Abstract
Background
Rotavirus is the leading cause of severe diarrhea disease in newborns and young children worldwide, estimated to be responsible for over 300,000 childhood deaths every year, mostly in developing countries. Rotavirus-related deaths represent approximately 5% of all deaths in children younger than 5 years of age worldwide. Saponins are readily soluble in water and are approved by the US FDA for inclusion in beverages intended for human consumption. The addition of saponins to existing water supplies offers a new form of intervention into the cycle of rotavirus infection. We believe that saponins will ‘coat’ the epithelium of the host's small intestine and prevent attachment of rotavirus.
Discussion
This experiment provides in vitro data for the possibility of including saponin in drinking water to prevent infections of rotavirus. We demonstrate that microgram amounts of extract, while exhibiting no cell cytotoxicity or direct virucidal activity, prevent rotavirus from infecting its host cells. In addition, the presence of residual amounts of extract continue to block viral infection and render cells resistant to infection for at least 16 h after the removal of the extract from the cell culture media.
Conclusion
We demonstrate that two Quillaja extracts possess strong antiviral activity at concentrations more than 1000-fold lower than concentrations exhibiting cell cytotoxicity. Extract concentrations as high as 1000 μg/ml are not cytotoxic, but concentrations as low as 1.0 μg/ml are able to block rotavirus and reovirus attachment and infection.
Saponins are natural detergents that form stable foams [1–4]. They contain a lipophilic nucleus and one or more side chains of hydrophilic carbo hydrate. Thus, the intact saponin molecule is a surfactant, with both fat- and water-soluble moieties. It has been known for many years that saponins form insoluble complexes with cholesterol [5–7]. Interactions of saponins with cholesterol and other sterols account for many of their biological effects, particularly those involving membrane activity. It was demonstrated years ago that dietary saponin reduces blood cholesterol level [8–13]. This effect is a result of the saponins binding to cholesterol excreted in bile, thus inhibiting entero hepatic cholesterol recycling.
In a similar manner, saponins demonstrate antiprotozoan activity by complexing with cholesterol in protozoan cell membranes, causing damage to the integrity of the membrane, and cell lysis. This has been well demonstrated with rumen protozoa [14–22]. The antiprotozoal (cholesterol-binding) activity requires the intact saponin structure with both nucleus and side chain present. Protozoan diseases, in which part of the life cycle occurs in the GI tract, respond to the antiprotozoan activity of saponins. Yucca saponins are as effective as the drug metronidazole in killing tropozoites of Giardia lamblia in the intestine [23].
Saponins have been suggested to have additional health benefits. According to work by Waterhouse [24], drinking red wine helps lower cholesterol and red wines contain approximately the same amount of saponin as they do resveratrol [25–28, 101]. However, while resveratrol is thought to block cholesterol oxidation by its antioxidant action, saponins are believed to work by binding to and preventing the absorption of cholesterol, he says. He also mentioned that saponins are known to affect inflammation pathways, an effect that could have implications in heart disease and cancer, according to published studies.
Triterpenoid saponins from other sources such as Maesa lanceolata, Maesa chisia and Maesa indica have also been reported to exhibit antiviral activity against Ranikhet disease virus, vaccinia virus and herpes simplex virus. Some saponins have also been shown to exhibit direct virucidal mechanisms of action, including destruction of viral envelopes and interaction with host cell membranes, leading to the loss of viral binding sites [29–36].
Natural, aqueous extracts of the Chilean soap bark tree (Quillaja Saponaria Molina) contain a number of physiologically active triterpenoid saponins [37]. These saponins have been shown to exhibit strong adjuvant activity that has been exploited for use in animal and human vaccines [38–44]. Quillaja extracts have strong immune-enhancing activity that may lead to a reduction in virus infection in vivo. We have demonstrated saponin antiviral activity in cell culture in the absence of an immune system. A large amount of research exists on saponin enhancement of the immune system and direct interaction with viral and cellular membranes. Extensive literature searches reveal that this mechanism has not been explored in detail. Saponins offer the possibility of a new virucidal mechanism of action, interaction with viral envelopes leading to their destruction and/or interaction with host cell membranes leading to a loss of viral binding sites [45]. We have previously demonstrated the activity of Ultra Dry 100 Q against reovirus [46] and we have included reovirus in this study to permit comparisons to be made between the earlier study and this current study reporting strong antiviral activity against a related virus, rotavirus. This current study presents new findings of antiviral activity against rotavirus, the leading single cause of severe diarrhea among infants and young children, and one of several viruses that cause infections commonly known as ‘stomach flu’, despite having no relation to influenza. This new study builds on the previous work with the saponin extract Ultra Dry 100 Q and explores the antiviral activity of the highly purified saponin extract, Vax Sap, against both of these viruses.
Key Terms.
Saponins
Diverse class of natural surfactants, or detergents, found in many plants, but which are most abundant in the desert plants Yucca and Quillaja. Extracts from these plants are commonly used as foaming agents for beverages such as root beer.
Quillaja saponaria Molina
Evergreen tree growing to 18 by 6 m at a slow rate, known commonly as the soap bark tree. It has a long history of medicinal use with the Andean people who used it especially as a treatment for various chest problems.
Ultra Dry 100 Q
Water extract from Quillaja saponaria Molina in powder form. It contains mainly triterpenic saponins larger than 65%.
