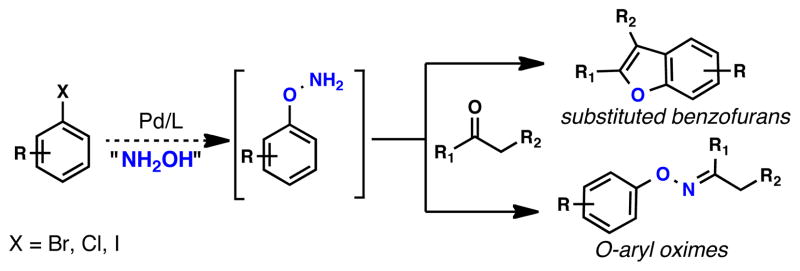
Abstract
An efficient Pd catalyst for the O-arylation of ethyl acetohydroximate with aryl chlorides, bromides, and iodides has been developed. Ethyl acetohydroximate serves as an efficient hydroxylamine equivalent for C-O cross-coupling, thereby allowing for the preparation of O-arylhydroxylamines from simple aryl halides. Short reaction times and broad substrate scope, including heteroaryl coupling partners, allows access to O-arylhydroxylamines that would be difficult to prepare in a single step by traditional methods. Moreover, the O-arylated products so formed can be directly transformed into substituted benzofurans in a single operation.
Owing to their facile incorporation into a variety of bioactive oxime linkages,1 use as tagging elements for library synthesis,2 as well as serving as key starting materials for the synthesis of benzofurans,3 O-arylhydroxylamines (aryloxyamines) represent valuable synthetic building blocks. Historically, this motif has been constructed via SNAr-type processes of various hydroxylamine equivalents (e.g. N-hydroxyphthalimide, ethyl acetohydroximate) with highly electron-deficient aromatic systems, including arene-metal complexes.4 In addition, N-transfer reagents have also been employed to form the N-O linkage from the corresponding phenol.5 Given the limited generality in these processes, recent emphasis has been placed on the copper-mediated construction of Ar—ON(R) bonds. Maitra and Wailes have reported the coupling of oximes with aryl iodides catalyzed by a CuI/1,10- Phenanthroline system.6 In addition, both Huang and Meyer have reported the coupling of oximes with arylboronic acids utilizing Cu(II) salts.7 To date, however, it is perhaps the copper-mediated coupling of aryl boronic acids with N-hydroxyphthalimide, reported by Sharpless and Kelly, that represents the most general route to O-arylhydroxylamines.8 We envisioned that a Pd-catalyzed coupling of simple aryl halides with a suitable hydroxylamine equivalent (Figure 1) could potentially address many of the shortcomings of the aforementioned Cu-based methodologies—namely low to moderate yields, long reaction times, difficulty with substrates containing ortho-substituents, lack of heterocyclic substrates, and the necessity to employ aryl iodides or arylboronic acids as coupling partners. Herein, we report a Pd-catalyzed method that utilizes commercially available ethyl acetohydroximate as the hydroxylamine equivalent. The O-arylated products so formed can easily be cleaved with aqueous acid to produce O-arylhydroxylamines or directly processed to substituted benzofurans.
Figure 1.
Desired Transformation.
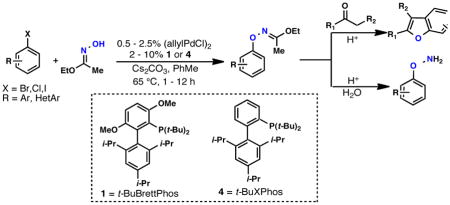
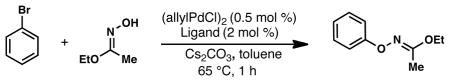
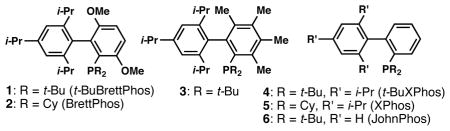
A survey of biarylphosphine ligands revealed that a catalyst based on t-BuBrettPhos9 (1) was highly active in the cross-coupling of PhBr with ethyl acetohydroximate (Table 1). Ligands 3 and 4 which have previously been employed in Pd-catalyzed C-O coupling processes between aryl halides and alcohols or phenols could be employed for this transformation, albeit with diminished efficiency.10 Ligand 6, which lacks the tri-i-propyl groups, as well as ligands 2 and 5 which do not contain the di-t-butyl phosphine moiety, were all ineffective under these conditions. The high activity displayed with 1 was crucial due to both the thermal sensitivity of the product N—O linkage,11 as well as the ability of Pd(0) to oxidatively add into this bond at elevated temperatures.12
Table 1.
