Abstract
A large number of novel therapeutics is currently undergoing clinical evaluation for the treatment of prostate cancer, and small molecule signal transduction inhibitors are a promising class of agents. These inhibitors have recently become a standard therapy in renal cell carcinoma and offer significant promise in prostate cancer. Through an understanding of the key pathways involved in prostate cancer progression, a rational drug design can be aimed at the molecules critical to cellular signaling. This may enable administration of selective therapies based on the expression of molecular targets, more appropriately individualizing treatment for prostate cancer patients.
One pathway with a prominent role in prostate cancer is the PI3K/Akt/mTOR pathway. Current estimates suggest that PI3K/Akt/mTOR signaling is upregulated in 30-50% of prostate cancers, often through loss of PTEN. Molecular changes in the PI3K/Akt/mTOR signaling pathway have been demonstrated to differentiate benign from malignant prostatic epithelium and are associated with increasing tumor stage, grade, and risk of biochemical recurrence. Multiple inhibitors of this pathway have been developed and are being assessed in the laboratory and in clinical trials, with much attention focusing on mTOR inhibition. Current clinical trials in prostate cancer are assessing efficacy of mTOR inhibitors in combination with multiple targeted or traditional chemotherapies, including bevacizumab, gefitinib, and docetaxel. Completion of these trials will provide substantial information regarding the importance of this pathway in prostate cancer and the clinical implications of its targeted inhibition. In this article we review the data surrounding PI3K/Akt/mTOR inhibition in prostate cancer and their clinical implications.
Keywords: Prostate cancer, targeted therapy, PI3K, Akt, mTOR
INTRODUCTION
Although the majority of the estimated 220,000 men diagnosed annually with prostate cancer do not die of this disease, the prognosis for men with advanced prostate cancer is very poor. Treatment for patients who experience recurrence of their disease after primary treatment or present with advanced disease typically involves androgen deprivation therapy. However, nearly all men on androgen deprivation therapy will progress within 18-24 months to castrate-resistant prostate cancer (CRPC), and no curative treatments currently exist for CRPC. As a result, prostate cancer remains the second leading cause of cancer-related deaths in men in the United States, with approximately 28,000 deaths each year [1]. New rational approaches to treatment of CRPC are needed, and signal transduction modulators offer significant promise. One signaling pathway with substantial therapeutic potential in prostate cancer is the PI3K/Akt/mTOR pathway. The role of this pathway in prostate cancer has been reviewed in the past [2-8], and it is a focus of constant and intensive investigations. The new findings regarding the biological rationale for inhibition of this pathway and the current status of PI3K/Akt/mTOR inhibitors in the treatment of prostate cancer are discussed in this review.
The current standard treatment for patients with CRPC derives from two large, prospective trials, TAX 327 [9] and SWOG 99-16 [10], which have placed docetaxel as the gold standard treatment for these men. In TAX 327 [9], over one-thousand men with CRPC were enrolled in a study of docetaxel versus mitoxantrone, which was considered standard therapy at that time [11, 12]. Men receiving docetaxel every 3 weeks had an increased median survival of 2.4 months over those receiving mitoxantrone (18.9 vs. 16.5 months, p=0.009). SWOG 99-16 [10] prospectively evaluated the effect of docetaxel plus estramustine compared with mitoxantrone and prednisone in men with metastatic CRPC. Patients receiving docetaxel had a significantly longer survival when compared with patients receiving mitoxantrone and prednisone (17.5 vs. 15.6 months, p=0.02), a greater prostate-specific antigen (PSA) decline (50% vs. 27% of patients with >50% reduction, p<0.001), and longer time to progression (6.3 vs. 3.2 months, p<0.001). While these two studies provided a clear standard of care for men with CRPC, they also demonstrated the need for new therapies capable of providing larger and more sustained benefits.
A number of drugs that rely on specific knowledge of cancer genetics and molecular pathways are emerging in prostate cancer. These molecular therapies include angio-genesis inhibitors, nucleotide-based targeted therapies, and small molecule signal transduction inhibitors. What these approaches have in common is a reliance on identification and inhibition of pathways critical to prostate cancer progression at the molecular level. In breast cancer, knowledge of molecular markers has already led to the development of effective, rational-based cancer therapies. A number of studies have demonstrated that targeting the receptor tyrosine kinase Her2/neu, which is overexpressed in approximately 30% of breast cancer, resulted in longer survival [13]. Patients with metastatic breast cancer overexpressing Her2/neu had a significant survival benefit when they received trastuzumab, a monoclonal antibody targeting Her2/neu, in addition to standard chemotherapy (25.1 vs. 20.3 month median survival, p=0.046) [13]. Trastuzumab has also demonstrated significant benefit as an adjuvant agent in women with surgically removed Her2/neu-positive breast cancer (HR 0.48 for recurrence, CI 0.39-0.59) [14]. In order to develop similar targeted therapies for prostate cancer, critical key molecules and signaling pathways need to be identified and the effects of inhibition investigated.
PI3K/Akt/mTOR PATHWAY
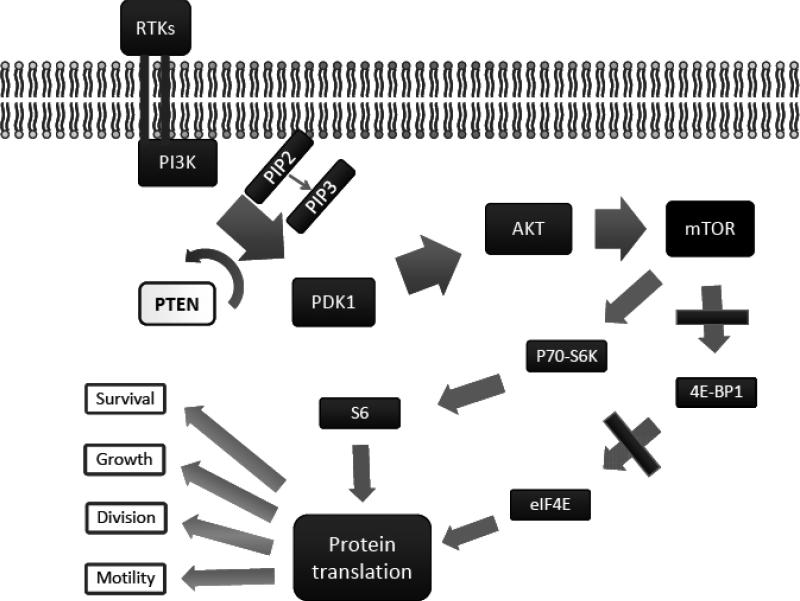
The PI3K/Akt/mTOR pathway is implicated in numerous cellular processes ranging from cell growth and survival to the promotion of angiogenesis. The basic outline of the PI3K/Akt/mTOR pathway is depicted in Fig. (1).
Fig. (1).
PIK3/Akt/mTOR Pathway.
A number of receptor tyrosine kinases can activate phosphatydidyl inositol-3-OH kinase (PI3K) at the cell membrane, initiating the signaling cascade. These receptor tyrosine kinases include the epidermal growth factor receptor (EGFR), insulin-like growth factor receptor (IGFR), and platelet derived growth factor receptor (PDGFR). Once activated, PI3K phosphorylates phosphatidylinositol-4,5-diphosphate (PIP2), leading to accumulation of phosphatidylinositol-3,4,5-triphosphate (PIP3) [15]. This lipid second messenger recruits Akt (also known as protein kinase B) and phosphoinositide dependent protein kinase 1 (PDK1) to the cell membrane, where Akt is phosphorylated by PDK1 [16]. Phosphorylated Akt regulates cellular processes by phosphorylation of a number of substrates, including checkpoint kinase 1 (Chk1), murine double minute (MDM2), BclxL/Bcl-2 associated death promoter (BAD), the forkhead box O (FOXO) family of transcription factors, and tuberous sclerosis complex 2 (TSC2) [17]. Most evidence to date, however, points to another Akt substrate, the mammalian target of rapamycin (mTOR), as having the most significant role in tumorigenesis. mTOR is a serine/threonine kinase that plays critical roles in the regulation of cell growth, survival, division, and motility [18]. mTOR acts through two separate complexes—mTORC1 and mTORC2. Each consists of mTOR bound to LST8 and either raptor (regulatory associated protein of mTOR) forming mTORC1 or rictor (rapamycin-insensitive companion of mTOR) forming mTORC2. When activated, mTORC1 increases mRNA translation by phosphorylation of the downstream molecules p70-S6K (S6K) and 4E binding protein 1 (4E-BP1). S6K phosphorylates the S6 component of the 40S ribosomal subunit, increasing translation of mRNA [19]. 4E-BP1 phosphorylation also leads to activation of translation, but by preventing association of 4E-BP-1 with the eukaryotic initiation factor 4F (eIF4F) complex [20]. mTORC2 functions are less understood. Recently, mTORC2 has been shown to be involved in phosphorylating Akt at Ser473 which, along with phosphorylation at Thr308 by PDK1, is required for full activation of Akt [21, 22].
The primary negative regulator of the PI3K/Akt/mTOR pathway is the tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN) [23]. This phosphatase acts on the D3 phosphorylated position of PIP3, promoting formation of PIP2 and directly opposing the action of PI3K [24, 25]. PTEN has a crucial role in controlling cell size, organ size, and proliferation, and loss of PTEN is the most common cause of overactivation of the PI3K pathway in human cancers [26].
PI3K/Akt/mTOR ACTIVITY IN PROSTATE CANCER
Alterations in the PI3K/Akt/mTOR pathway have been detected in prostatic tissues in multiple studies, suggesting that this pathway plays a prominent role in the development and progression of prostate cancer. It is estimated that upregulation occurs in 30-50% of prostate cancers, and aberrant signaling of the molecules in this pathway have also been detected in prostate cancer cell lines and xenografts.
