Abstract
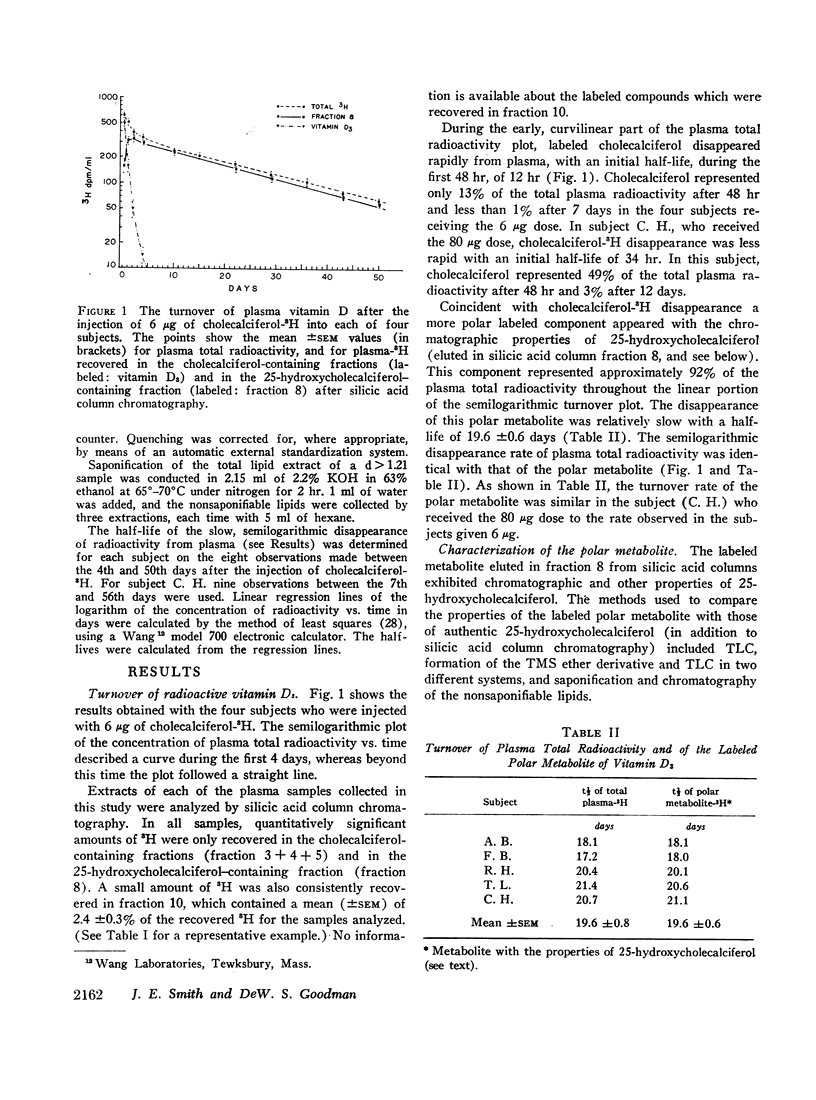
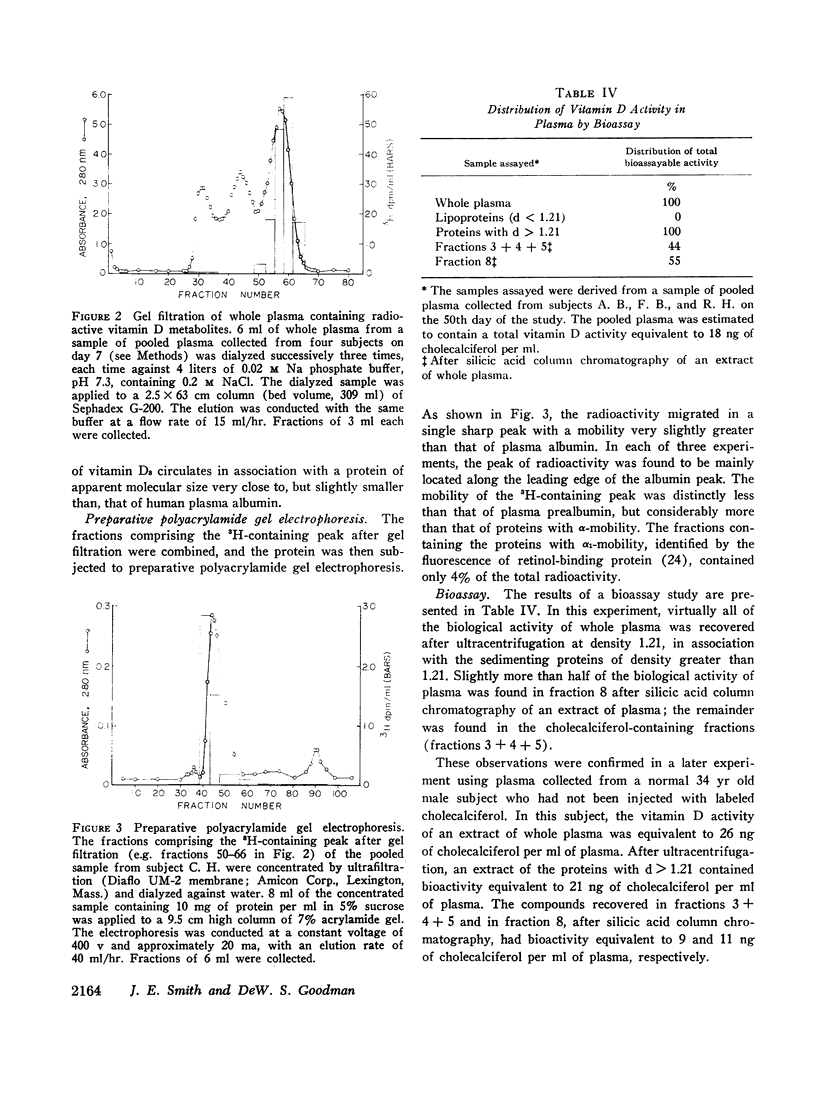
Four normal men were injected intravenously with physiological doses (6 μg) of vitamin D3-1,2-3H. Serial samples of plasma were collected for 50 days. Total lipid extracts were chromatographed on silicic acid columns or thin-layer plates in order to characterize the radioactive components. Labeled vitamin D3 disappeared rapidly from plasma (initial half-life approximately 12 hr); after 7 days unchanged vitamin D3 represented less than 1% of circulating radioactivity. Coincident with vitamin D3 disappearance a more polar labeled metabolite appeared with chromatographic and other properties identical with those of 25-hydroxycholecalciferol. The disappearance of the more polar metabolite was relatively slow with a half-life of 19.6 ±0.6 days. A similar half-life was seen in a fifth subject, injected with 80 μg of vitamin D3-3H. Most (approximately 92%) of the plasma total radioactivity was represented by this component throughout the study. Plasma samples collected at various times were adjusted to density (d) 1.21 and were ultracentrifuged to separate plasma lipoproteins from proteins with d > 1.21. In all samples, almost all (mean 94%) of the radioactivity was found in association with proteins of d > 1.21. This observation was confirmed by bioassay, measuring uptake of 45Ca by intestinal slices. All plasma bioassayable vitamin D was found in association with proteins of d > 1.21; 55% of bioactivity was found in the chromatographic fraction corresponding to 25-hydroxycholecalciferol and 44% in the fractions representing vitamin D3. Since both vitamin D3 and its 25-hydroxy metabolite are lipid-soluble sterol derivatives, the finding that these compounds do not circulate in association with the known plasma lipoproteins provides presumptive evidence for the existence of a specific transport protein of d > 1.21. The transport protein for the polar metabolite has been partly characterized by gel filtration on Sephadex G-200 and by electrophoresis on polyacrylamide gel. The protein has an apparent size slightly smaller than plasma albumin (approximate mol wt 50,000-60,000) and an electrophoretic mobility very slightly greater than that of albumin. Studies are in progress to fractionate further and to characterize the transport protein.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avioli L. V., Lee S. W., McDonald J. E., Lund J., DeLuca H. F. Metabolism of vitamin D3-3H in human subjects: distribution in blood, bile, feces, and urine. J Clin Invest. 