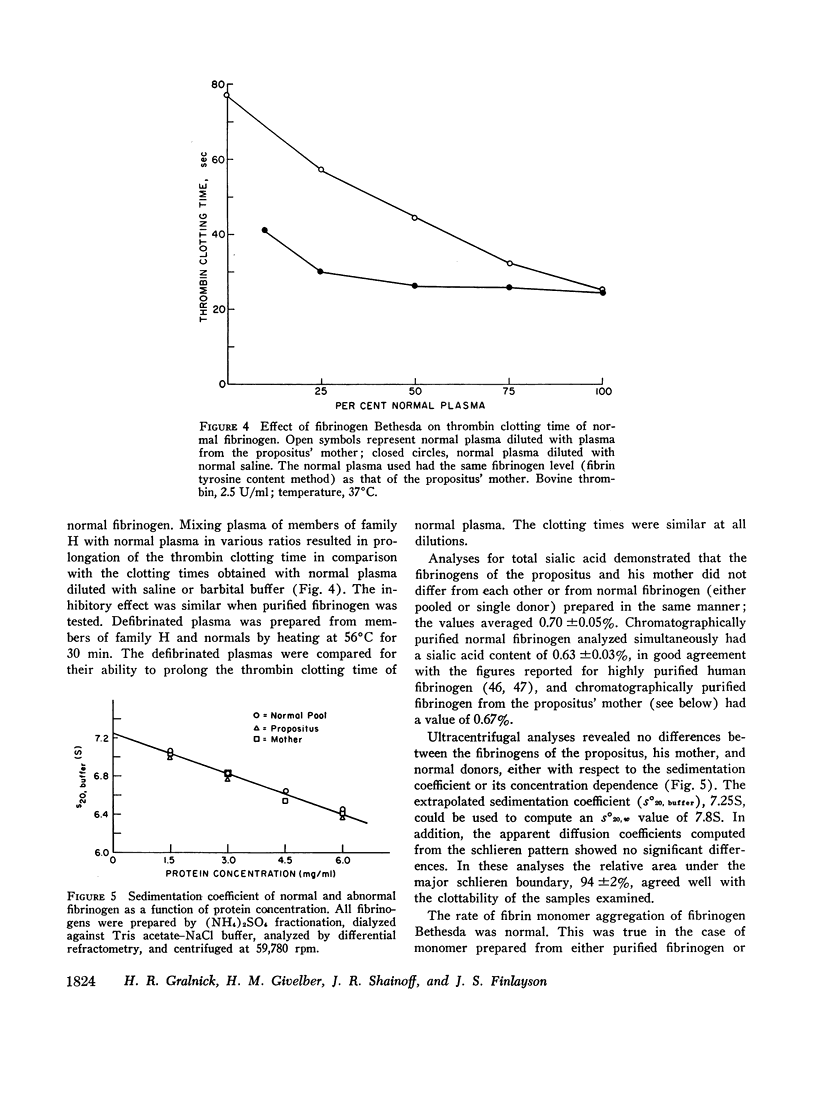
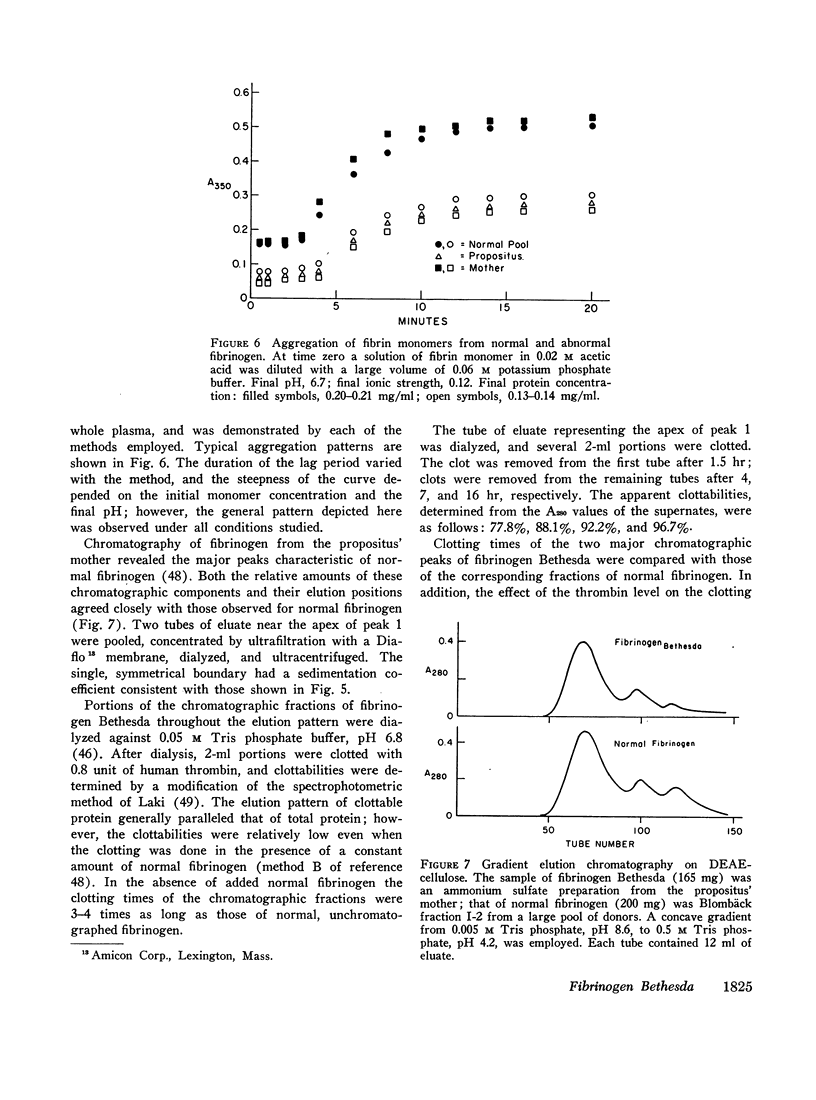
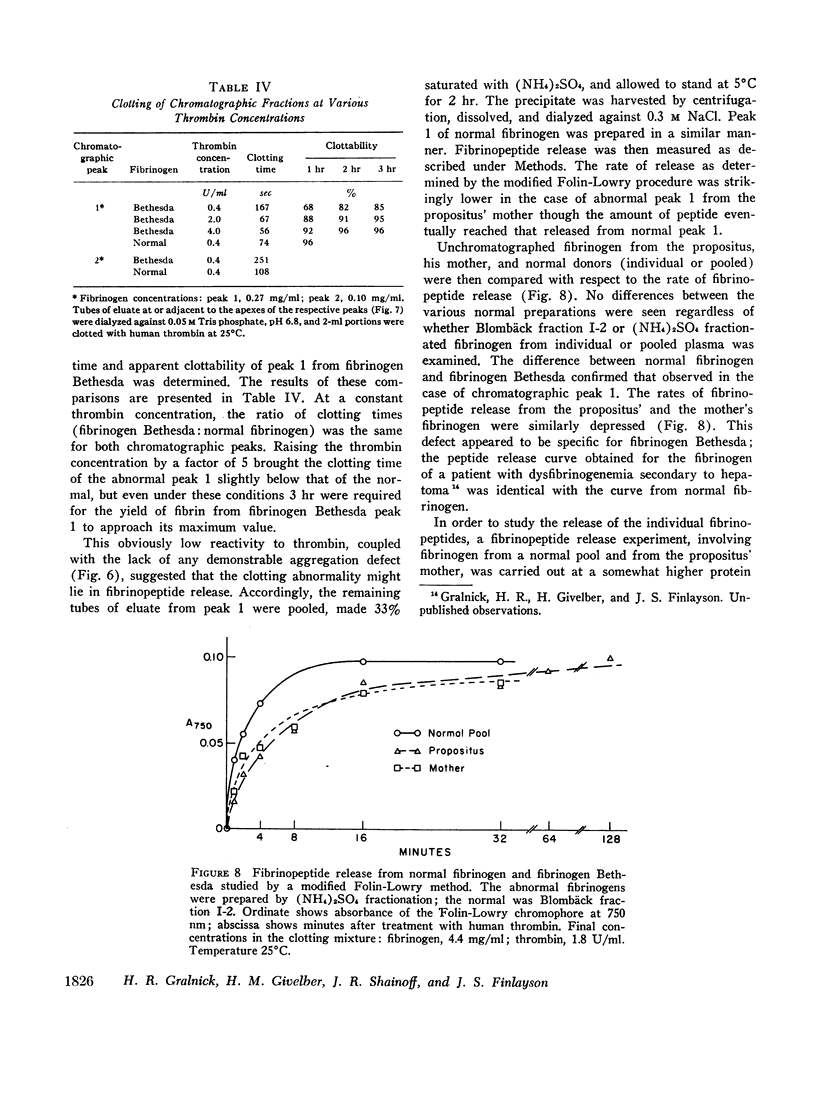
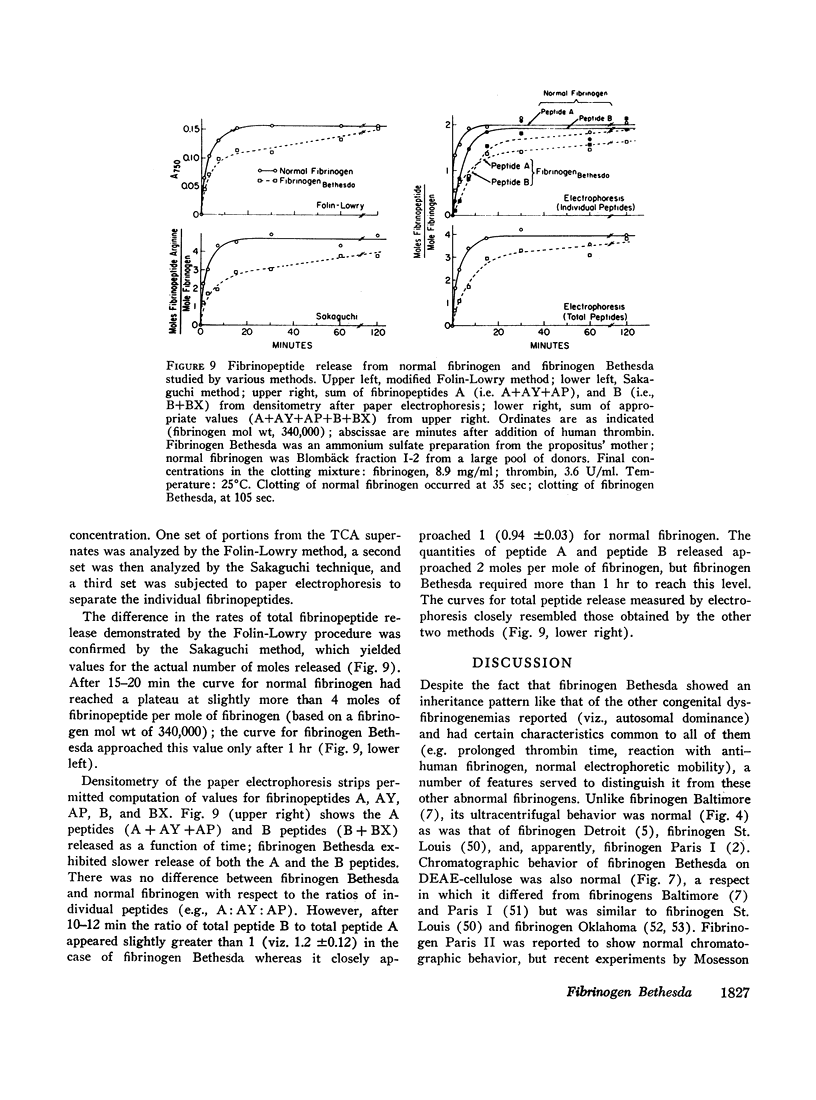
A dysfibrinogenemia (fibrinogen Bethesda) was detected in a 9 yr old male of Mexican-English extraction who had a lifelong history of mild bleeding diathesis. The prothrombin and partial thromboplastin times were moderately prolonged; the thrombin and Reptilase times were markedly prolonged. The plasma fibrinogen level was normal by conventional methods but was markedly reduced by the Clauss method. Results of all other tests for clotting factors, fibrinolysis, antithrombin levels, clot stabilization, and fibrin(ogen) degradation products were normal. The patient's plasma and fibrinogen inhibited the clotting of normal plasma or fibrinogen by thrombin. Family studies revealed that the propositus' mother and two siblings exhibited these abnormalities to a lesser degree and indicated an autosomal dominant inheritance. Fibrinogen Bethesda was similar to normal fibrinogen in the following respects: metabolic turnover time (measured in the propositus' mother); immunodiffusion, ultracentrifugal, electrophoretic (on cellulose acetate or polyacrylamide gel), and chromatographic (on DEAE-cellulose) characteristics; sialic acid content; and aggregation of fibrin monomers. By contrast, fibrinogen Bethesda gave an abnormal immunoelectrophoretic pattern especially when whole plasma (as opposed to purified fibrinogen) was examined, and it showed a pronounced decrease in the rate of fibrinopeptide release by thrombin. This decrease, which was shown to involve both fibrinopeptides A and B, distinguishes fibrinogen Bethesda from previously reported dysfibrinogenemias.