PTEN is a negative regulator of activity of the PI3K/Akt/mTOR pathway. PTEN deletions and mutations that result in expression of inactive protein lead to increased activity of the PI3K/Akt/mTOR pathway. Mutations in the PTEN tumor suppressor are common events in prostate cancer, with studies showing loss of heterozygosity at the PTEN locus in up to 60% of prostate cancer samples [27-30]. Decreased expression of PTEN has been found in 85% of primary tumors relative to normal tissues from the same patients, and PTEN expression was also reduced in cancer relative to prostatic intraepithelial neoplasia (PIN) [31]. Alterations in PTEN expression are associated with a number of clinico-pathologic variables in prostate cancer. Loss of PTEN expression correlated with Gleason score and pathologic stage of primary tumors [30, 32] and increased the incidence of development of lymph node metastases [33]. Moreover, when combined with detection of phospho-Akt, PTEN status of the primary tumor was a better predictor of PSA recurrence than phospho-Akt alone (AUC 0.890) [34]. Importantly, 90% of the patients with PTEN-negative primary tumors with high levels of phospho-Akt experienced a biochemical recurrence, while 88% of PTEN-positive tumors with low phospho-Akt did not recur within the study period.
In vitro and preclinical studies have also shown that inactivation of PTEN leads to constitutively activated Akt and mTOR, as well as deregulation of cell size and cell growth [35]. A number of commonly employed prostate cancer-cell lines, including PC-3, LNCaP, and C4-2, are PTEN-negative or express inactive PTEN. Mice heterozygous for PTEN develop PIN with 100% incidence. PTEN homozygous knockouts die in utero, while mice with prostate-specific deletion of PTEN develop invasive prostate cancer [36].
Changes in expression and activation of Akt have also been reported in prostate cancer. Akt protein was detected in virtually every sample in a study of 56 prostatectomy specimens, with cancer cells having greater staining intensity and an increased percentage of positive-staining cells compared to non-neoplastic cells (p<0.001) [37]. Furthermore, phospho-Akt levels were also significantly greater in high-grade prostate tumors vs. low- or intermediate-grade tumors; phospho-Akt was detected in 14% of samples with Gleason score ≤6, 36% of samples with Gleason score 7, and 92% of samples with Gleason score ≥8 tumors (p<0.001) [38]. Levels of phospho-Akt were significantly increased in cancer cells relative to normal prostate epithelium and benign prostatic hyperplasia (45.8% vs. 8.4%) [38]. Phospho-Akt was found to be an independent predictor of biochemical recurrence (HR 3.44, CI 1.83-6.43) [39], and increased levels of phospho-Akt were detected in primary tumors of patients who eventually suffered PSA recurrence (p<0.001) while no correlation was found between Akt expression and biochemical recurrence [40]. Furthermore, increased levels of phospho-Akt were detected in CRPC tissues when compared with hormone-sensitive tissues and were associated with decreased disease-specific survival (HR 2.89, CI 1.43-5.8) [41]. Results of a study evaluating expression of Akt iso-forms with respect to prostate cancer recurrence showed that only high cytoplasmic Akt-1 combined with low nuclear Akt-1 independently predicted time to biochemical failure (HR 2.2, CI 1.12-3.99) [42].
Levels of mTOR and cytoplasmic phospho-mTOR were greater in prostate cancer tissue vs. normal prostatic epithelium, with mTOR levels in cancer cells twice that of benign tissue [31]. Phospho-mTOR was detected at low levels in the cytoplasm and at moderate to high levels along the membrane in normal prostatic epithelium, while in cancer cells strong immunoreactivity of phospho-mTOR was detected both at the membrane and in the cytoplasm. Comparisons of levels of signaling molecules downstream of mTOR, such as 4E-BP1 and S6, also showed higher levels in prostate cancer vs. normal cells [31]. Further evidence surrounding the activity of mTOR in prostate cancer is indirect and comes from the use of mTOR inhibitors, which will be discussed below.
PI3K/Akt/mTOR PATHWAY INHIBITION IN PROSTATE CANCER
PI3K Inhibition
Multiple small molecule inhibitors of the PI3K/Akt/ mTOR pathway have been investigated in both in vitro and in vivo models of prostate cancer (see Table 1). The most studied PI3K inhibitors to date are LY294002 and wortmannin. LY294002 is a potent inhibitor and a competitive antagonist of PI3K. LY294002 treatment resulted in cell-cycle arrest of LNCaP cells and sensitized these cells to radiation [43]; decreased the invasive properties of LNCaP, PC-3, and DU 145 cells [44]; and inhibited angiogenesis in PC-3 cells via decreased levels of HIF1-α and VEGF [45]. LY294002 also lowered levels of phospho-Akt in PC-3 and LNCaP cells [46]. However, in addition to PI3K inhibition, LY294002 inhibits DNA-dependent protein kinase, ataxia teleangectasia mutated, estrogen receptor, mTOR, and even voltage gated K+ channels [47-50]. Therefore, some of the effects of LY294002 may not be directly related to its ability to inhibit PI3K. Wortmannin is a fungicide that was originally isolated from soil and is an irreversible inhibitor of PI3K [51]. Treatment with wortmannin decreased levels of phospho-Akt in PC-3 and LNCaP cells [52, 53], and induced apoptosis and radiosensitized DU 145 cells [54, 55]. Wortmannin, similar to LY294002, is not specific to PI3K and inhibits multiple other signaling molecules [48]. Unfortunately, in vivo use of both, LY294002 and wortmannin, have met with significant negative side effects [56]. Nonetheless, in vivo LY294002 decreased phosphorylation of eIF4F and translation of downstream proteins in the prostates of transgenic mice constitutively expressing an active catalytic subunit of PI3K [57].
Table 1.
Pre-Clinical Studies of Inhibition of PI3K/Akt/mTOR Pathway in Prostate Cancer
| PI3K Inhibitors | Other Drugs Tested | Experimental Model | Results | Reference |
|---|---|---|---|---|
| LY294002 | p110 transgenic | Decreased pAkt by 47%; significant decreases in eIF4G, Mst1 and RanBP2 | [57] | |
| Curcumin | TRAMP | Reduced formation of PIN and adenocarcinoma | [58] | |
| Curcumin | TRAIL | LNCaP s.c. | Decreased tumor growth and cell proliferation, increased apoptosis | [59] |
| Curcumin | Gemcitabine Radiation tx. | PC-3 s.c. | Curcumin inhibited growth; additional benefits seen when used in combination | [61] |
| Akt Inhibitors | ||||
| Deguelin | PC-3 s.c. | 38 % reduction in tumor volume at 15 days | [80] | |
| GSK690693 | LNCaP s.c. | Significant inhibition of tumor growth by ~50% | [81] | |
| Celecoxib | Atorvastatin | PC-3 s.c. | Inhibited formation of tumors used in combination | [74] |
| Celecoxib | Exisulind | Wister-Unilever Rats | Decreased incidence of PIN and adenocarcinoma | [72] |
| Celecoxib | green tea polyphenol | CWR22Rnu1 s.c. | 57% growth inhibition with celecoxib alone | [75] |
| Celecoxib | TRAMP | Reduced formation of PIN and adenocarcinoma | [73] | |
| Celecoxib | PC-3 s.c. | Reduced tumor volumes by 52% at highest dose | [76] | |
| Celecoxib DMC | PC-3 s.c. | No inhibition with celecoxib – DMC with significant tumor growth inhibition | [77] | |
| Genistein | Orthotopic PC-3 | Reduced lung metastasis between 40-60% | [69] | |
| Genistein | TRAMP | Reduced development of poorly differentiated CaP | [68] | |
| Genistein | Docetaxel | SCID/hu PC-3 | Inhibition of tumor growth with genistein alone - benefits in combination | [70] |
| Genistein | Radiation tx. | Orthotopic PC-3 | Genistein – 30% tumor volume reduction; 84% reduction in combination; genistein alone increased metastasis | [65] |
| Genistein | LNCaP s.c. | Reduced tumor volume and incidence | [67] | |
| mTOR Inhibitors | ||||
| RAD-001 | Docetaxel Zoledronic acid | C4-2 intra-tibial | Significant decreases in tumor growth with addition of drugs in combination | [100] |
| RAD-001 | Akt1 transgenic | Reversed high grade PIN lesions | [94] | |
| Rapamycin | IRS-1 ASO | PC-3 s.c. | Significant growth inhibition by rapamycin; additive effect with IRS-1 ASO; IHC - 20% decreased proliferative index by rapamycin | [97] |
| Rapamycin | HDAC inhibitor | PC-3 s.c | 53% tumor volume reduction alone; 80% reduction in combination | [98] |
| CCI-779 | Docetaxel | PC-3 & DU 145 s.c. | Inhibited growth – significant decrease in Ki-67 index | [91] |
| CCI-779 | Doxorubicin | PC-3 s.c. | Reduced tumor growth by 40% alone and by 69% in combination with doxorubicin on doxorubicin resistant cells | [35] |
s.c. subcutaneous.
(HR 3.44, CI 1.83-6.43) [39], and increased levels of phospho-Akt were detected in primary tumors of patients who eventually suffered PSA recurrence (p<0.001) while no correlation was found between Akt expression and biochemical recurrence [40]. Furthermore, increased levels of phospho-Akt were detected in CRPC tissues when compared with hormone-sensitive tissues and were associated with decreased disease-specific survival (HR 2.89, CI 1.43-5.8) [41]. Results of a study evaluating expression of Akt iso-forms with respect to prostate cancer recurrence showed that only high cytoplasmic Akt-1 combined with low nuclear Akt-1 independently predicted time to biochemical failure (HR 2.2, CI 1.12-3.99) [42].
Levels of mTOR and cytoplasmic phospho-mTOR were greater in prostate cancer tissue vs. normal prostatic epithelium, with mTOR levels in cancer cells twice that of benign tissue [31]. Phospho-mTOR was detected at low levels in the cytoplasm and at moderate to high levels along the membrane in normal prostatic epithelium, while in cancer cells strong immunoreactivity of phospho-mTOR was detected both at the membrane and in the cytoplasm. Comparisons of levels of signaling molecules downstream of mTOR, such as 4E-BP1 and S6, also showed higher levels in prostate cancer vs. normal cells [31]. Further evidence surrounding the activity of mTOR in prostate cancer is indirect and comes from the use of mTOR inhibitors, which will be discussed below.