1967 Jun;46(6):983–992. doi: 10.1172/JCI105605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avioli L. V., Williams T. F., Lund J., DeLuca H. F. Metabolism of vitamin D3-3H in vitamin D-resistant rickets and familial hypophosphatemia. J Clin Invest. 1967 Dec;46(12):1907–1915. doi: 10.1172/JCI105680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEEKEN W. L., VOLWILER W., GOLDSWORTHY P. D., GARBY L. E., REYNOLDS W. E., STOGSDILL R., STEMLER R. S. Studies of I-131-albumin catabolism and distribution in normal young male adults. J Clin Invest. 1962 Jun;41:1312–1333. doi: 10.1172/JCI104594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunt J. W., DeLuca H. F., Schnoes H. K. 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry. 1968 Oct;7(10):3317–3322. doi: 10.1021/bi00850a001. [DOI] [PubMed] [Google Scholar]
- CHALK K. J., KODICEK E. The association of 14C-labelled vitamin D2 with rat serum proteins. Biochem J. 1961 Apr;79:1–7. doi: 10.1042/bj0790001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. S., Jr, Lane K. Serum protein binding of vitamin D3. Arch Biochem Biophys. 1965 Oct;112(1):70–75. doi: 10.1016/0003-9861(65)90011-1. [DOI] [PubMed] [Google Scholar]
- Cousins R. J., DeLuca H. F., Gray R. W. Metaboism of 25-hydroxycholecalciferol in target and nontarget tissues. Biochemistry. 1970 Sep 15;9(19):3649–3652. doi: 10.1021/bi00821a001. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. Recent advances in the metabolism and function of vitamin D. Fed Proc. 1969 Sep-Oct;28(5):1678–1689. [PubMed] [Google Scholar]
- DeLuca H. F. Role of kidney tissue in metabolism of vitamin D. N Engl J Med. 1971 Mar 11;284(10):554–555. doi: 10.1056/NEJM197103112841014. [DOI] [PubMed] [Google Scholar]
- FREDRICKSON D. S., GORDON R. S., Jr Transport of fatty acids. Physiol Rev. 1958 Oct;38(4):585–630. doi: 10.1152/physrev.1958.38.4.585. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Fredrickson D. S., Levy R. I., Lees R. S. Fat transport in lipoproteins--an integrated approach to mechanisms and disorders. N Engl J Med. 1967 Jan 5;276(1):34–contd. doi: 10.1056/NEJM196701052760107. [DOI] [PubMed] [Google Scholar]
- GORDAN G. S., SCHACHTER D. Vitamin Dactivity of normal and neoplastic human breast tissue. Proc Soc Exp Biol Med. 1963 Jul;113:760–761. doi: 10.3181/00379727-113-28483. [DOI] [PubMed] [Google Scholar]
- GRUNDY S. M., AHRENS E. H., Jr, MIETTINEN T. A. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL FECAL BILE ACIDS. J Lipid Res. 1965 Jul;6:397–410. [PubMed] [Google Scholar]