Ligand Evaluation.a
 | ||
|---|---|---|
| Entry | Ligand | Conversionb |
| 1 | 1 | 100% |
| 2 | 3 | 70% |
| 3 | 4 | 51% |
| 4 | 2 | 0% |
| 5 | 5 | 0% |
| 6 | 6 | 0% |
PhBr (1.5 mmol), ethyl acetohydroximate (1.9 mmol), Cs2CO3 (2.3 mmol), (allylPdCl)2 (0.5 mol %), Ligand (2 mol %), toluene (3 ml), 65 °C, 1 h.
determined by GC.
The generality of the coupling process is shown by the examples in Table 2. Aryl chlorides, bromides, and iodides could all be employed, with aryl bromides being optimal and electron-rich aryl chlorides being most problematic.13 Using 1% Pd,14 2% 1, and Cs2CO3 as base, many electron-neutral or- deficient aryl bromides were found to undergo complete conversion within 1 hour at 65°C in toluene. The couplings of 1,4-bromochlorobenzene and 1,2-bromofluorobenzene were performed on scales of 5 and 10 mmol, respectively. In addition, a variety of heteroaryl halides including pyridinyl, quinolinyl, pyrimidinyl, and benzothiazolyl were found to readily undergo coupling. Heterocycles containing acidic N-H groups, such as indoles and imidazoles, have proven problematic to date. We have found that for aryl bromides containing ortho-alkyl substituents, the use of ligand 4 gives superior results to that with 1, presumably due to its smaller size. Using this system even hindered substrates with an i-propyl or phenyl group in the ortho position undergo efficient coupling (Table 2). The O-arylated products can be easily hydrolyzed to the free oxyamines by exposure to aqueous HCl (Table 3).
Table 2.
Palladium-Catalzyed O-Arylation of Ethyl Acetohydroximate.a
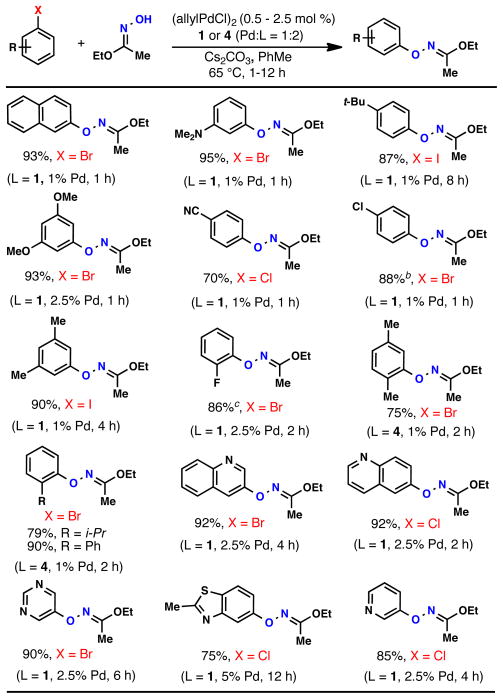 |
ArX (1 mmol), Ethyl Acetohydroximate (1.25 mmol), Cs2CO3 (1.5 mmol), (allylPdCl)2 (0.5 – 2.5 mol %), 1 or 4 (2 – 10 mol %), PhMe (2 ml), 65 °C, 1–12 h; isolated yields, average of 2 or more runs.
yield on a 10 mmol scale = 87%.
yield on a 5 mmol scale = 88%.