PI3K/Akt/mTOR PATHWAY INHIBITION IN PROSTATE CANCER
PI3K Inhibition
Multiple small molecule inhibitors of the PI3K/Akt/ mTOR pathway have been investigated in both in vitro and in vivo models of prostate cancer (see Table 1). The most studied PI3K inhibitors to date are LY294002 and wortmannin. LY294002 is a potent inhibitor and a competitive antagonist of PI3K. LY294002 treatment resulted in cell-cycle arrest of LNCaP cells and sensitized these cells to radiation [43]; decreased the invasive properties of LNCaP, PC-3, and DU 145 cells [44]; and inhibited angiogenesis in PC-3 cells via decreased levels of HIF1- and VEGF [45]. LY294002 also lowered levels of phospho-Akt in PC-3 and LNCaP cells [46]. However, in addition to PI3K inhibition, LY294002 inhibits DNA-dependent protein kinase, ataxia teleangectasia mutated, estrogen receptor, mTOR, and even voltage gated K+ channels [47-50]. Therefore, some of the effects of LY294002 may not be directly related to its ability to inhibit PI3K. Wortmannin is a fungicide that was originally isolated from soil and is an irreversible inhibitor of PI3K [51]. Treatment with wortmannin decreased levels of phospho-Akt in PC-3 and LNCaP cells [52, 53], and induced apoptosis and radiosensitized DU 145 cells [54, 55]. Wortmannin, similar to LY294002, is not specific to PI3K and inhibits multiple other signaling molecules [48]. Unfortunately, in vivo use of both, LY294002 and wortmannin, have met with significant negative side effects [56]. Nonetheless, in vivo LY294002 decreased phosphorylation of eIF4F and translation of downstream proteins in the prostates of transgenic mice constitutively expressing an active catalytic subunit of PI3K [57].
Recently, curcumin has been shown to inhibit PI3K activity, in addition to other mechanisms of action. Curcumin treatment induced apoptosis of LNCaP, PC-3, and DU 145 cells, inhibited growth of LNCaP and PC-3 xenografts, and inhibited PIN formation in TRAMP mice [58-61]. Because of the critical nature of PI3K in cancer and limited availability of specific inhibitors, there are several new PI3K inhibitors in development that may have improved selectivity against PI3K; however, these have not yet been evaluated in prostate cancer [62].
Akt Inhibition
Multiple Akt inhibitors have been investigated in prostate cancer. Perifosine is an alkylphospholipid that decreases Akt phosphorylation and upregulates expression of the tumor suppressor p21 [63]. Perifosine inhibited growth and induced cell-cycle arrest of PC-3 cells, and it also induced differentiation of PC-3 and LNCaP cells through activation of the GSK-3β pathway [64]. Although there are no published pre-clinical studies investigating perifosine activity against prostate cancer, perifosine has gone on to clinical trials in prostate cancer.
Genistein, an isoflavinoid found in soy, is another compound with Akt inhibitory activity. In vitro studies have showed that genistein inhibits activity of Akt and causes significant growth inhibition and apoptosis of prostate cancer cells [65, 66]. However, genistein also inhibits mTOR, AR, and a wide range of other targets (i.e. tyrosine kinases, topoisomerases, and telomerases). In vivo, genistein has shown significant potential [67, 68], sensitizing prostate tumors to radiation [65] and decreasing the incidence of lung metastasis in an orthotopic model using PC-3 cells [69]. In an experimental model of bone metastasis, genistein in combination with docetaxel further inhibited growth over either agent alone [70]. Interestingly, in one report, genistein increased the size of metastatic lymph nodes in a PC-3 orthotopic model [65].
Celecoxib, a well-known cyclooxygenase-2 (COX-2) inhibitor, also inhibits phosphorylation of Akt in prostate cancer cells [71], and may have chemopreventive properties as demonstrated by its ability to reduce formation of PIN and adenocarcinoma in murine and rat models of prostate cancer [72-76]. However, because of the multiple activities of celecoxib, the observed effects cannot be solely attributed to inhibition of Akt. To further delineate anti-Akt activities of celecoxib, a structural analogue of celecoxib, dimethylcelecoxib (DMC), which does not posses COX-2 inhibitory activity has been developed. Administration of DMC to animals bearing PC-3 tumors resulted in inhibition of Akt phosphorylation but also in inhibition of PDK1 and was accompanied by decreased tumor growth [77].
There are two other recent additions to the family of Akt inhibitors, deguelin and GSK690693. Deguelin, a rotenoid, has been shown to inhibit Akt activation in vitro [78, 79] and PC-3 tumor growth in vivo [80]. However, as with other naturally occurring compounds, deguelin appears to have activity against multiple other molecules (i.e., heat-shock protein (HSP 90); nuclear factor kappa B (NFκB)) [79-82]. GSK690693, a compound that competes for ATP-binding sites on Akt, inhibited proliferation of PC-3 and DU 145 cells in vitro and caused inhibition of LNCaP tumor growth in vivo [81].
mTOR Inhibition
mTOR inhibitors have met with the most success among the inhibitors of the PI3K/Akt/mTOR pathway in treating solid tumors and have also received the most attention in the treatment of prostate cancer. The most extensively studied mTOR inhibitors are rapamycin and its analogues, including RAD-001 (everolimus), CCI-779 (temsirolimus), and AP23573 (deforolimus). A number of studies have demonstrated efficacy of these inhibitors against prostate cancer cell lines and xenografts.
Rapamycin
Rapamycin is a macrolide antibiotic with immunosuppressive and anticancer activities. It was originally found in the soil on Easter Island (known by natives as Rapa Nui) and was eventually isolated from the bacteria Streptomyces hygroscopicus [82]. The precise mechanisms of mTOR inhibition by rapamycin are not fully understood; however, it is known that rapamycin associates with FK506 binding protein 12 (FKBP12), and this complex then binds mTOR, resulting in inhibition of mTOR kinase activity. Chronic exposure to rapamycin also decreases levels of phosphorylated Akt [83]. In contrast, short-term treatment with rapamycin increases levels of phospho-Akt, potentially representing activation of the Akt survival pathway, a means for rapamycin resistance [84].
Studies of mTOR inhibition have increased our understanding of the roles of mTOR and its function in several cellular pathways important for prostate cancer development and progression. Rapamycin treatment decreased levels of the phosphorylated substrates of mTOR (i.e., activated S6K) and led to cell-cycle arrest in PC-3 and DU 145 cells [84-87]. Rapamycin also decreased levels of p4E-BP1, leading to an increase in bound (and inactive) eIF4E [84, 86, 87]. Several studies have focused on the changes in gene expression that occur after treatment with rapamycin: increased expression of bone morphogenetic protein 4 (BMP4), suppression of follistatin and a resultant increase in Smad activity have been detected in LNCaP and PC-3 cells treated with rapamycin, implicating the effects on transforming growth factor beta (TGF[notdef]) signaling [88]. Rapamycin has also decreased HIF-1α expression in PC-3 cells despite placement in hypoxic environments, with further decreases observed when rapamycin was used in combination with histone deacytelase inhibitors [89].
There are also emerging data suggesting that mechanism of rapamycin action may be cell-specific. From the two mTOR complexes (mTORC1 and mTORC2) only mTORC1 has been thought to be sensitive to rapamycin. mTORC2 has been considered resistant to mTOR inhibition. However, new evidence suggests that mTORC2 is inhibited by prolonged exposure to rapamycin, but only in certain cells. Interestingly, suppression of mTORC2 was demonstrated in PC-3 prostate cancer [22, 90]. Thus, while mTORC1 is inhibited by rapamycin in all cells, mTORC2 inhibition is likely cell-dependent. This differential effect lends some insight into why mTOR inhibition may be an effective therapy for some tumors and not others, and the identification of the molecular characteristics associated with mTORC2 susceptibility to rapamycin remains an important goal. This could further inform the use of mTOR inhibitors in future clinical trials.
Rapamycin Analogues
Although limited, there are reports on in vitro and pre-clinical investigations demonstrating the efficacy of the rapamycin analogs CCI-779 and RAD-001 in the treatment of prostate cancer. CCI-779 inhibited growth of PC-3 and DU 145 cells in a dose-dependent manner in vitro, and in vivo, reduced tumor volumes in PC-3 and DU 145 xenografts [91]. RAD-001 treatment resulted in decreased proliferation of prostate cancer cells in vitro [92, 93] and reversed PIN lesions in vivo through HIF-1α-dependent pathways in Akt-1 transgenic mice [94]. There are no published reports on the rapamycin analog AP23573 (deforolimus) in prostate cancer at present. Nonetheless, all three analogs, along with rapamycin, are currently under investigation in clinical trials for treatment of prostate cancer (Table 2).
Table 2.