- Goodman D. S., Shiratori T. Fatty acid composition of human plasma lipoprotein fractions. J Lipid Res. 1964 Jul;5(3):307–313. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler M. R., Boyce D. W., Littledike E. T., Rasmussen H. A rapidly acting metabolite of vitamin D3. Proc Natl Acad Sci U S A. 1971 Jan;68(1):177–181. doi: 10.1073/pnas.68.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M., Raz A., Goodman D. S. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968 Sep;47(9):2025–2044. doi: 10.1172/JCI105889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawer E. B., Lumb G. A., Stanbury S. W. Long biological half-life of vitamin D3 and its polar metabolites in human serum. Nature. 1969 May 3;222(5192):482–483. doi: 10.1038/222482a0. [DOI] [PubMed] [Google Scholar]
- Olson E. B., DeLuca H. F. 25-hydroxycholecalciferol: direct effect on calcium transport. Science. 1969 Jul 25;165(3891):405–407. doi: 10.1126/science.165.3891.405. [DOI] [PubMed] [Google Scholar]
- Ponchon G., Kennan A. L., DeLuca H. F. "Activation" of vitamin D by the liver. J Clin Invest. 1969 Nov;48(11):2032–2037. doi: 10.1172/JCI106168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Shiratori T., Goodman D. S. Studies on the protein-protein and protein-ligand interactions involved in retinol transport in plasma. J Biol Chem. 1970 Apr 25;245(8):1903–1912. [PubMed] [Google Scholar]
- Rikkers H., DeLuca H. F. An in vivo study of the carrier proteins of 3H-vitamins D3 and D4 in rat serum. Am J Physiol. 1967 Aug;213(2):380–386. doi: 10.1152/ajplegacy.1967.213.2.380. [DOI] [PubMed] [Google Scholar]
- SCHACHTER D., KIMBERG D. V., SCHENKER H. Active transport of calcium by intestine: action and bio-assay of vitamin D. Am J Physiol. 1961 Jun;200:1263–1271. doi: 10.1152/ajplegacy.1961.200.6.1263. [DOI] [PubMed] [Google Scholar]
- Schaeffer K., Koch H. U., Opitz A., von Herrath D., Knoop H. Vitamin D-Stoffwechsel und Niereninsuffizienz. Klin Wochenschr. 1970 Sep 15;48(18):1129–1131. doi: 10.1007/BF01496406. [DOI] [PubMed] [Google Scholar]
- Smith F. R., Raz A., Goodman D. S. Radioimmunoassay of human plasma retinol-binding protein. J Clin Invest. 1970 Sep;49(9):1754–1761. doi: 10.1172/JCI106393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer S., Eder H. A. Transport of lysolecithin by albumin in human and rat plasma. J Lipid Res. 1965 Oct;6(4):506–511. [PubMed] [Google Scholar]
- Trummel C. L., Raisz L. G., Blunt J. W., Deluca H. F. 25-Hydroxycholecalciferol: stimulation of bone resorption in tissue culture. Science. 1969 Mar 28;163(3874):1450–1451. doi: 10.1126/science.163.3874.1450. [DOI] [PubMed] [Google Scholar]
- WHYTE M., KARMEN A., GOODMAN D. S. FATTY ACID ESTERIFICATION AND CHYLOMICRON FORMATION DURING FAT ABSORPTION. 2. PHOSPHOLIPIDS. J Lipid Res. 1963 Jul;4:322–329. [PubMed] [Google Scholar]
- de Crousaz P., Blanc B., Antener I. Vitamin D activity in normal human serum and serum proteins. Helv Odontol Acta. 1965 Oct;9(2):151–155. [PubMed] [Google Scholar]