Table 3.
Oxime Hydrolysis.a
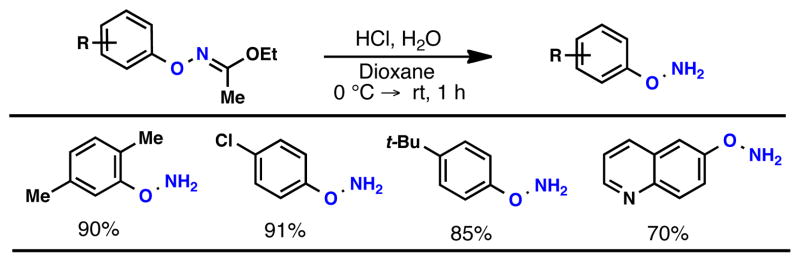 |
Oxime (1 equiv), HCl (6 M in H2O, 2 equiv), 1,4-dioxane (0.5 M), 0 °C → rt, 1 h; isolated yields, average of 2 runs on a 0.5 – 1.0 mmol scale.
O-arylketoximes bearing acidic α-hydrogens are known to rearrange to benzofurans via a [3,3] sigmatropic process, closely paralleling the venerable Fischer indole synthesis.3 Of interest to us was the prospect of directly converting the products of the Pd-coupling into benzofurans in a process reminiscent of our prior work in the Fischer indolization.15 After significant experimentation, we found that exposure of the O-arylated ethyl acetohydroximate product to an exogenous ketone and H2O in HCl/dioxane at 70 °C produces the corresponding benzofuran in synthetically useful yields (Table 4).
Table 4.
One-Pot Synthesis of Benzofuransa
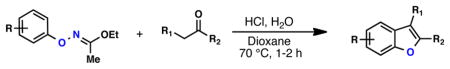 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Ketone | Product | Yield [%] | Time [h] |
| 1 |  |
86% | 1 | ||
| 2 | 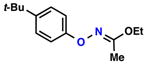 |
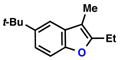 |
68% | 1 | |
| 3 | 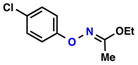 |
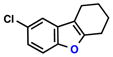 |
88% | 1 | |
| 4 | 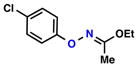 |
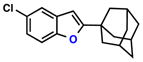 |
83% | 2 | |
| 5 | 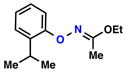 |
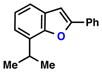 |
68% | 2 | |
| 6 | 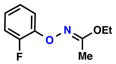 |
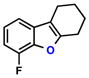 |
55% | 2 | |
Oxime (1 equiv), ketone (2 equiv), H2O (5 equiv), HCl (4 M in dioxane, 5 equiv), 1,4-dioxane (0.2 M), 70 °C, 1–2 h; isolated yield, average of 2 runs on a 0.5 – 1.0 mmol scale.
In summary, a hydroxylamine equivalent has been developed for Pd-catalyzed C-O cross-coupling. Key to the success of this reaction was the use of bulky biarylphosphine ligands 1 and 4, which promote C-O reductive elimination under relatively mild conditions. Broad substrate scope and short reaction times makes this an attractive method to prepare highly substituted O-arylhydroxylamines and benzofurans from simple aryl halides.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (NIH) for financial support of this project (GM-58160) and a postdoctoral fellowship to T.J.M. (1F32GM088931). We thank Chemetall for a generous gift of Cs2CO3.