Clinical Trials of mTOR Inhibitors in Prostate Cancer
| mTOR Inhibitor | Other Drugs Tested | Institution/Sponsor | Patients | Outcomes | Phase | Status |
|---|---|---|---|---|---|---|
| RAD-001a | Docetaxel Bevacizumab | Cedars-Sinai Medical Center, Novartis Pharmaceuticals, Genentech | Metastatic CRPC | Survival/response, safety | I/II | Enrolling |
| RAD-001b | Docetaxel | Dana-Farber Cancer Institute, Novartis Pharmaceuticals, Massachusetts General Hospital, Beth Israel Deaconess Medical Center, Oregon Health and Science University | Metastatic CRPC | Survival/response, safety | I/II | Enrolling |
| RAD-001c | Duke University | Metastatic CRPC | PSA response, changes in mTOR pathway (tissue bx) | II | Enrolling | |
| RAD-001d | Bicalutamide | Dana-Farber Cancer Institute, Beth Israel Deaconess Medial Center, Novartis | CRPC | Survival/response, safety | II | Enrolling |
| RAD-001e | Gefitinib | Memorial Sloan-Kettering Cancer Center, NCI | Glioblastoma multiforme or metastatic CRPC | Survival/response, safety | I/II | Ongoing, not enrolling |
| AP23573f | Ariad Pharmaceuticals | Taxane-resistant CRPC | Survival/response, safety, molecular markers | II | Ongoing, not enrolling | |
| CCI-779g | Jonsson Comprehensive Cancer Center, NCI | High risk newly dx'd CaP undergoing RP | mTOR pathway markers | II | Ongoing, not enrolling | |
| CCI-779h | Wyeth Pharmaceuticals | Newly dx'd CaP undergoing RP | mTOR pathway markers | II | Completed | |
| RAD-001i | Androgen deprivation | Sheba Medical Center | Intermediate or high-risk CaP undergoing RP or RadRx | mTOR pathway markers, time to biochemical failure | II | Not yet open |
| Rapamycinj | Sidney Kimmel Comprehensive Cancer Center, NCI | Intermediate or high-risk CaP undergoing RP | Safety, mTOR pathway markers, PSA response | I/II | Enrolling |
Gross, M.E. NCT00574769.
Taplin, M.E. NCT00459186.
George, D.J. NCT00629525.
Taplin, ME. NCT00630344.
Scher, H.I.; Rosen, N.; Abrey, L.E. NCT00085566.
Bedrosian, C. NCT00110188.
Sawyers, C. NCT00071968.
Carducci, M.A. NCT00311623.
In attempts to find improved efficacy, much focus has been placed on finding therapies for advanced prostate cancer with synergistic or additive effects. A key challenge with the use of mTOR and other signal transduction inhibitors is the overlap of signaling pathways, enabling cells to bypass the targeted molecule when exposed to these inhibitors. Resistance to signal transduction inhibitors likely arises from either mutations of key factors in the pathway that allow the signaling cascade to proceed or through upregulation of alternative pathways which enable cell growth and survival via different mechanisms [95]. Therefore, a large number of studies have focused on mTOR inhibition as part of a combination regimen rather than as monotherapy (see Table 1).
A combination of rapamycin and receptor tyrosine kinase inhibitors decreased survival of LNCaP and CWR22Rv1 cells in vitro [93], and a combination of rapamycin and D-glucosamine (an inhibitor of p70-S6K) increased growth inhibition of DU 145 cells [96]. Rapamycin, in combination with an insulin receptor substrate (IRS-1) antisense oligodeoxinucleotide exhibited a more pronounced inhibition of PC-3 tumor growth than treatment with the IRS-1 antisense alone [97]. Growth inhibition of PC-3 and C4-2 tumors was increased with the combination of rapamycin and histone deactylase inhibitors over either agent alone [98]. CCI-779 reversed doxorubicin resistance of PC-3 and DU 145 tumors [35] and had additive effects when used in combination with docetaxel [91]. RAD-001 used in combination with an epidermal growth factor receptor inhibitor (gefitinib) and a novel anti-androgen, VN/124-1, had additive inhibitory effects on growth of LNCaP cells in vitro [99]. RAD-001 also sensitized prostate cancer cells to radiation [92]. We have recently shown that RAD-001 in combination with zoledronic acid and docetaxel more effectively inhibited growth of prostate tumor cells in the bone environment over any of these agents alone [100].
INTERACTION BETWEEN PI3K/Akt/mTOR PATHWAY AND ANDROGEN RECEPTOR
Detailed investigations have shown that crosstalk exists between the PI3K/Akt/mTOR and AR signaling pathways. AR is a key modulator of growth and development of the prostate and of prostate cancer progression [101-104]. AR-mediated transcription is tightly controlled and mechanisms of regulation of AR-transcriptional activity include association with transcriptional cofactors as well as phosphorylation and acetylation [105-110]. A better understanding of the molecular interactions and crosstalk between AR and other signaling pathways might have a dramatic positive impact on strategies to treat prostate cancer [111, 112].
Increasing evidence suggests that key factors of the PI3K/Akt/mTOR pathway may directly regulate the expression and transcriptional activity of AR [113, 114]. In particular, it has been demonstrated that AR phosphorylation and activation by Akt occurs predominantly at low androgen concentrations, suggesting a significant role of Akt in stimulating cell growth in the castrate state [115, 116]. Inhibition of the PI3K/Akt pathway with LY294002 decreased DHT-induced expression of AR in LNCaP cells, while expression of a dominant-negative Akt blocked AR expression [116]. Conversely, stimulation of LNCaP cells with DHT led to AR-mediated activation of mTOR independent of PI3K/Akt stimulation [117]. Recent data has also shown that androgen-dependent LNCaP cells respond weakly to mTOR inhibition in vitro, while growth of the castrate-resistant C4-2 cells is significantly reduced [118]. Reintroduction of PTEN in C4-2 cells increased their sensitivity to androgen ablation with bicalutimide [119]. Furthermore, increased levels of phospho-mTOR and phospho-Akt have been detected in high-passage LNCaP cells after treatment with an androgen inhibitor [99]. Interestingly, treatment with the mTOR inhibitors RAD-001 or rapamycin has resulted in increased AR-transcriptional activity in both high-passage/androgen-independent and low passage/androgen-dependent LNCaP cells [120, 121].
A recent report has demonstrated the clinical relevance of these in vitro results. A comparison of matched hormone-sensitive and hormone-resistant tissues from patients who progressed to CRPC revealed that upregulation of the PI3K/Akt pathway was associated with AR phosphorylation during transition from a hormone-sensitive to a hormone-refractory state [41]. Furthermore, increases in phospho-Akt and phospho-AR were each associated with decreased disease-specific survival. These results together suggest that, as clinical trials with inhibitors of the PI3K/Akt/mTOR pathway move forward, efficacy may be highly dependent upon patient populations in terms of exposure to hormonal therapies and resistance to castration.
CLINICAL USE OF PI3K/Akt/mTOR INHIBITORS IN PROSTATE CANCER
The results of in vitro and preclinical studies suggest that, due to adverse effects, current inhibitors of PI3K and Akt may have limited use in clinical practice. At present, the most promising inhibitors of PIK3/Akt/mTOR pathway for the treatment of prostate cancer are mTOR inhibitors, some of which are already in clinical use for other pathological conditions as well as for other malignancies.
Only two of the compounds that inhibit Akt activation, perifosine and celecoxib, have been investigated in the clinical setting. In a trial investigating the effects of perifosine in patients with metastatic CRPC (n=19), no complete or partial responses were detected and only four patients had a PSA stabilization for 12 weeks or more [122]. There was, however, a decrease in the detection of circulating tumor cells in 11/14 of these patients after treatment. These results may be significant since circulating tumor cells are considered evidence of disseminated disease [123], and decreases in circulating tumor cells have been shown to correlate with increased survival in patients with metastatic breast cancer [124]. Long term follow up is needed to determine whether these effects of perifosine will result in clinical improvements. In a phase II study in men with biochemically recurrent, hormone-sensitive prostate cancer, perifosine administration resulted in PSA decreases in 5/24 patients; however, no patients met the predefined criteria for a true response (a 50% or greater reduction in PSA) [125]. A phase II clinical trial investigating the use of celecoxib in patients with biochemically recurrent prostate cancer (n=40) after radiation or radical prostatectomy showed a significant inhibition of PSA doubling time [126]. Three months after treatment initiation, 90% of patients had a lower PSA doubling time with 11/40 experiencing a decrease in absolute PSA levels. However, a trial of celecoxib vs. placebo in a similar patient population (n=78) did not show any differences in PSA doubling time [127]. Celecoxib in combination with docetaxel and estramustine in CRPC patients resulted in a median survival of 19.2 months, relatively similar to TAX 327 and SWOG 99-16 [128]. Further trials, such as STAMPEDE, will help to determine the role of COX-2 inhibition in treating advanced prostate cancer and whether any anti-cancer activity is due to its Akt-inhibiting properties.
Clinical investigation of mTOR inhibitors in the oncologic setting is a relatively new, but promising area of investigation that started within the past decade. Rapamycin was initially developed as an immunosuppressive agent and was approved by the FDA in 1998 for this purpose [33]. The pharmacokinetics of this drug is well known, with excellent absorption after oral dosing and peak concentrations at approximately 1.5 hours after administration. The incidence of severe toxicity reactions has been rare, and only mild adverse effects including hyperlipidemia, thrombocytopenia, leukopenia, diarrhea, skin rash, pneumonitis, and electrolyte abnormalities have been reported. There are also quickly accumulating data regarding pharmacologic profiles of rapamycin analogs showing that these analogs are well-tolerated and exhibit minimal negative side effects [129-134].
Efficacy of mTOR inhibition was demonstrated in early phase clinical trials in a number of malignancies, and mTOR inhibitors are now in clinical development in endometrial cancer, breast cancer, glioblastoma, lymphoma, and sarcomas [135]. CCI-779 was investigated in a large phase III trial in advanced renal cell carcinoma, and median overall survival was significantly increased vs. IFN-α (10.9 months vs. 7.3 months, p=0.008) [136]. CCI-779 (temsirolimus) was subsequently approved by the FDA in 2007 for the treatment of advanced renal cell carcinoma.
