Abstract
In isolated fiber bundles of external intercostal muscle from each of 13 normal volunteers and each of 6 patients with myotonia congenita, some or all of the following were measured: concentrations of Na+, K+, and Cl-, extracellular volume, water content, K+ efflux, fiber size, fiber cable parameters, and fiber resting potentials.
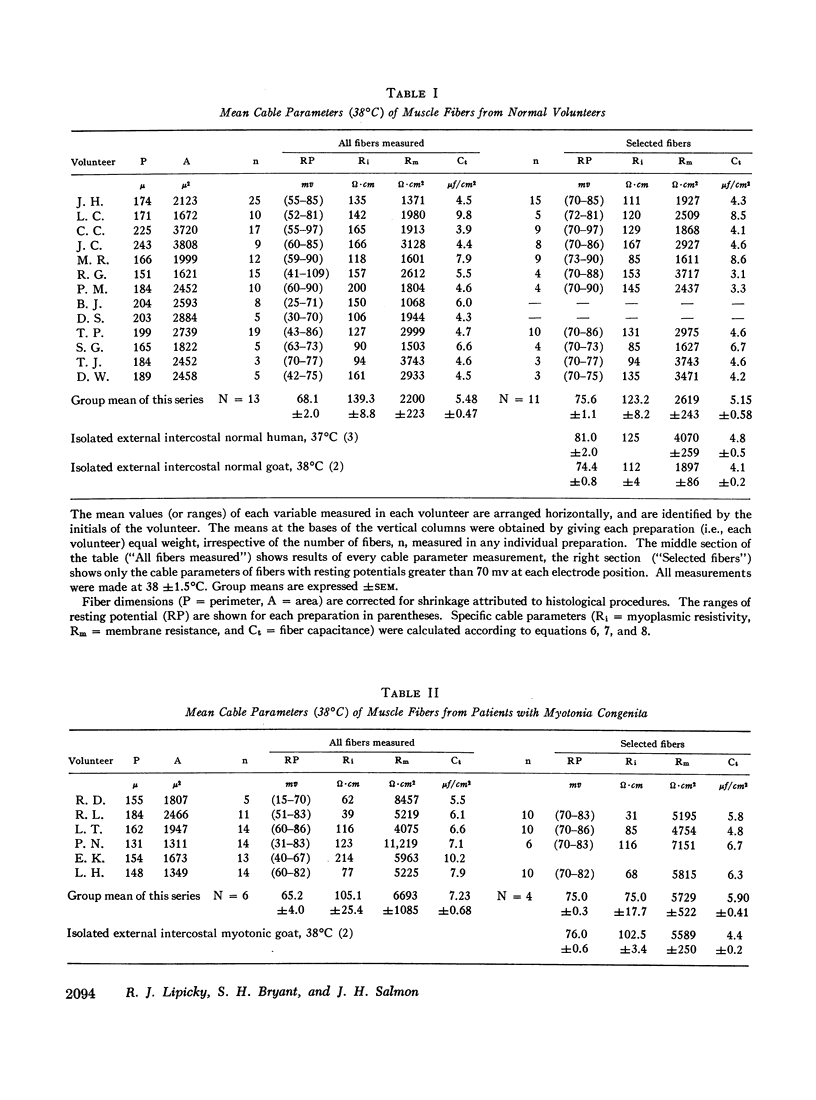
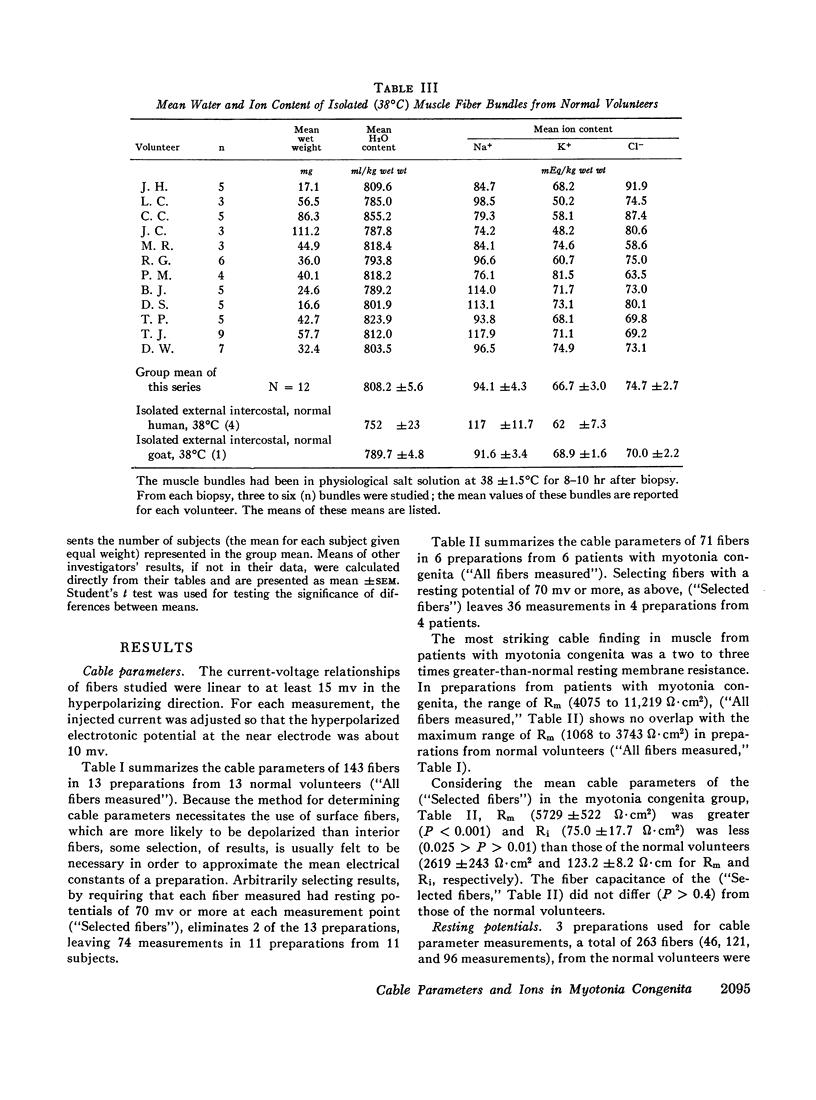