Footnotes
SUPPORTING INFORMATION AVAILABLE: Procedural and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Johnson SM, Petrassi HM, Palaninathan SK, Mohamedmohaideen NN, Purkey HE, Nichols C, Chiang KP, Walkup T, Sacchettini JC, Sharpless KB, Kelly JW. J Med Chem. 2005;48:1576. doi: 10.1021/jm049274d. [DOI] [PubMed] [Google Scholar]
- 2.Nazarpack-Kandlousy N, Chernushevich IV, Meng L, Yang Y, Eliseev AV. J Am Chem Soc. 2000;122:3358. [Google Scholar]
- 3.(a) Sheradsky T. Tetrahedron Lett. 1966;7:5225. [Google Scholar]; (b) Castellino AJ, Rapoport H. J Org Chem. 1984;49:4399. [Google Scholar]; (c) Takeda N, Miyata O, Naito T. Eur J Org Chem. 2007:1491. [Google Scholar]
- 4.(a) Miyazawa E, Sakamoto T, Kikugawa Y. Org Prep Proced Int. 1997;29:594. [Google Scholar]; (b) Cadogan JIG, Rowley AG. Synth Comm. 1977;7:365. [Google Scholar]; (c) Baldoni C, Del Bettero P, Licandro E, Maiorana S. Synthesis. 1988:344. [Google Scholar]
- 5.(a) Castellino AJ, Rapoport H. J Org Chem. 1984;49:1348. [Google Scholar]; (b) Tamura Y, Minamikawa J, Sumoto K, Fujii S, Ikeda M. Synthesis. 1977:1. doi: 10.1021/jo00946a045. [DOI] [PubMed] [Google Scholar]; (c) Foot OF, Knight DW. Chem Comm. 2000:975. [Google Scholar]
- 6.Prithwiraj D, Nonappa, Pandurangan K, Maitra U, Wailes S. Org Lett. 2007;9:2767. doi: 10.1021/ol0709578. [DOI] [PubMed] [Google Scholar]
- 7.Feng XH, Zhang GZ, Chen CQ, Yang MY, Xu XY, Huang GS. Synth Comm. 2009;39:1768.Abdelselam A, Meyer AG, Tuck KL. Synlett. 2009:955.A polymer supported Cu-catalyst has also been shown to couple oximes with aryl boronic acids, see: Wang L, Huang C, Cai C. Catalysis Comm. 2010;11:532.
- 8.Petrassi HM, Sharpless KB, Kelly JW. Org Lett. 2001;3:139. doi: 10.1021/ol0003533. [DOI] [PubMed] [Google Scholar]
- 9.For use of 1 see: Fors BP, Dooleweerdt K, Zeng Q, Buchwald SL. Tetrahedron. 2009;65:6576. doi: 10.1016/j.tet.2009.04.096.Watson DA, Su M, Teverovskiy G, Zhang Y, Garcia-Fortanet J, Kinzel T, Buchwald SL. Science. 2009;325:1661. doi: 10.1126/science.1178239.Fors BP, Buchwald SL. J Am Chem Soc. 2009;131:12898. doi: 10.1021/ja905768k.
- 10.(a) Vorogushin AV, Huang X, Buchwald SL. J Am Chem Soc. 2005;127:8146. doi: 10.1021/ja050471r. [DOI] [PubMed] [Google Scholar]; (b) Burgos CH, Barder TE, Huang X, Buchwald SL. Angew Chem, Int Ed. 2006;45:4321. doi: 10.1002/anie.200601253. [DOI] [PubMed] [Google Scholar]
- 11.Blake JA, Pratt DA, Lin S, Walton JC, Mulder P, Ingold KU. J Org Chem. 2004;69:3112. doi: 10.1021/jo049927y. [DOI] [PubMed] [Google Scholar]
- 12.For many examples of oxidative addition of Pd(0) into polarized oxime N—O bonds see: Narasaka K, Kitamura M. Eur J Org Chem. 2005:4505.
- 13.We have observed the following trend in rate of C-O coupling: PhBr > PhI > PhCl.
- 14.The use of Pd2(dba)3 gives similar results to (allylPdCl)2.
- 15.(a) Wagaw S, Yang BH, Buchwald SL. J Am Chem Soc. 1998;120:6621. [Google Scholar]; (b) Wagaw S, Yang BH, Buchwald SL. J Am Chem Soc. 1999;121:10251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.