In prostate cancer, there are several ongoing phase I and II clinical trials with mTOR inhibitors (Table 2). Some of these trials are designed in the neoadjuvant and/or the adjuvant setting. These trials are focused on analysis of key factors in mTOR signaling and their alterations in response to mTOR inhibition. Certainly, the ability to assess the molecular response to therapy is one of the central advantages of cell signaling modifiers. Molecular stratification of patients to mTOR inhibitor therapy may help to identify those patients most likely to benefit from treatment while sparing those patients who are unlikely to respond. Neoadjuvant use of mTOR inhibition could also provide an opportunity to target tumor cells before they have accumulated the large number of mutations that typically arise with advanced disease. PTEN mutations and deletions within primary tumors have been associated with an increased risk of metastasis [33] and early targeting may prove to be beneficial in preventing metastatic spread. Additionally, it has been suggested that mTOR inhibitors could be used as chemopreventive agents in patients who have deleted or inactivated PTEN in benign prostate epithilium or PIN at the time of prostate needle biopsy [7]. Because the clinical trials testing mTOR inhibitors are ongoing or still accruing patients, there are only limited results available at this time. A preliminary analysis of a phase II clinical trial of neoadjuvant administration of RAD-001 in patients prior to radical prostatectomy has not only showed that the drug is well tolerated but also that it decreases the levels of activated mTOR substrates in the primary tumor.1
A majority of the ongoing trials in prostate cancer are assessing mTOR inhibition in the setting of CRPC. One study in patients with metastatic CRPC is evaluating the cellular and molecular responses to RAD-001 by comparing pre- and post-treatment bone-derived tumor biopsies.2 Results of this trial, similar to the neoadjuvant studies assessing phenotypic changes in the primary tumor, will provide important information regarding the efficacy of these therapies on a molecular level.
Because of the heterogeneity of prostate cancer [137, 138] and the ability of tumor cells to undergo cellular alterations that allow survival under changing conditions, most of the trials investigating mTOR inhibition in CRPC utilize combinations of drugs. The majority of these trials are designed to provide a horizontal blockade within the cancer cell. Horizontal blockade refers to the simultaneous inhibition of multiple different targets. For example, inhibiting a MAP kinase at the same time as mTOR may block one of the key pathways that overlaps with the PI3K/Akt/mTOR pathway. Another approach to horizontal blockade involves targeting different cell types, such as targeting endothelial cells with a VEGF inhibitor, pericytes with a PDGF inhibitor, and/or osteoblasts with an endothelin A inhibitor, while also targeting the tumor cell directly [139]. The second approach to combination therapy is to administer agents according to a vertical blockade rationale. A vertical blockade is designed to target multiple key factors within one specific pathway. For example, simultaneous inhibition of PI3K, Akt, and mTOR may be required to fully suppress activity of this pathway. Since upstream molecules in the mTOR pathway may be upregulated with administration of mTOR inhibitors—proposed as mechanism for mTOR inhibitor resistance [84, 95]—vertical blockade may prevent the shunting of upstream molecules down alternative signaling pathways. However, initial analysis of AP23573 used in combination with the epidermal growth factor inhibitor gefitinib in patients with advanced prostate cancer showed that only 5/29 patients had no disease progression at 12 weeks.3
CONCLUSIONS
Increasing molecular evidence from in vitro studies, prostate cancer animal models, and staining of human prostate tissues demonstrates that the PI3K/Akt/mTOR pathway plays a critical role in prostate cancer development and progression. Data generated using clinical samples have shown that increased expression of key factors in this pathway correlates with disease stage and lower survival rates. Inhibition of the PI3K/Akt/mTOR pathway in prostate cancer models has lent significant insight into the mechanisms behind the development of advanced prostate cancer. Unfortunately the in vitro studies have also demonstrated that present inhibitors of PI3K and Akt are not highly specific, and preclinical studies have shown that use of these compounds is associated with significant negative side effects. More promising, currently, are the inhibitors of mTOR. These have been shown to inhibit proliferation of prostate tumor cells and show high specificity for mTOR in vitro, and these inhibitors have inhibited tumor growth in the preclinical setting with minimal negative side effects.
The in vitro and preclinical results are encouraging, and multiple phase I and phase II clinical trials are underway to evaluate the efficacy of mTOR inhibitors in both the neoadjuvant setting and in advanced prostate cancer patients. Because of the ability of tumor cells to adapt to new conditions, mTOR inhibitors are also being investigated in combination with other drugs. Since the clinical trials in prostate cancer are in their early stages, it remains unclear what role mTOR inhibitors will have in the care of patients with prostate cancer. The results from these trials will help to determine the efficacy of mTOR inhibition in prostate cancer, further the mechanistic understanding of these therapies, and move prostate cancer treatment towards rational strategies based on tumor-specific markers. Given the tremendous heterogeneity of prostate cancers, particularly metastatic cancers, therapies based on protein expression profiles of individual tumors may provide the best chance for success. Further work will determine whether the growing knowledge of tumor biology can be translated into real clinical gains.
ACKNOWLEDGEMENTS
Research in the E.C. laboratory is supported by National Institutes of Health grants PO1 CA85859-01A and 5R01CA125395-02, and the Pacific Northwest SPORE P50 CA097186 Pilot Award. T.M.M. and T.D.K. were supported by Ruth L. Kirschstein National Research Training Grant. While we have attempted to highlight key findings in the field of PIK3/Akt/mTOR signaling in the context of prostate cancer, we apologize for the inevitable and inadvertent omissions of important work.
ABBREVIATIONS
- AR
androgen receptor
- BAD
Bcl-xL/Bcl-2 associated death promoter
- BMP4
bone morphogenetic protein 4
- Chk1
checkpoint kinase 1
- CRPC
castration resistant prostate cancer
- DMC
dimethyl-celecoxib
- EGFR
epidermal growth factor receptor
- eIF4F
eukaryotic initiation factor 4F
- FKB12
FK506 binding protein
- FOXO
forkhead box O
- HIF-1α
hypoxia inducible transcription factor 1 alpha
- HSP 90
heat shock protein 90
- IGFR
insulin-like growth factor receptor
- IRS-1
insulin receptor substrate
- MAP
mitogen activated protein
- MDM2
murine double minute
- mTOR
mammalian target of rapamycin
- PDGFR
platelet derived growth factor receptor
- PDK1
phosphoinositide dependent protein kinase 1
- PIN
prostatic intraepithelial neoplasia
- PIP2
phosphatidylinositol-4,5-diphosphate
- PIP3
phosphatidylinositol-3,4,5-triphosphate
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- PSA
prostate specific antigen
- PI3K
phosphatydidyl inositol-3-OH kinase
- S6K
p70-S6 kinase
- TGFβ
transforming growth factor beta
- TSC2
tuberous sclerosis complex 2
- 4E-BP1
4E binding protein 1
Footnotes
Lerut, E; Roskams, T; Goossens, E; Bootle, D; Dimitrijevic, S; Stumm, M; Shand, N; van Poppel H. Molecular pharmacodynamic (MPD) evaluation of dose and schedule of RAD001 (everolimus) in patients with operable prostate carcinoma (PC). J Clin Oncol. 2005, 25, 3071.
Geoge, D.A. Molecular, genetic, and genomic assessments from patients treated with RAD001. NCT00636090.
Shaffer, D.R.; Abrey, L; Beekman K. A phase I/II trial of RAD001 with gefitinib in patients with castrate metastatic prostate cancer and gliobastoma multiforme. J Clin Oncol. 2006, 24, 14520.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer Statistics, 2008. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Garcia J, Danielpour D. Mammalian target of rapamycin inhibition as a therapeutic strategy in the management of urologic malignancies. Mol. Cancer Ther. 2008;7:1347–1354. doi: 10.1158/1535-7163.MCT-07-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Ittmann M, Ayala G, Tsai M, Amat OR, Wheeler T, Miles B, Kadmon D, Thompson T. The emerging role of the PI3-K-Akt pathway in prostate cancer progression. Prostate Cancer Prostatic Dis. 2005;8:108–118. doi: 10.1038/sj.pcan.4500776. [DOI] [PubMed] [Google Scholar]
- 4.Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 5.Nelson E, Evans CP, Mack PC, Devere-White R, Lara PJ. Inhibition of Akt pathways in the treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2007;10:331–339. doi: 10.1038/sj.pcan.4500974. [DOI] [PubMed] [Google Scholar]
- 6.Pommery N, Henichart J. Involvement of PI3K/Akt pathway in prostate cancer--potential strategies for developing targeted therapies. Mini. Rev. Med. Chem. 2005;5:1125–1132. doi: 10.2174/138955705774933356. [DOI] [PubMed] [Google Scholar]
