Abstract
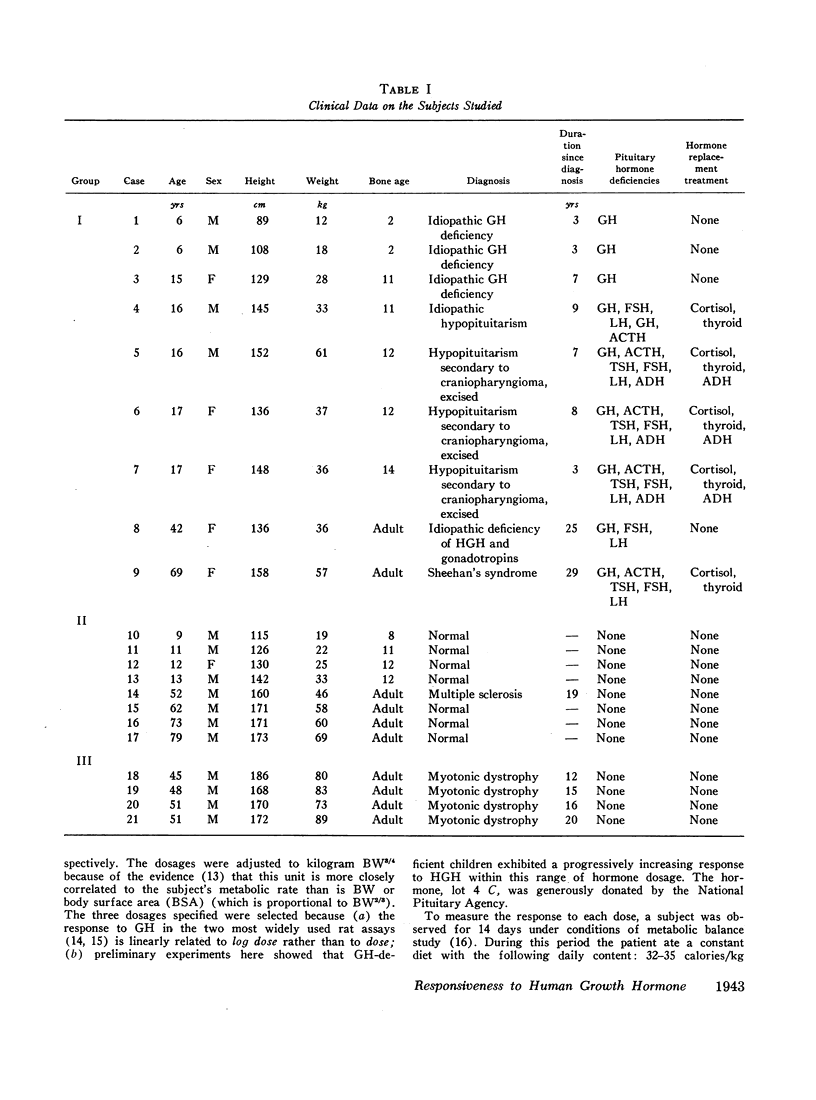
The effect of human growth hormone (HGH) on the N, P, Na, and K balance, and on the body weight (BW) of three groups of subjects was measured. In group I were nine cases (age 6-69) with HGH deficiency; in group II, eight cases (age 9-79) with normal endogenous HGH; in group III, four cases with myotonic dystrophy (age 45-51). After a 7 day control period, the hormone was administered for 7 days. Each subject was tested with three doses of HGH: dose A, 0.0168 U/kg BW3/4 per day; dose B, 0.0532 U/kg BW3/4 per day; dose C, 0.168 U/kg BW3/4 per day.