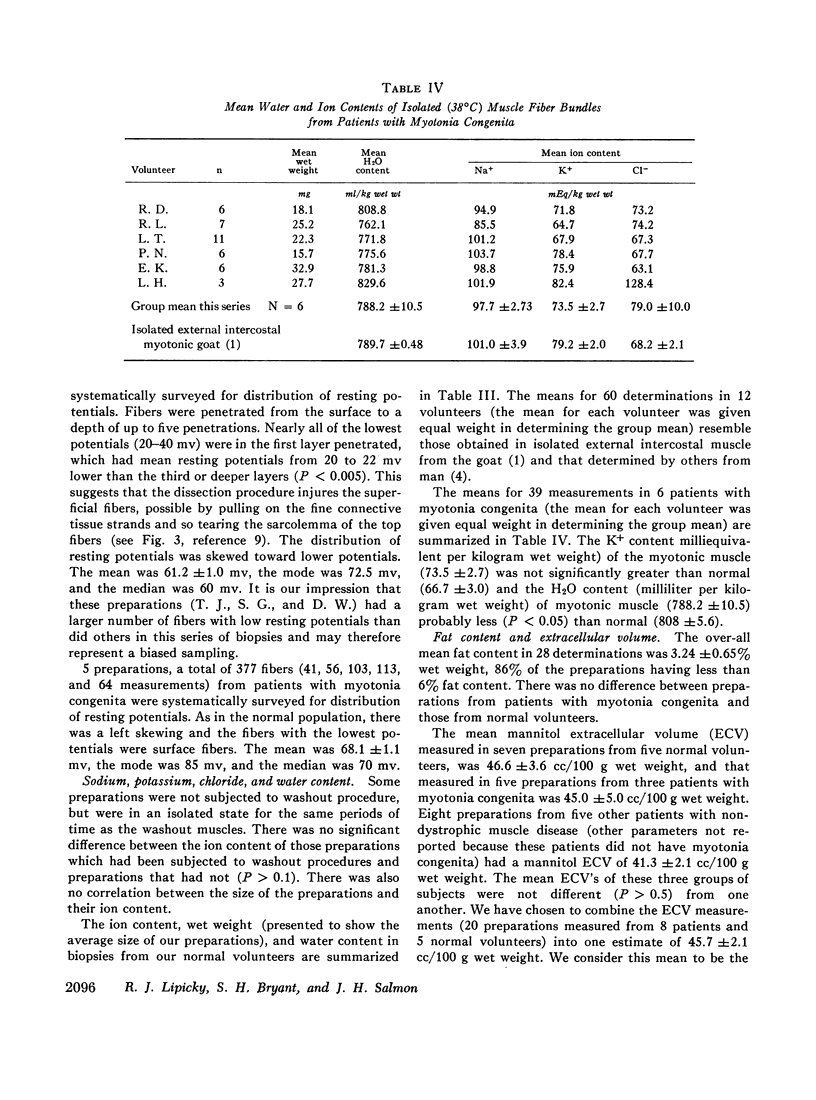
Muscle from patients with myotonia congenita differed significantly (0.001 <P< 0.025) with respect to the following mean values (myotonia congenita vs. normal): the membrane resistance was greater (5729 vs. 2619 ω·cm2), the internal resistivity was less (75.0 vs. 123.2 ω·cm), the water content was less (788.2 vs. 808.2 ml/kg wet weight), and the mean resting potential was greater (68 vs. 61 mv).