- 7.Tolcher AW. Novel therapeutic molecular targets for prostate cancer: the mTOR signaling pathway and epidermal growth factor receptor. J. Urol. 2004;171:S41–44. doi: 10.1097/01.ju.0000108100.53239.b7. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Kreisberg J, Ghosh P. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr. Cancer Drug Targets. 2007;7:591–604. doi: 10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 9.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 10.Petrylak DP, Tangen CM, Hussain MHA, Lara PN, Jr., Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 11.Berry W, Dakhil S, Modiano M, Gregurich M, Asmar L. Phase III study of mitoxantrone plus low dose prednisone versus low dose prednisone alone in patients with asymptomatic hormone refractory prostate cancer. J. Urol. 2002;168:2439–2443. doi: 10.1016/S0022-5347(05)64163-8. [DOI] [PubMed] [Google Scholar]
- 12.Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, Armitage GR, Wilson JJ, Venner PM, Coppin CM, Murphy KC. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J. Clin. Oncol. 1996;14:1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 13.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 14.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr., Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 15.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 16.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell. Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 17.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 18.Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proc. Natl. Acad. Sci. USA. 2001;98:7037–7044. doi: 10.1073/pnas.121145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5'TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbert TP, Tee AR, Proud CG. The extracellular signal-regulated kinase pathway regulates the phosphorylation of 4E-BP1 at multiple sites. J. Biol. Chem. 2002;277:11591–11596. doi: 10.1074/jbc.M110367200. [DOI] [PubMed] [Google Scholar]
- 21.Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiol. Mol. Biol. Rev. 2005;69:79–100. doi: 10.1128/MMBR.69.1.79-100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Fiovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, brease, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 24.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 25.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sansal I, Sellers WR. The Biology and Clinical Relevance of the PTEN Tumor Suppressor Pathway. J. Clin. Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 27.Cairns P, Okami K, Halachmi S, Halchmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- 28.Gray IC, Phillips SMA, Lee SJ, Neoptolemos JP, Weissenbach J, Spurr NK. Loss of the chromosomal region 10q23-25 in prostate cancer. Cancer Res. 1995;55:4800–4803. [PubMed] [Google Scholar]
- 29.Komiya A, Suzuki H, Ueda T, Yatani R, Emi M, Ito H, Shimazaki J. Allelic losses at loci on chromosome 10 are associated with metastasis and progression of human prostate cancer. Genes Chromosomes Cancer. 1996;17:245–253. doi: 10.1002/(SICI)1098-2264(199612)17:4<245::AID-GCC6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 30.McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59:4291–4296. [PubMed] [Google Scholar]
- 31.Kremer CL, Klein RR, Mendelson J, Browne W, Samadzedeh LK, Vanpatten K, Highstrom L, Pestano GA, Nagle RB. Expression of mTOR signaling pathway markers in prostate cancer progression. Prostate. 2006;66:1203–1212. doi: 10.1002/pros.20410. [DOI] [PubMed] [Google Scholar]
- 32.Dreher T, Zentgraf H, Abel U, Kappeler A, Michel MS, Bleyl U, Grobholz R. Reduction of PTEN and p27kip1 expression correlates with tumor grade in prostate cancer. Analysis in radical prostatectomy specimens and needle biopsies. Virchows Arch. 2004;444:509–517. doi: 10.1007/s00428-004-1004-6. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz M, Grignard G, Margue C, Dippel W, Capesius C, Mossong J, Nathan M, Giacchi S, Scheiden R, Kieffer N. Complete loss of PTEN expression as a possible early prognostic marker for prostate cancer metastasis. Int. J. Cancer. 2007;120:1284–1292. doi: 10.1002/ijc.22359. [DOI] [PubMed] [Google Scholar]
- 34.Bedolla R, Prihoda TJ, Kreisberg JI, Malik SN, Krishnegowda NK, Troyer DA, Ghosh PM. Determining risk of biochemical recurrence in prostate cancer by immunohistochemical detection of PTEN expression and Akt activation. Clin. Cancer Res. 2007;13:3860–3867. doi: 10.1158/1078-0432.CCR-07-0091. [DOI] [PubMed] [Google Scholar]
- 35.Grünwald V, DeGraffenried L, Russel D, Friedrichs WE, Ray RB, Hidalgo M. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 2002;62:6141–6145. [PubMed] [Google Scholar]
- 36.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Buman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 37.Liao Y, Grobholz R, Abel U, Trojan L, Michel MS, Angel P, Mayer D. Increase of AKT/PKB expression correlates with Gleason pattern in human prostate cancer. Int. J. Cancer. 2003;107:676–680. doi: 10.1002/ijc.11471. [DOI] [PubMed] [Google Scholar]
- 38.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, Kreisberg JI. Immunohistochemical demonstration of phospho-Akt in high gleason grade prostate cancer. Clin. Cancer Res. 2002;8:1168–1171. [PubMed] [Google Scholar]
- 39.Ayala G, Thompson T, Yang G, Frolov A, Li R, Scardino P, Ohori M, Wheeler T, Harper W. High levels of phosphorylated form of Akt-1 in prostate cancer and non-neoplastic prostate tissues are strong predictors of biochemical recurrence. Clin. Cancer Res. 2004;10:6472–6578. doi: 10.1158/1078-0432.CCR-04-0477. [DOI] [PubMed] [Google Scholar]
- 40.Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S, Ghosh PM. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;64:5232–5236. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 41.McCall P, Gemell LK, Mukherjee R, Bartlett JMS, Edwards J. Phosphorylation of the androgen receptor is associated with reduced survival in hormone-refractory prostate cancer patients. Br. J. Cancer. 2008;98:1094–1101. doi: 10.1038/sj.bjc.6604152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Page C, Koumakpayi IH, Alam-Fahmy M, Mes-Masson AM, Saad F. Expression and localisation of Akt-1, Akt-2 and Akt-3 correlate with clinical outcome of prostate cancer patients. Br. J. Cancer. 2006;94:1906–1912. doi: 10.1038/sj.bjc.6603184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottschalk AR, Doan A, Nakamura JL, Stokoe D, Haas-Kogan DA. Inhibition of phosphatidylinositol-3-kinase causes increased sensitivity to radiation through a PKB-dependent mechanism. Int. J. Radiat. Oncol. Biol. Phys. 2005;63:1221–1227. doi: 10.1016/j.ijrobp.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 44.Shukla S, Maclennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int. J. Cancer. 2007;121:1424–1432. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- 45.Fang J, Ding M, Yang L, Liu LZ, Jiang BH. PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell. Signal. 2007;19:2487–2497. doi: 10.1016/j.cellsig.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beresford SA, Davies MA, Gallick GE, Donato NJ. Differential effects of phosphatidylinositol-3/Akt-kinase inhibition on apoptotic sensitization to cytokines in LNCaP and PCc-3 prostate cancer cells. J. Interferon Cytokine Res. 2001;21:313–322. doi: 10.1089/107999001300177501. [DOI] [PubMed] [Google Scholar]
- 47.Brunn G, Williams J, Sabers C, Wiederrecht G, Lawrence JJ, Abraham R. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 48.Stein R. Prospects for phosphoinositide 3-kinase inhibition as a cancer treatment. Endocr. Relat. Cancer. 2001;8:237–248. doi: 10.1677/erc.0.0080237. [DOI] [PubMed] [Google Scholar]
- 49.El-Kholy W, Macdonald PE, Lin JH, Wang J, Fox JM, Light PE, Wang Q, Tsushima RG, Wheeler MB. The phosphatidylinositol 3-kinase inhibitor LY294002 potently blocks K(V) currents via a direct mechanism. FASEB J. 2003;17:720–722. doi: 10.1096/fj.02-0802fje. [DOI] [PubMed] [Google Scholar]
- 50.Pasapera Limón A, Herrera-Muñoz J, Gutiérrez-Sagal R, Ulloa-Aguirre A. The phosphatidylinositol 3-kinase inhibitor LY294002 binds the estrogen receptor and inhibits 17beta-estradiol-induced transcriptional activity of an estrogen sensitive reporter gene. Mol. Cell. Endocrinol. 2003;200:199–202. doi: 10.1016/s0303-7207(02)00421-5. [DOI] [PubMed] [Google Scholar]
- 51.Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindey G. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 52.Reinhold WC, Kouros-Mehr H, Kohn KW, Maunakea AK, Lababidi S, Roschke A, Stover K, Alexander J, Pantazis P, Miller L, Liu E, Kirsch IR, Urasaki Y, Pommier Y, Weinstein JN. Apoptotic susceptibility of cancer cells selected for camptothecin resistance: gene expression profiling, functional analysis, and molecular interaction mapping. Cancer Res. 2003;63:1000–1011. [PubMed] [Google Scholar]
- 53.Collis SJ, Swartz MJ, Nelson WG, DeWeese TL. Enhanced radiation and chemotherapy-mediated cell killing of human cancer cells by small inhibitory RNA silencing of DNA repair factors. Cancer Res. 2003;63:1550–1554. [PubMed] [Google Scholar]
- 54.Lin J, Adam RM, Santiestevan E, Freeman MR. The phosphatidylinositol 3'-kinase pathway is a dominant growth factor-activated cell survival pathway in LNCaP human prostate carcinoma cells. Cancer Res. 1999;59:2891–2897. [PubMed] [Google Scholar]
- 55.Seol JW, Lee YJ, Kang HS, Kim IS, Kim NS, Kwak YG, Kim TH, Seol DW, Park SY. Wortmannin elevates tumor necrosis factor-related apoptosis-inducing ligand sensitivity in LNCaP cells through down-regulation of IAP-2 protein. Exp. Oncol. 2005;27:120–124. [PubMed] [Google Scholar]
- 56.Gupta AK, Cerniglia GJ, Mick R. Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. Int. J. Radiat. Oncol. Biol. Phys. 2003;56:846–853. doi: 10.1016/s0360-3016(03)00214-1. [DOI] [PubMed] [Google Scholar]
- 57.Renner O, Fominaya J, Alonso S, Blanco-Aparicio C, Leal JF, Carnero A. Mst1, RanBP2 and eIF4G are new markers for in vivo PI3K activation in murine and human prostate. Carcinogenesis. 2007;28:1418–1425. doi: 10.1093/carcin/bgm059. [DOI] [PubMed] [Google Scholar]