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALKJAERSIG N., FLETCHER A. P., SHERRY S. The mechanism of clot dissolution by plasmin. J Clin Invest. 1959 Jul;38(7):1086–1095. doi: 10.1172/JCI103885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E. A., Charache P., Jackson D. P. A new inherited coagulation disorder caused by an abnormal fibrinogen ('fibrinogen Baltimore'). Nature. 1965 Oct 9;208(5006):143–145. doi: 10.1038/208143a0. [DOI] [PubMed] [Google Scholar]
- Belitser V. A., Varetskaja T. V., Malneva G. V. Fibrinogen-fibrin interaction. Biochim Biophys Acta. 1968 Feb 19;154(2):367–375. doi: 10.1016/0005-2795(68)90051-2. [DOI] [PubMed] [Google Scholar]
- Blombäck M., Blombäck B., Mammen E. F., Prasad A. S. Fibrinogen Detroit--a molecular defect in the N-terminal disulphide knot of human fibrinogen? Nature. 1968 Apr 13;218(5137):134–137. doi: 10.1038/218134a0. [DOI] [PubMed] [Google Scholar]
- Bull B. S., Schneiderman M. A., Brecher G. Platelet counts with the Coulter counter. Am J Clin Pathol. 1965 Dec;44(6):678–688. doi: 10.1093/ajcp/44.6.678. [DOI] [PubMed] [Google Scholar]
- CLAUSS A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957 Apr;17(4):237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Egeberg O. Inherited fibrinogen abnormality causing thrombophilia. Thromb Diath Haemorrh. 1967 Feb 28;17(1-2):176–187. [PubMed] [Google Scholar]
- FEINBERG J. G. A new quantitative method for antigen-antibody titrations in gels. Nature. 1956 Mar 17;177(4507):530–531. doi: 10.1038/177530a0. [DOI] [PubMed] [Google Scholar]
- FINLAYSON J. S., SUCHINSKY R. T., DAYTON A. L. Effects of long-term storage on human serum albumin. I. Chromatographic and ultracentrifugal aspects. J Clin Invest. 1960 Dec;39:1837–1840. doi: 10.1172/JCI104207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman W. B., Ratnoff O. D., Boyer M. H. An inherited qualitative abnormality in plasma fibrinogen: fibrinogen Cleveland. J Lab Clin Med. 1968 Sep;72(3):455–472. [PubMed] [Google Scholar]