No significant differences were found with respect to the following variables: K+ content (73.5 vs. 66.7 mEq/kg wet weight) and the calculated intracellular K+ concentration (215 vs. 191 mEq/liter fiber water), fiber capacitance (5.90 vs. 5.15 μf/cm2), Na+ content (97.7 vs. 94.1 mEq/kg wet weight), Cl- content (79.0 vs. 74.7 mEq/kg wet weight), mannitol extracellular volume (45.1 vs. 46.6 cc/100 g wet weight), and K+ efflux (23.2 vs. 21.5 moles × 10-12 cm-2·sec-1).
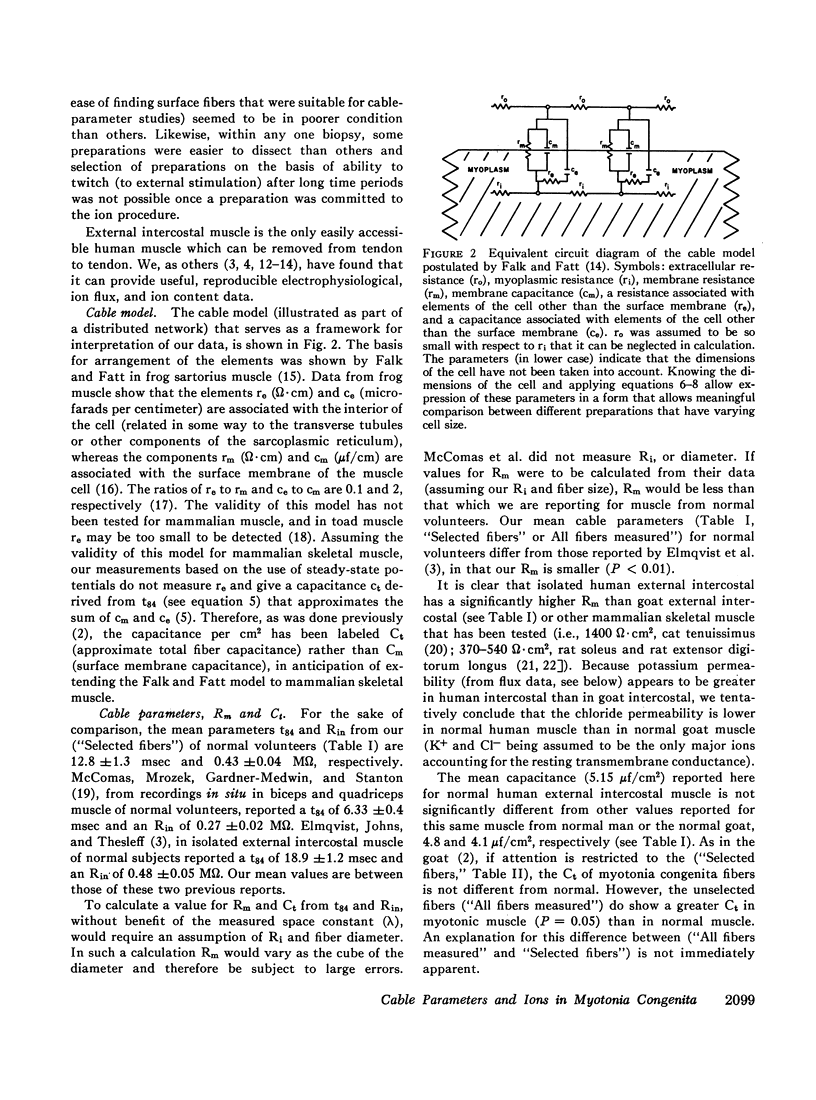
These abnormalities of skeletal muscle in human myotonia congenita are like those of skeletal muscle in goats with hereditary myotonia. We tentatively conclude that a decreased Cl- permeability accounts for some of the abnormal electrical properties of skeletal muscle in myotonia congenita.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. Internal chloride concentration and chloride efflux of frog muscle. J Physiol. 1961 May;156:623–632. doi: 10.1113/jphysiol.1961.sp006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Thesleff S. A comparative study of membrane properties of innervated and chronically denervated fast and slow skeletal muscles of the rat. Acta Physiol Scand. 1968 Aug;73(4):471–480. doi: 10.1111/j.1365-201x.1968.tb10886.x. [DOI] [PubMed] [Google Scholar]
- BOHR D. F. ELECTROLYTES AND SMOOTH MUSCLE CONTRACTION. Pharmacol Rev. 1964 Mar;16:85–127. [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. Membrane constants of mammalian muscle fibres. J Physiol. 1959 Oct;147:450–457. doi: 10.1113/jphysiol.1959.sp006255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURR L. H., McLENNAN H. The apparent extracellular space of mammalian skeletal muscle. A comparison of the inulin space in normal and dystrophic mouse tissues. Can J Biochem Physiol. 1960 Aug;38:829–835. [PubMed] [Google Scholar]
- Bryant S. H. Cable properties of external intercostal muscle fibres from myotonic and nonmyotonic goats. J Physiol. 1969 Oct;204(3):539–550. doi: 10.1113/jphysiol.1969.sp008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant S. H., Lipicky R. J., Herzog W. H. Variability of myotonic signs in myotonic goats. Am J Vet Res. 1968 Dec;29(12):2371–2381. [PubMed] [Google Scholar]
- CREESE R., DILLON J. B., MARSHALL J., SABAWALA P. B., SCHNEIDER D. J., TAYLOR D. B., ZINN D. E. The effect of neuromuscular blocking agents on isolated human intercostal muscles. J Pharmacol Exp Ther. 1957 Apr;119(4):485–494. [PubMed] [Google Scholar]
- Caughey J. E. Transitional forms between myotonia congenita, dystrophia myotonica and paramyotonia congenita. N Z Med J. 1968 Mar;67(429):347–350. [PubMed] [Google Scholar]