- 58.Barve A, Khor TO, Hao X, Keum YS, Yang CS, Reddy B, Kong AN. Murine prostate cancer inhibition by dietary phytochemicals-curcumin and phenyethylisothiocyanate. Pharm. Res. 2008;25:2181–2189. doi: 10.1007/s11095-008-9574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shankar S, Ganapathy S, Chen Q, Srivastava RK. Curcumin sensitizes TRAIL-resistant xenografts: molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol. Cancer. 2008;7:16. doi: 10.1186/1476-4598-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21(/WAF1/CIP1). Cell Cycle. 2007;6:2953–2961. doi: 10.4161/cc.6.23.4951. [DOI] [PubMed] [Google Scholar]
- 61.Li M, Zhang Z, Hill D, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 62.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochem. Biophys. Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol. Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 64.Floryk D, Thompson TC. Perifosine induces differentiation and cell death in prostate cancer cells. Cancer Lett. 2008;266:216–226. doi: 10.1016/j.canlet.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hillman GG, Wang Y, Kucuk O, Che M, Doerge DR, Yudelev M, Joiner MC, Marples B, Forman JD, Sarkar FH. Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model. Mol. Cancer Ther. 2004;3:1271–1279. [PubMed] [Google Scholar]
- 66.Li Y, Sarkar F. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin. Cancer Res. 2002;8:2369–2377. [PubMed] [Google Scholar]
- 67.Bemis DL, Capodice JL, Desai M, Buttyan R, Katz AE. A concentrated aglycone isoflavone preparation (GCP) that demonstrates potent anti-prostate cancer activity in vitro and in vivo. Clin. Cancer Res. 2004;10:5282–5292. doi: 10.1158/1078-0432.CCR-03-0828. [DOI] [PubMed] [Google Scholar]
- 68.Wang JE, Eltoum IE, Lamartiniere CA. Genistein chemoprevention of prostate cancer in TRAMP mice. J. Carcinog. 2007;6:3. doi: 10.1186/1477-3163-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lakshman M, Xu L, Ananthanarayanan V, Cooper J, Takimoto C, Helenowski I, Pelling J, Bergan R. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008;68:2024–2032. doi: 10.1158/0008-5472.CAN-07-1246. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Kucuk O, Hussain M, Abrams J, Cher M, Sarkar F. Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res. 2006;66:4816–4825. doi: 10.1158/0008-5472.CAN-05-3752. [DOI] [PubMed] [Google Scholar]
- 71.Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J. Biol Chem. 2000;275:11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- 72.Narayanan BA, Reddy BS, Bosland MC, Nargi D, Horton L, Randolph C, Narayanan NK. Exisulind in combination with celecoxib modulates epidermal growth factor receptor, cyclooxygenase-2, and cyclin D1 against prostate carcinogenesis: in vivo evidence. Clin. Cancer Res. 2007;13:5965–5973. doi: 10.1158/1078-0432.CCR-07-0744. [DOI] [PubMed] [Google Scholar]
- 73.Narayanan BA, Narayanan NK, Pttman B, Reddy BS. Adenocarcina of the mouse prostate growth inhibition by celecoxib: downregulation of transcription factors involved in COX-2 inhibition. Prostate. 2006;16:257–265. doi: 10.1002/pros.20331. [DOI] [PubMed] [Google Scholar]
- 74.Zheng X, Cui XX, Avila GE, Huang MT, Liu Y, Patel J, Kong AN, Paulino R, Shih WJ, Lin Y, Rabson AB, Reddy BS, Conney AH. Atorvastatin and celecoxib inhibit prostate PC-3 tumors in immunodeficient mice. Clin. Cancer Res. 2007;13:5480–5487. doi: 10.1158/1078-0432.CCR-07-0242. [DOI] [PubMed] [Google Scholar]
- 75.Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, Syed DN, Afaq F, Pasha FS, Saleem M, Mukhtar H. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin. Cancer Res. 2007;13:1611–1619. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- 76.Patel MI, Subbaramaiah K, Du B, Chang M, Yang P, Newman RA, Cordon-Cardo C, Thaler HT, Dannenberg AJ. Celecoxib inhibits prostate cancer growth: evidence of a cyclooxygenase-2-independent mechanism. Clin. Cancer Res. 2005;11:1999–2007. doi: 10.1158/1078-0432.CCR-04-1877. [DOI] [PubMed] [Google Scholar]
- 77.Kulp S, Yang Y, Hung C, Chen K, Lai J, Tseng P, Fowble J, Ward P, Chen C. 3-phosphoinositide-dependent protein kinase-1/Akt signaling represents a major cyclooxygenase-2-independent target for celecoxib in prostate cancer cells. Cancer Res. 2004;64:1444–1451. doi: 10.1158/0008-5472.can-03-2396. [DOI] [PubMed] [Google Scholar]
- 78.Peng XH, Karna P, O'Regan RM, Liu X, Naithani R, Moriarty RM, Wood WC, Lee HY, Yang L. Down-regulation of inhibitor of apoptosis proteins by deguelin selectively induces apoptosis in breast cancer cells. Mol. Pharmacol. 2007;71:101–111. doi: 10.1124/mol.106.027367. [DOI] [PubMed] [Google Scholar]
- 79.Nair AS, Shishodia S, Ahn KS, Kunnumakkara AB, Sethi G, Aggarwal BB. Deguelin, an Akt inhibitor, suppresses IkappaBalpha kinase activation leading to suppression of NF-kappaB-regulated gene expression, potentiation of apoptosis, and inhibition of cellular invasion. J. Immunol. 2006;177:5612–5622. doi: 10.4049/jimmunol.177.8.5612. [DOI] [PubMed] [Google Scholar]
- 80.Oh SH, Woo JK, Yazici YD, Myers JN, Kim WY, Jin Q, Hong SS, Park HJ, Suh YG, Kim KW, Hong WK, Lee HY. Structural basis for depletion of heat shock protein 90 client proteins by deguelin. J. Natl. Cancer Inst. 2007;99:949–961. doi: 10.1093/jnci/djm007. [DOI] [PubMed] [Google Scholar]
- 81.Rhodes N, Heerding DA, Duckett DR, Eberwein DJ, Knick VB, Lansing TJ, McConnell RT, Gilmer TM, Zhang SY, Robell K, Kahana JA, Geske RS, Kleymenova EV, Choudhry AE, Lai Z, Leber JD, Minthorn EA, Strum SL, Wood ER, Huang PS, Copeland RA, Kumar R. Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 2008;68:2366–2374. doi: 10.1158/0008-5472.CAN-07-5783. [DOI] [PubMed] [Google Scholar]
- 82.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 83.Edinger A, Linardic C, Chiang G, Thompson C, Abraham R. Differential effects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res. 2003;63:8451–8460. [PubMed] [Google Scholar]
- 84.Sun S, Rosenberg L, Wang X, Zhou Z, Yue P, Fu H, Khuri F. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 85.Gao N, Zhang Z, Jiang B, Shi X. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem. Biophys. Res. Commun. 2003;310:1124–1132. doi: 10.1016/j.bbrc.2003.09.132. [DOI] [PubMed] [Google Scholar]
- 86.Peffley D, Sharma C, Hentosh P, Buechler R. Perillyl alcohol and genistein differentially regulate PKB/Akt and 4E-BP1 phosphorylation as well as eIF4E/eIF4G interactions in human tumor cells. Arch. Biochem. Biophys. 2007;465:266–273. doi: 10.1016/j.abb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 87.MacManus C, Pettigrew J, Seaton A, Wilson C, Maxwell P, Berlingeri S, Purcell C, McGurk M, Johnston P, Waugh D. Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol. Cancer Res. 2007;5:737–748. doi: 10.1158/1541-7786.MCR-07-0032. [DOI] [PubMed] [Google Scholar]
- 88.van der Poel H, Hanrahan C, Zhong H, Simons J. Rapamycin induces Smad activity in prostate cancer cell lines. Urol. Res. 2003;30:380. doi: 10.1007/s00240-002-0282-1. [DOI] [PubMed] [Google Scholar]
- 89.Hudson C, Liu M, Chiang G, Otterness D, Loomis D, Kaper F, Giaccia A, Abraham R. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 91.Wu L, Birle D, Tannock I. Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res. 2005;65:2825–2831. doi: 10.1158/0008-5472.CAN-04-3137. [DOI] [PubMed] [Google Scholar]
- 92.Cao C, Subhawong T, Albert JM, Kim KW, Geng L, Sekhar KR, Gi YJ, Lu B. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66:10040–10047. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- 93.Masiello D, Mohi M, McKnight N, Smith B, Neel B, Balk S, Bubley G. Combining an mTOR antagonist and receptor tyrosine kinase inhibitors for the treatment of prostate cancer. Cancer Biol. Ther. 2007;6:195–201. doi: 10.4161/cbt.6.2.3588. [DOI] [PubMed] [Google Scholar]
- 94.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 95.Huang S, Houghton P. Mechanisms of resistance to rapamycins. Drug Resist. Updat. 2001;4:378–391. doi: 10.1054/drup.2002.0227. [DOI] [PubMed] [Google Scholar]
- 96.Oh HJ, Lee JS, Song DK, Shin DH, Jang BC, Suh SI, Park JW, Suh MH, Baek WK. D-glucosamine inhibits proliferation of human cancer cells through inhibition of p70S6K. Biochem. Biophys. Res. Commun. 2007;360:840–845. doi: 10.1016/j.bbrc.2007.06.137. [DOI] [PubMed] [Google Scholar]
- 97.Oliveira JC, Souza KK, Dias MM, Faria MC, Ropelle ER, Flores MB, Ueno M, Velloso LA, Saad ST, Saad MJ, Carvalheira JB. Antineoplastic effect of rapamycin is potentiated by inhibition of IRS-1 signaling in prostate cancer cells xenografts. J. Cancer Res. Clin. Oncol. 2008;134:833–839. doi: 10.1007/s00432-008-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Verheul HM, Salumbides B, Van Erp K, Hammers H, Qian DZ, Sanni T, Atadja P, Pili R. Combination strategy targeting the hypoxia inducible factor-1 alpha with mammalian target of rapamycin and histone deacetylase inhibitors. Clin. Cancer Res. 2008;14:3589–3597. doi: 10.1158/1078-0432.CCR-07-4306. [DOI] [PubMed] [Google Scholar]
- 99.Schayowitz A, Sabnis G, Njar VCO, Brodie AMH. Synergistic effect of a novel antiandrogen, VN/124-1, and signal transduction inhibitors in prostate cancer progression to hormone independence in vitro. Mol. Cancer Ther. 2008;7:121–132. doi: 10.1158/1535-7163.MCT-07-0581. [DOI] [PubMed] [Google Scholar]
- 100.Morgan TM, Pitts TE, Gross TS, Poliachik SL, Vessella RL, Corey E. RAD001 (Everolimus) inhibits growth of prostate cancer in the bone and the inhibitory effects are increased by combination with docetaxel and zoledronic acid. Prostate. 2008;68:861–871. doi: 10.1002/pros.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 1: Modifications to the androgen receptor. BJU Int. 2005;95:1320–1326. doi: 10.1111/j.1464-410X.2005.05526.x. [DOI] [PubMed] [Google Scholar]
