Abstract
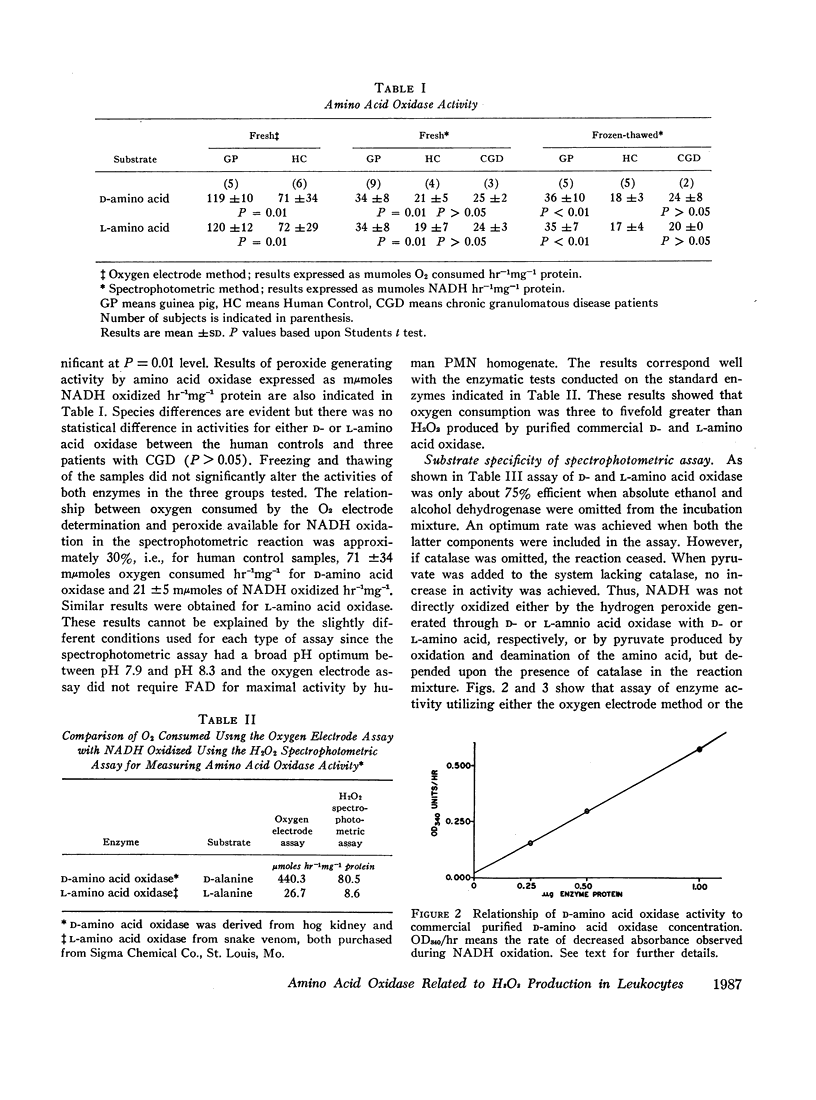
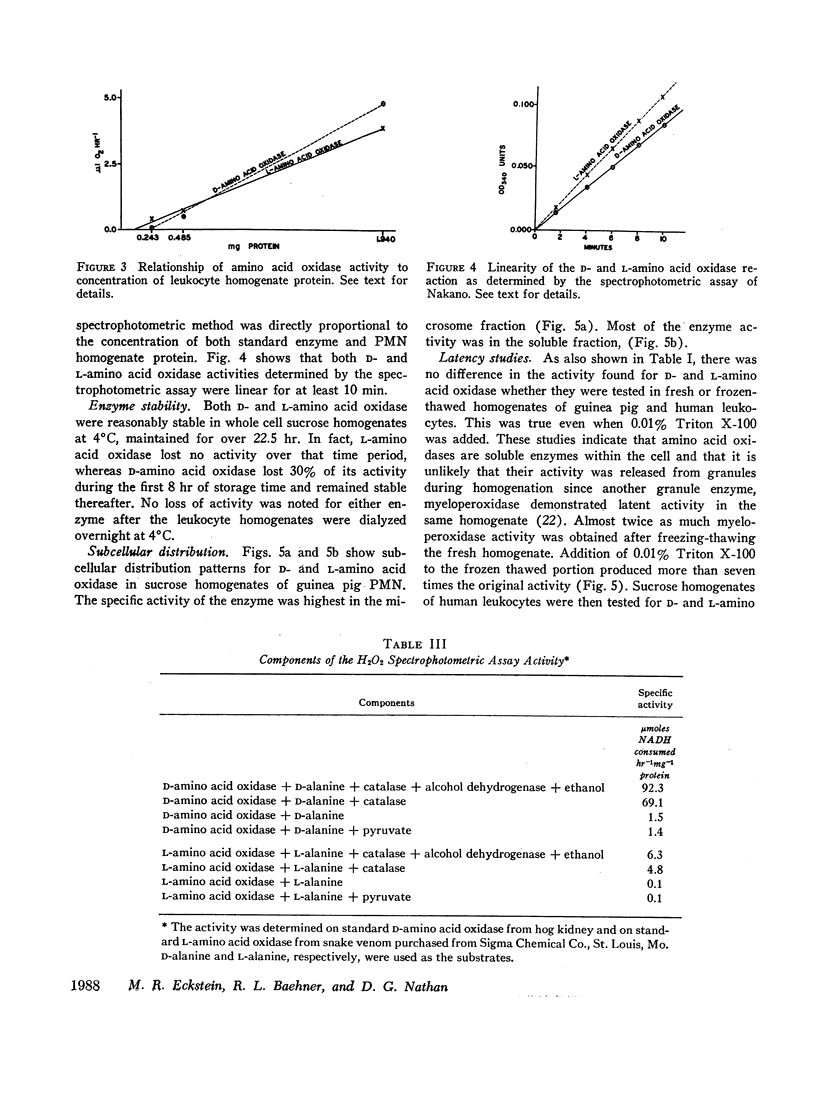
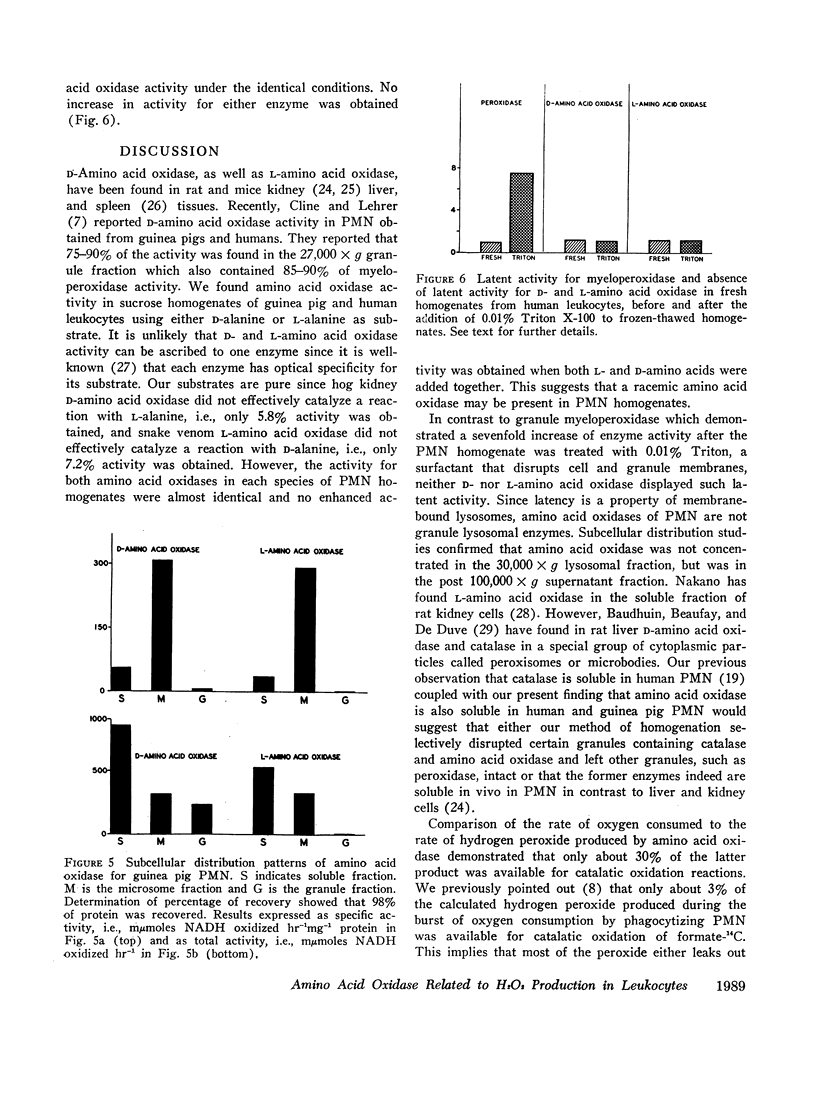
D-Amino acid oxidase and L-amino acid oxidase have been measured in sucrose homogenates of polymorphonuclear leukocytes (PMN) obtained from guinea pigs and humans. Subcellular distribution patterns and studies on latency indicate that these oxidases are soluble enzymes. Their hydrogen peroxide-generating capacity was verified. Chronic granulomatous disease PMN, which lack a respiratory burst and fail to generate H2O2 during phagocytosis and do not kill catalase positive bacteria, had peroxide-generating amino acid oxidase activity equal to that found in PMN homogenates from patients with bacterial infections. The precise metabolic and bactericidal role of amino acid oxidases in PMN remains uncertain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L., Gilman N., Karnovsky M. L. Respiration and glucose oxidation in human and guinea pig leukocytes: comparative studies. J Clin Invest. 1970 Apr;49(4):692–700. doi: 10.1172/JCI106281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. J., Karnovsky M. L. Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Jan;48(1):187–192. doi: 10.1172/JCI105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. L. Deficiency of reduced nicotinamide-adenine dinucleotide oxidase in chronic granulomatous disease. Science. 1968 Dec 13;162(3859):1277–1279. doi: 10.1126/science.162.3859.1277. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G., Karnovsky M. L. Correction of metabolic deficiencies in the leukocytes of patients with chronic granulomatous disease. J Clin Invest. 1970 May;49(5):865–870. doi: 10.1172/JCI106305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science. 1967 Feb 17;155(3764):835–836. doi: 10.1126/science.155.3764.835. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., De Duve C. Combined biochemical and morphological study of particulate fractions from rat liver. Analysis of preparations enriched in lysosomes or in particles containing urate oxidase, D-amino acid oxidase, and catalase. J Cell Biol. 1965 Jul;26(1):219–243. doi: 10.1083/jcb.26.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAGAN R. H., KARNOVSKY M. L. ENZYMATIC BASIS OF THE RESPIRATORY STIMULATION DURING PHAGOCYTOSIS. Nature. 1964 Oct 17;204:255–257. doi: 10.1038/204255a0. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. D-amino acid oxidase in leukocytes: a possible D-amino-acid-linked antimicrobial system. Proc Natl Acad Sci U S A. 1969 Mar;62(3):756–763. doi: 10.1073/pnas.62.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth A., Dounce A. L. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970 Jul;50(3):319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Baehner R. L. Improvement of leukocyte bactericidal activity in chronic granulomatous disease. Blood. 1970 Mar;35(3):350–355. [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., White L. R. Iodination defect in the leukocytes of a patient with chronic granulomatous disease of childhood. N Engl J Med. 1969 Feb 27;280(9):460–466. doi: 10.1056/NEJM196902272800902. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lyle L. R., Jutila J. W. D-amino acid oxidase induction in the kidneys of germ-free mice. J Bacteriol. 1968 Sep;96(3):606–608. doi: 10.1128/jb.96.3.606-608.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAEHLY A. C., CHANCE B. The assay of catalases and peroxidases. Methods Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- McMenamy R. H., Lund C. C., Neville G. J., Wallach D. F. STUDIES OF UNBOUND AMINO ACID DISTRIBUTIONS IN PLASMA, ERYTHROCYTES, LEUKOCYTES AND URINE OF NORMAL HUMAN SUBJECTS. J Clin Invest. 