- GRABAR P., WILLIAMS C. A. Méthode permettant l'étude conjuguée des proprietés électrophorétiques et immunochimiques d'un mélange de protéines; application au sérum sanguin. Biochim Biophys Acta. 1953 Jan;10(1):193–194. doi: 10.1016/0006-3002(53)90233-9. [DOI] [PubMed] [Google Scholar]
- HASSELBACK R., MARION R. B., THOMAS J. W. Congenital hypofibrinogenemia in five members of a family. Can Med Assoc J. 1963 Jan 5;88:19–22. [PMC free article] [PubMed] [Google Scholar]
- IMPERATO C., DETTORI A. G. Ipofibrinogenemia congenita con fibrinoastenia. Helv Paediatr Acta. 1958 Oct;13(4):380–399. [PubMed] [Google Scholar]
- Israels E. D., Rayner H., Israels L. G., Zipursky A. A microhemagglutination-inhibition assay for the quantitation of fibrinogen breakdown products. J Lab Clin Med. 1968 Feb;71(2):333–337. [PubMed] [Google Scholar]
- Jackson D. P., Beck E. A., Charache P. Congenital disorders of fibrinogen. Fed Proc. 1965 Jul-Aug;24(4):816–821. [PubMed] [Google Scholar]
- LAKI K. The polymerization of proteins; the action of thrombin on fibrinogen. Arch Biochem Biophys. 1951 Jul;32(2):317–324. doi: 10.1016/0003-9861(51)90277-9. [DOI] [PubMed] [Google Scholar]
- LANGDELL R. D., WAGNER R. H., BRINKHOUS K. M. Effect of antihemophilic factor on one-stage clotting tests; a presumptive test for hemophilia and a simple one-stage antihemophilic factor assy procedure. J Lab Clin Med. 1953 Apr;41(4):637–647. [PubMed] [Google Scholar]