- 102.Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 2: Androgen-receptor cofactors and bypass pathways. BJU Int. 2005;95:1327–1335. doi: 10.1111/j.1464-410X.2005.05527.x. [DOI] [PubMed] [Google Scholar]
- 103.Culig Z. Androgen receptor cross-talk with cell signaling pathways. Growth Factors. 2004;22:179–184. doi: 10.1080/08977190412331279908. [DOI] [PubMed] [Google Scholar]
- 104.Gioeli D. Signal transduction in prostate cancer progression. Clin. Sci. 2005;108:293–308. doi: 10.1042/CS20040329. [DOI] [PubMed] [Google Scholar]
- 105.Abreu-Martin MT, Chari A, Palladino AA, Craft NA, Sawyers CL. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol. Cell. Biol. 1999;19:5143–5154. doi: 10.1128/mcb.19.7.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Culig Z, Comuzzi B, Steiner H, Bartsch G, Hobisch A. Expression and function of androgen receptor coactivators in prostate cancer. J. Steroid Biochem. Mol. Biol. 2004;92:265–271. doi: 10.1016/j.jsbmb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 107.Fu M, Rao M, Wang C, Sakamaki T, Wang J, Di Vizio D, Zhang X, Albanese C, Balk S, Chang C, Fan S, Rosen E, Palvimo JJ, Janne OA, Muratoglu S, Avantaggiati ML, Pestell RG. Acetylation of androgen receptor enhances coactivator binding and promotes prostate cancer cell growth. Mol. Cell. Biol. 2003;23:8563–8575. doi: 10.1128/MCB.23.23.8563-8575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fu M, Wang C, Reutens AT, Wang J, Angeletti RH, Siconolfi-Baez L, Ogryzko V, Avantaggiati ML, Pestell RG. p300 and p300/cAMP-response element-binding protein-associated factor acetylate the androgen receptor at sites governing hormone-dependent transactivation. J. Biol. Chem. 2000;275:20853–20860. doi: 10.1074/jbc.M000660200. [DOI] [PubMed] [Google Scholar]
- 109.Fu M, Wang C, Wang J, Zhang X, Sakamaki T, Yeung YG, Chang C, Hopp T, Fuqua SA, Jaffray E, Hay RT, Palvimo JJ, Janne OA, Pestell RG. Androgen receptor acetylation governs trans activation and MEKK1-induced apoptosis without affecting in vitro sumoylation and trans-repression function. Mol. Cell. Biol. 2002;22:3373–3388. doi: 10.1128/MCB.22.10.3373-3388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fu M, Wang C, Zhang X, Pestell RG. Acetylation of nuclear receptors in cellular growth and apoptosis. Biochem. Pharmacol. 2004;68:1199–1208. doi: 10.1016/j.bcp.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 111.Danielpour D. Functions and regulation of transforming growth factor-beta (TGF-beta) in the prostate. Eur. J. Cancer. 2005;41:846–857. doi: 10.1016/j.ejca.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 112.Quinn D, Henshall S, Sutherland RL. Molecular markers of prostate cancer outcome. Eur. J. Cancer. 2005;41:858–887. doi: 10.1016/j.ejca.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 113.Lin HK, Yeh S, Kang HY, Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc. Natl. Acad. Sci. USA. 2001;98:7200–7205. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang L, Xie S, Jamaluddin MS, Altuwaijri S, Ni J, Kim E, Chen YT, Hu YC, Wang L, Chuang KH, Wu CT, Chang C. Induction of androgen receptor expression by phosphatidylinositol 3-kinase/Akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. J. Biol. Chem. 2005;280:33558–33565. doi: 10.1074/jbc.M504461200. [DOI] [PubMed] [Google Scholar]
- 115.Wen Y, Hu MCT, Makino K, Spohn B, Bartholomeusz G, Yan D-H, Hung M-C. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- 116.Manin M, Baon S, Goossens K, Beaudoin C, Jean C, Veyssiere G, Verhoeven G, Morel L. Androgen receptor expression is regulated by the phosphoinositide 3-kinase/Akt pathway in normal and tumoral epithelial cells. Biochem. J. 2002;366:729–736. doi: 10.1042/BJ20020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–7792. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- 118.Ghosh PM, Malik SN, Bedolla RG, Wang Y, Mikhailova M, Prihoda TJ, Troyer DA, Kreisberg JI. Signal transduction pathways in androgen-dependent and independent prostate cancer cell proliferation. Endocr. Relat. Cancer. 2005;12:119–134. doi: 10.1677/erc.1.00835. [DOI] [PubMed] [Google Scholar]
- 119.Wu Z, Conaway M, Gioeli D, Weber MJ, Theodorescu D. Conditional expression of PTEN alters the androgen responsiveness of prostate cancer cells. Prostate. 2006;66:1114–1123. doi: 10.1002/pros.20447. [DOI] [PubMed] [Google Scholar]
- 120.Cinar B, De Benedetti A, Freeman MR. Post-transcriptional regulation of the androgen receptor by mammalian target of rapamycin. Cancer Res. 2005;65:2547–2553. doi: 10.1158/0008-5472.CAN-04-3411. [DOI] [PubMed] [Google Scholar]
- 121.Lin H, Hu Y, Yang L, Altuwaijri S, Chen Y, Kang H, Chang C. Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. J. Biol. Chem. 2003;278:50902–50907. doi: 10.1074/jbc.M300676200. [DOI] [PubMed] [Google Scholar]
- 122.Posadas E, Gulley J, Arlen P, Trout A, Parnes H, Wright J, Lee M, Chung E, Trepel J, Sparreboom A, Chen C, Jones E, Steinberg S, Daniels A, Figg W, Dahut W. A phase II study of perifosine in androgen independent prostate cancer. Cancer Biol. Ther. 2005;4:1133–1137. doi: 10.4161/cbt.4.10.2064. [DOI] [PubMed] [Google Scholar]
- 123.Morgan TM, Lange PH, Vessella RL. Detection and characterization of circulating and disseminated prostate cancer cells. Front. Biosci. 2007;12:3000–3009. doi: 10.2741/2290. [DOI] [PubMed] [Google Scholar]
- 124.Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen LW, Hayes DF. Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. J. Clin. Oncol. 2006;12:6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 125.Chee K, Longmate J, Quinn D, Chatta G, Pinski J, Twardowski P, Pan C, Cambio A, Evans C, Gandara D, Lara PJ. The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase II California/Pittsburgh cancer consortium trial. Clin. Genitourin. Cancer. 2007;5:433–437. doi: 10.3816/CGC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 126.Pruthi RS, Derksen JE, Moore D, Carson CC, Grigson G, Watkins C, Wallen E. Phase II trial of celecoxib in prostate-specific antigen recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. Clin. Cancer Res. 2006;12:2172–2177. doi: 10.1158/1078-0432.CCR-05-2067. [DOI] [PubMed] [Google Scholar]
- 127.Smith M, Manola J, Kaufman D, Oh W, Bubley G, Kantoff P. Celecoxib versus placebo for men with prostate cancer and a rising serum prostate-specific antigen after radical prostatectomy and/or radiation therapy. J. Clin. Oncol. 2006;24:2723–2728. doi: 10.1200/JCO.2005.03.7804. [DOI] [PubMed] [Google Scholar]
- 128.Carles J, Font A, Mellado B, Domenech M, Gallardo E, Gonzalez-Larriba JL, Catalan G, Alfaro J, Gonzalez Del Alba A, Nogue M, Lianes P, Tello JM. Weekly administration of docetaxel in combination with estramustine and celecoxib in patients with advanced hormone-refractory prostate cancer: final results from a phase II study. Br. J. Cancer. 2007;97:1206–1210. doi: 10.1038/sj.bjc.6604030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hidalgo M, Buckner JC, Erlichman C, Pollack MS, Boni JP, Dukart G, Marshall B, Speicher L, Moore L, Rowinsky EK. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin. Cancer Res. 2006;12:5755–5763. doi: 10.1158/1078-0432.CCR-06-0118. [DOI] [PubMed] [Google Scholar]
- 130.Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, Park Y, Liou SH, Marshall B, Boni JP, Dukart G, Sherman ML. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J. Clin. Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 131.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, Martinelli E, Ramon y Cajal S, Jones S, Vidal L, Shand N, Macarulla T, Ramos FJ, Dimitrijevic S, Zoellner U, Tang P, Stumm M, Lane HA, Lebwohl D, Baselga J. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J. Clin. Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 132.O'Donnell A, Faivre S, Burris HA, 3rd, Rea D, Papadimitrakopoulou V, Shand N, Lane HA, Hazell K, Zoellner U, Kovarik JM, Brock C, Jones S, Raymond E, Judson I. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J. Clin. Oncol. 2008;26:1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 133.Mita MM, Mita AC, Chu QS, Rowinsky EK, Fetterly GJ, Goldston M, Patnaik A, Mathews L, Ricart AD, Mays T, Knowles H, Rivera VM, Kreisberg J, Bedrosian CL, Tolcher AW. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J. Clin. Oncol. 2008;26:361–367. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- 134.Rizzieri DA, Feldman E, Dipersio JF, Gabrail N, Stock W, Strair R, Rivera VM, Albitar M, Bedrosian CL, Giles FJ. A phase 2 clinical trial of deforolimus (AP23573, MK-8669), a novel mammalian target of rapamycin inhibitor, in patients with relapsed or refractory hematologic malignancies. Clin. Cancer Res. 2008;14:2756–2762. doi: 10.1158/1078-0432.CCR-07-1372. [DOI] [PubMed] [Google Scholar]
- 135.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 136.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O'Toole T, Lustgarten S, Moore L, Motzer RJ, Trial GA. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 137.Roudier MP, True LD, Higano CS, Vesselle H, Ellis W, Lange P, Vessella RL. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum. Pathol. 2003;34:646–653. doi: 10.1016/s0046-8177(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 138.Chau CH, Figg WD. Molecular and phenotypic heterogeneity of metastatic prostate cancer. Cancer Biol. Ther. 2005;4:166–167. doi: 10.4161/cbt.4.2.1571. [DOI] [PubMed] [Google Scholar]
- 139.Sosman JA, Puzanov I, Atkins MB. Opportunities and obstacles to combination targeted therapy in renal cell cancer. Clin. Cancer Res. 2007;13:764s–769s. doi: 10.1158/1078-0432.CCR-06-1975. [DOI] [PubMed] [Google Scholar]