1960 Nov;39(11):1675–1687. doi: 10.1172/JCI104191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Karnovsky M. J., Karnovsky M. L. The distributions of some granule-associated enzymes in guinea-pig polymorphonuclear leucocytes. Biochem J. 1970 Jan;116(2):207–216. doi: 10.1042/bj1160207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Saga M., Tsutsumi Y. Distribution and immunochemical properties of rat kidney L-amino-acid oxidase, with a note on peroxisomes. Biochim Biophys Acta. 1969 Jul 8;185(1):19–30. doi: 10.1016/0005-2744(69)90278-2. [DOI] [PubMed] [Google Scholar]
- Nakano M., Tsutsumi Y., Danowski T. S. Crystalline L-amino-acid oxidase from the soluble fraction of rat-kidney cells. Biochim Biophys Acta. 1967 May 16;139(1):40–48. doi: 10.1016/0005-2744(67)90111-8. [DOI] [PubMed] [Google Scholar]
- Nakano M., Tsutsumi Y., Danowski T. S. Enzymatic determination of small quantities of hydrogen peroxide. Proc Soc Exp Biol Med. 1968 Dec;129(3):960–963. doi: 10.3181/00379727-129-33468. [DOI] [PubMed] [Google Scholar]
- Reed P. W. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem. 1969 May 10;244(9):2459–2464. [PubMed] [Google Scholar]
- Rossi F., Zatti M. Mechanism of the respiratory stimulation in saponine-treated leucocytes. The KCN-insensitive oxidation of NADPH. Biochim Biophys Acta. 1968 Jan 15;153(1):296–299. doi: 10.1016/0005-2728(68)90174-6. [DOI] [PubMed] [Google Scholar]
- STAHELIN H., KARNOVSKY M. L., FARNHAM A. E., SUTER E. Studies on the interaction between phagocytes and tubercle bacilli. III. Some metabolic effects in guinea pigs associated with infection with tubercle bacilli. J Exp Med. 1957 Mar 1;105(3):265–277. doi: 10.1084/jem.105.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes R. C. L-amino-acid oxidase, a bactericidal system. Nature. 1970 Mar 14;225(5237):1072–1073. doi: 10.1038/2251072a0. [DOI] [PubMed] [Google Scholar]
- Waldschmidt M. Das Verhalten von 1-Aminosäurenoxydase und Acyl-CoA-Dehydrogenasen in den Mitochondrien sowie der Gehalt an Flavinadenindinukleotid in Leber und Milz von Ratten nach Bestrahlung in vivo. Strahlentherapie. 1967 Mar;132(3):463–471. [PubMed] [Google Scholar]