- Creese R. Sodium fluxes in diaphragm muscle and the effects of insulin and serum proteins. J Physiol. 1968 Jul;197(2):255–278. doi: 10.1113/jphysiol.1968.sp008558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DILLON J., FIELDS J., GUMAS T., JENDEN D. J., TAYLOR D. B. An isolated human voluntary muscle preparation. Proc Soc Exp Biol Med. 1955 Nov;90(2):409–409. doi: 10.3181/00379727-90-22048. [DOI] [PubMed] [Google Scholar]
- ELMQVIST D., JOHNS T. R., THESLEFF S. A study of some electrophysiological properties of human intercostal muscle. J Physiol. 1960 Dec;154:602–607. doi: 10.1113/jphysiol.1960.sp006599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALK G., FATT P. LINEAR ELECTRICAL PROPERTIES OF STRIATED MUSCLE FIBRES OBSERVED WITH INTRACELLULAR ELECTRODES. Proc R Soc Lond B Biol Sci. 1964 Apr 14;160:69–123. doi: 10.1098/rspb.1964.0030. [DOI] [PubMed] [Google Scholar]
- FALK G., LANDA J. F. Effects of potassium on frog skeletal muscle in a chloride-deficient medium. Am J Physiol. 1960 Jun;198:1225–1231. doi: 10.1152/ajplegacy.1960.198.6.1225. [DOI] [PubMed] [Google Scholar]
- FATT P. AN ANALYSIS OF THE TRANSVERSE ELECTRICAL IMPEDANCE OF STRIATED MUSCLE. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:606–651. doi: 10.1098/rspb.1964.0023. [DOI] [PubMed] [Google Scholar]
- Falk G. Predicted delays in the activation of the contractile system. Biophys J. 1968 May;8(5):608–625. doi: 10.1016/S0006-3495(68)86511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freygang W. H., Jr, Rapoport S. I., Peachey L. D. Some relations between changes in the linear electrical properties of striated muscle fibers and changes in ultrastructure. J Gen Physiol. 1967 Nov;50(10):2437–2458. doi: 10.1085/jgp.50.10.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Capacitance of the surface and transverse tubular membrane of frog sartorius muscle fibers. J Gen Physiol. 1969 Mar;53(3):265–278. doi: 10.1085/jgp.53.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKANSSON C. H. Conduction velocity and amplitude of the action potential as related to circumference in the isolated fibre of frog muscle. Acta Physiol Scand. 1956 Jul 17;37(1):14–34. doi: 10.1111/j.1748-1716.1956.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Hofmann W. W., Alston W., Rowe G. A study of individual neuro-muscular junctions in myotonia. Electroencephalogr Clin Neurophysiol. 1966 Dec;21(6):521–537. doi: 10.1016/0013-4694(66)90171-4. [DOI] [PubMed] [Google Scholar]
- Kiyohara T., Sato M. Membrane constants of red and white muscle fibers in the rat. Jpn J Physiol. 1967 Dec 15;17(6):720–725. doi: 10.2170/jjphysiol.17.720. [DOI] [PubMed] [Google Scholar]