- LORAND L. Fibrino-peptide. Biochem J. 1952 Oct;52(2):200–203. doi: 10.1042/bj0520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOSOWSKY M. S., HALL R., GOLDIE W. CONGENITAL DEFICIENCY OF FIBRIN-STABILISING FACTOR; A REPORT OF THREE UNRELATED CASES. Lancet. 1965 Jul 24;2(7404):156–158. doi: 10.1016/s0140-6736(65)90233-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOSESSON M. W., FINLAYSON J. S. SUBFRACTIONS OF HUMAN FIBRINOGEN; PREPARATION AND ANALYSIS. J Lab Clin Med. 1963 Oct;62:663–674. [PubMed] [Google Scholar]
- MOSESSON M. W. The preparation of human fibrinogen free of plasminogen. Biochim Biophys Acta. 1962 Feb 26;57:204–213. doi: 10.1016/0006-3002(62)91112-5. [DOI] [PubMed] [Google Scholar]
- Mammen E. F., Prasad A. S., Barnhart M. I., Au C. C. Congenital dysfibrinogenemia: fibrinogen Detroit. J Clin Invest. 1969 Feb;48(2):235–249. doi: 10.1172/JCI105980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane A. S. IN VIVO BEHAVIOR OF I-FIBRINOGEN. J Clin Invest. 1963 Mar;42(3):346–361. doi: 10.1172/JCI104721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mester L., Szabados L. Différences constitutionnelles et fonctionnelles entre les fragments glucidiques du fibrinogène humain normal et d'un fibrinogène humain anormal. Bull Soc Chim Biol (Paris) 1968;50(12):2561–2566. [PubMed] [Google Scholar]
- Mosesson M. W., Beck E. A. Chromatographic, ultracentrifugal, and related studies of fibrinogen "Baltimore". J Clin Invest. 1969 Sep;48(9):1656–1662. doi: 10.1172/JCI106130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- OWREN P. A., AAS K. The control of dicumarol therapy and the quantitative determination of prothrombin and proconvertin. Scand J Clin Lab Invest. 1951;3(3):201–208. doi: 10.3109/00365515109060600. [DOI] [PubMed] [Google Scholar]
- PEDEN J. C., Jr, McFARLAND J. A. Use of the plasma thrombin time to assess the adequacy of in vivo neutralization of heparin: comparative studies following operations employing extracorporeal circulation. Blood. 1959 Nov;14:1230–1236. [PubMed] [Google Scholar]