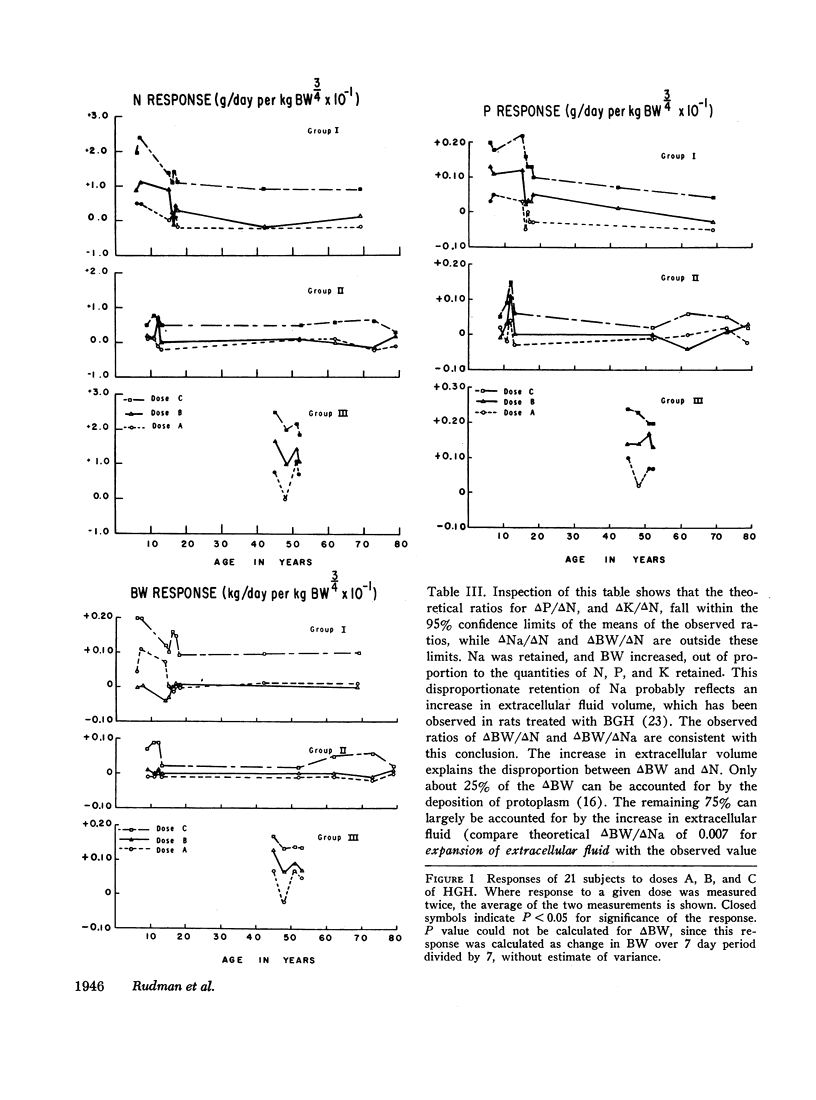
In group I, the responsiveness to HGH declined with age. Two subjects aged 6 yr responded to all three doses of HGH with positive balances in N, P, Na, and K and increases in BW. At ages 15-17, responses were obtained only to doses B and C in three cases, and only to dose C in two cases. Two subjects, aged 42 and 69, responded only to dose C. Not only did the threshold dose increase with age in group I, but the magnitude of the responses declined with age as well.
Patients of group II were less responsive to all doses of HGH than were patients of group I at comparable age. None responded to dose A or dose B. All responded to dose C, but the increments in balances and in BW stimulated by this dose were only one-third to one-half as great as in HGH-deficient subjects of similar age.
Three patients of group III responded to all three doses of HGH, and one responded to doses B and C. The responses were similar in magnitude to those in the 6-yr old HGH-deficient children, and greater than those in all other subjects studied.
These data show that responsiveness to exogenous HGH rises with deficiency of endogenous HGH, and that individuals with myotonic dystrophy are hyperresponsive to exogenous HGH.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATTS A. A., BENNETT L. L., GARCIA J., STEIN J. The effect of growth hormone on muscle potassium and on extracellular fluid. Endocrinology. 1954 Oct;55(4):456–465. doi: 10.1210/endo-55-4-456. [DOI] [PubMed] [Google Scholar]
- Birge C. A., Peake G. T., Mariz I. K., Daughaday W. H. Radioimmunoassayable growth hormone in the rat pituitary gland: effects of age, sex and hormonal state. Endocrinology. 1967 Aug;81(2):195–204. doi: 10.1210/endo-81-2-195. [DOI] [PubMed] [Google Scholar]
- Brown G. A., Stimmler L., Lines J. G. Growth hormone-induced nitrogen retention in children of short stature. Arch Dis Child. 1967 Jun;42(223):239–244. doi: 10.1136/adc.42.223.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAFTS R. C., MEINEKE H. A. The effect of growth hormone on hematopoiesis in hypophysectomized adult female rats. Endocrinology. 1956 Oct;59(4):444–453. doi: 10.1210/endo-59-4-444. [DOI] [PubMed] [Google Scholar]
- FRUHMAN G. J., GERSTNER R., GORDON A. S. Effects of growth hormone upon erythropoiesis in the hypophysectomized rat. Proc Soc Exp Biol Med. 1954 Jan;85(1):93–96. doi: 10.3181/00379727-85-20796. [DOI] [PubMed] [Google Scholar]
- GREENSPAN F. S., LI C. H. Bioassay of hypophyseal growth hormone; the tibia test. Endocrinology. 1949 Nov;45(5):455-63, illust. doi: 10.1210/endo-45-5-455. [DOI] [PubMed] [Google Scholar]
- Goodman H. G., Grumbach M. M., Kaplan S. L. Growth and growth hormone. II. A comparison of isolated growth-hormone deficiency and multiple pituitary-hormone deficiencies in 35 patients with idiopathic hypopituitary dwarfism. N Engl J Med. 1968 Jan 11;278(2):57–68. doi: 10.1056/NEJM196801112780201. [DOI] [PubMed] [Google Scholar]