- Kuhn E., Schneider V. Dickdarmonomalien bei autosomal rezessiv vererbter Myotonia congenita. Schweiz Med Wochenschr. 1968 Jan;98(4):114–116. [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Ling G. N., Kromash M. H. The extracellular space of voluntary muscle tissues. J Gen Physiol. 1967 Jan;50(3):677–694. doi: 10.1085/jgp.50.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipicky R. J., Bryant S. H. Sodium, potassium, and chloride fluxes in intercostal muscle from normal goats and goats with hereditary myotonia. J Gen Physiol. 1966 Sep;50(1):89–111. doi: 10.1085/jgp.50.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludin H. P. Microelectrode study of normal human skeletal muscle. Eur Neurol. 1969;2(6):340–347. doi: 10.1159/000113810. [DOI] [PubMed] [Google Scholar]
- MAAS O., PATERSON A. S. Myotonia congenita, dystrophia myotonica and paramyotonia; reaffirmation of their identity. Brain. 1950;73(3):318–336. doi: 10.1093/brain/73.3.318. [DOI] [PubMed] [Google Scholar]
- McComas A. J., Mrozek K., Gardner-Medwin D., Stanton W. H. Electrical properties of muscle fibre membranes in man. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):434–440. doi: 10.1136/jnnp.31.5.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComas A. J., Mrozek K. The electrical properties of muscle fiber membranes in dystrophia myotonica and myotonia congenita. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):441–447. doi: 10.1136/jnnp.31.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Narahashi T. Tetrodotoxin's highly selective blockage of an ionic channel. Fed Proc. 1967 Nov-Dec;26(6):1655–1663. [PubMed] [Google Scholar]
- NARAHASHI T., DEGUCHI T., URAKAWA N., OHKUBO Y. Stabilization and rectification of muscle fiber membrane by tetrodotoxin. Am J Physiol. 1960 May;198:934–938. doi: 10.1152/ajplegacy.1960.198.5.934. [DOI] [PubMed] [Google Scholar]
- NORRIS F. H., Jr Unstable membrane potential in human myotonic muscle. Electroencephalogr Clin Neurophysiol. 1962 Apr;14:197–201. doi: 10.1016/0013-4694(62)90029-9. [DOI] [PubMed] [Google Scholar]
- Nichols B. L., Hazlewood C. F., Barnes D. J. Percutaneous needle biopsy of quadriceps muscle: potassium analysis in normal children. J Pediatr. 1968 Jun;72(6):840–852. doi: 10.1016/s0022-3476(68)80437-8. [DOI] [PubMed] [Google Scholar]
- PAGE E. Cat heart muscle in vitro. III. The extracellular space. J Gen Physiol. 1962 Nov;46:201–213. doi: 10.1085/jgp.46.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley I. D. Contribution of the transverse tubular system to the membrane capacitance of striated muscle of the toad (Bufo marinus). Aust J Exp Biol Med Sci. 1966 Feb;44(1):9–22. doi: 10.1038/icb.1966.2. [DOI] [PubMed] [Google Scholar]
- RIECKER G., DOBBELSTEIN H., ROEHL D., BOLTE H. D. MESSUNGEN DES MEMBRANPOTENTIALS EINZELMER QUERGESTREIFTER MUSKELZELLEN BEI MYOTONIA CONGENITA (THOMSEN) Klin Wochenschr. 1964 Jun 1;42:519–522. doi: 10.1007/BF01486678. [DOI] [PubMed] [Google Scholar]