- RATNOFF O. D., MENZIE C. A new method for the determination of fibrinogen in small samples of plasma. J Lab Clin Med. 1951 Feb;37(2):316–320. [PubMed] [Google Scholar]
- Radzevich I. M., Khodorova E. L. Oderzhannia fibryn-monomeru z fibrynu nefraktsionovanoi plazmy krovi. Ukr Biokhim Zh. 1969;41(4):367–370. [PubMed] [Google Scholar]
- STEFANINI M. New one-stage procedures for the quantitative determination of prothrombin and labile factor. Am J Clin Pathol. 1950 Mar;20(3):233–240. doi: 10.1093/ajcp/20.3.233. [DOI] [PubMed] [Google Scholar]
- Samama M., Soria J., Soria C., Bousser J. Dysfibrinogénémie congénitale et familiale sans tendance hémorragique. Nouv Rev Fr Hematol. 1969 Nov-Dec;9(6):817–832. [PubMed] [Google Scholar]
- Sasaki T., Page I. H., Shainoff J. R. Stable complex of fibrinogen and fibrin. Science. 1966 May 20;152(3725):1069–1071. doi: 10.1126/science.152.3725.1069. [DOI] [PubMed] [Google Scholar]
- Simone J. V., Vanderheiden J., Abildgaard C. F. A semiautomatic one-stage factor 8 assay with a commercially prepared standard. J Lab Clin Med. 1967 Apr;69(4):706–712. [PubMed] [Google Scholar]
- Von Felten A., Duckert F., Frick P. G. Familial disturbance of fibrin monomer aggregation. Br J Haematol. 1966 Nov;12(6):667–677. doi: 10.1111/j.1365-2141.1966.tb00152.x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- von Felten A., Frick P. G., Straub P. W. Studies on fibrin monomer aggregation in congenital dysfibrinogenaemia (fibrinogen "Zürich"): separation of a pathological from a normal fibrin fraction. Br J Haematol. 1969 Apr;16(4):353–361. doi: 10.1111/j.1365-2141.1969.tb00412.x. [DOI] [PubMed] [Google Scholar]