- HENNEMAN P. H., FORBES A. P., MOLDAWER M., DEMPSEY E. F., CARROLL E. L. Effects of human growth hormone in man. J Clin Invest. 1960 Aug;39:1223–1238. doi: 10.1172/JCI104138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins J. N., Garland J. T., Daughaday W. H. Incorporation of 35S-sulfate into rat cartilage explants in vitro. Effects of aging on responsiveness to stimulation by sulfation factor. Endocrinology. 1970 Oct;87(4):688–692. doi: 10.1210/endo-87-4-688. [DOI] [PubMed] [Google Scholar]
- LILIENFELD A. M., JACOBS M., WILLIS M. A study of the reproducibility of muscle testing and certain other aspects of muscle scoring. Phys Ther Rev. 1954 Jun;34(6):279–289. doi: 10.1093/ptj/34.6.279. [DOI] [PubMed] [Google Scholar]
- Merimee T. J., Hall J., Rabinowitz D., McKusick V. A., Rimoin D. L. An unusual variety of endocrine dwarfism: subresponsiveness to growth hormone in a sexually mature dwarf. Lancet. 1968 Jul 27;2(7561):191–193. doi: 10.1016/s0140-6736(68)92623-8. [DOI] [PubMed] [Google Scholar]
- Merimee T. J., Rabinowitz D., Riggs L., Burgess J. A., Rimoin D. L., McKusick V. A. Plasma growth hormone after arginine infusion. Clinical experiences. N Engl J Med. 1967 Feb 23;276(8):434–439. doi: 10.1056/NEJM196702232760803. [DOI] [PubMed] [Google Scholar]
- Morris H. G., Jorgensen J. R., Elrick H., Goldsmith R. E. Metabolic effects of human growth hormone in corticosteroid-treated children. J Clin Invest. 1968 Mar;47(3):436–451. doi: 10.1172/JCI105740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRADER A., ILLIG R., SZEKY J., WAGNER H. THE EFFECT OF HUMAN GROWTH HORMONE IN HYPOPITUITARY DWARFISM. Arch Dis Child. 1964 Dec;39:535–544. doi: 10.1136/adc.39.208.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlow A. F., Wilhelmi A. E., Reichert L. E., Jr Further studies on the fractionation of human pituitary glands. Endocrinology. 1965 Dec;77(6):1126–1134. doi: 10.1210/endo-77-6-1126. [DOI] [PubMed] [Google Scholar]
- ROTH J., GLICK S. M., YALOW R. S., BERSONSA Hypoglycemia: a potent stimulus to secretion of growth hormone. Science. 1963 May 31;140(3570):987–988. doi: 10.1126/science.140.3570.987. [DOI] [PubMed] [Google Scholar]
- Ray R. D., Evans H. M., Becks H. Effect of the pituitary growth hormone on the epiphyseal disk of the tibia of the rat. Am J Pathol. 1941 Jul;17(4):509–528.5. [PMC free article] [PubMed] [Google Scholar]
- Rimoin D. L., Merimee T. J., Rabinowitz D., Cavalli-Sforza L. L., McKusick V. A. Peripheral subresponsiveness to human growth hormone in the African pygmies. N Engl J Med. 1969 Dec 18;281(25):1383–1388. doi: 10.1056/NEJM196912182812502. [DOI] [PubMed] [Google Scholar]
- Scow R. O., Hagan S. N. Effect of testosterone propionate and growth hormone on growth and chemical composition of muscle and other tissues in hypophysectomized male rats. Endocrinology. 1965 Nov;77(5):852–858. doi: 10.1210/endo-77-5-852. [DOI] [PubMed] [Google Scholar]