Reovirus
Any one of three ubiquitous, double-stranded RNA viruses found in the respiratory and alimentary tracts of both healthy and sick people. Reoviruses have been implicated in some cases of upper respiratory tract disease and infantile gastroenteritis. Reo indicates respiratory enteric orphan.
Rotavirus
Discovered in 1973 and taking its name from its wheel-like appearance (rota means wheel in latin). Rotavirus is a double-stranded RNA virus in the family Reoviridae that causes diarrhea in the young of many species, including the human gastroenteritis viruses that cause infant diarrhea. Also called gastroenteritis virus type B.
Vax sap
Highly purified Quillaja saponaria Molina water extract in powder form. It contains mainly triterpenic saponins greater than 90%.
Experimental
■ Virus & cell lines
The viruses used in this study were reovirus sero-type 3 (ST3) strain Dearing (MRV-3DE) [47] and rhesus rotavirus (MMU 18006) (RRV-ATCC VR-954). Mouse L929 fibroblasts (ATCC CCL-1) were used to support the growth of reovirus and the Vervent monkey kidney cell line MA-104 clone 1 (ATCC CRL-2378.1) to support the growth of RR virus. All cell lines were propagated in monolayer cultures using minimal essential medium (MEM) with Earles’ salts, supplemented with 10% fetal bovine serum (FBS). Cells were passaged at 1:2 to 1:10 dilutions according to conventional procedures using trypsin 0.05% with EDTA 0.02%.
■ Quillaja extract preparation
The Quillaja extract used was obtained from Desert King International, San Diego, CA, USA. The material, Ultra Dry 100-Q is the spray-dried purified aqueous extract of the Chilean Soap bark tree (Quillaja Saponaria Molina), consisting of larger than 65% saponins and moisture content of less than 7%. The extract was prepared by dissolving the dried material into MEM at a concentration of 1.07 g 100-Q/100 ml and filter sterilized to yield a 10 mg/ml stock. From this working stock, material was transferred to MEM with 10% FBS to yield the indicated final concentrations, and the media added to the cell cultures.
The extract is a complex mixture of triterpenoids (saponins) [48–50]. It has been established that these terpenes are built around a common quillaic acid, which is decorated with oligo saccharides at C3 and in most cases C28. The differences in the members of this family arise in the level of oxidation around the quillaic acid skeleton (typically at C23 and C30), the type, location and number of sugars and the number, location and types of acyl moieties (most often on the C28 fuctose – at C3′ and C4′). Presumably, the quillaic acid moiety simply serves as a scaffolding element, which then presents the oligosaccharides in the appropriate orientation and spatial distribution for interaction with the cellular target(s). See Figures 1 & 2 for the general structure of the Quillaja saponins.
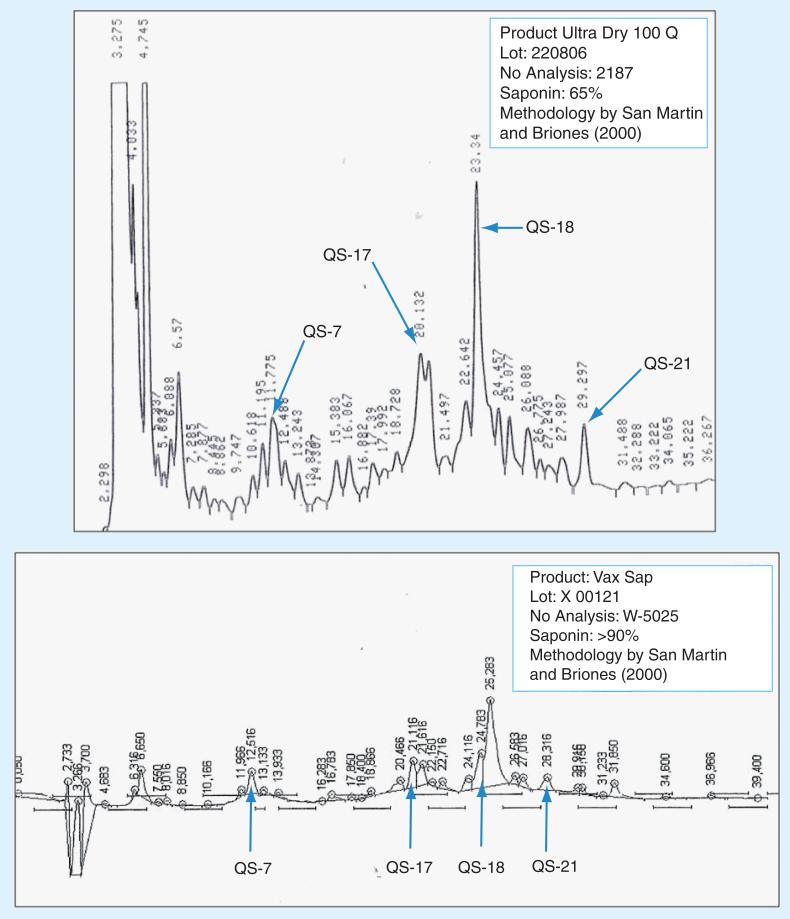
Figure 1. HPLC analysis of Ultra Dry 100 Q and Vax Sap water extracts used in this study.
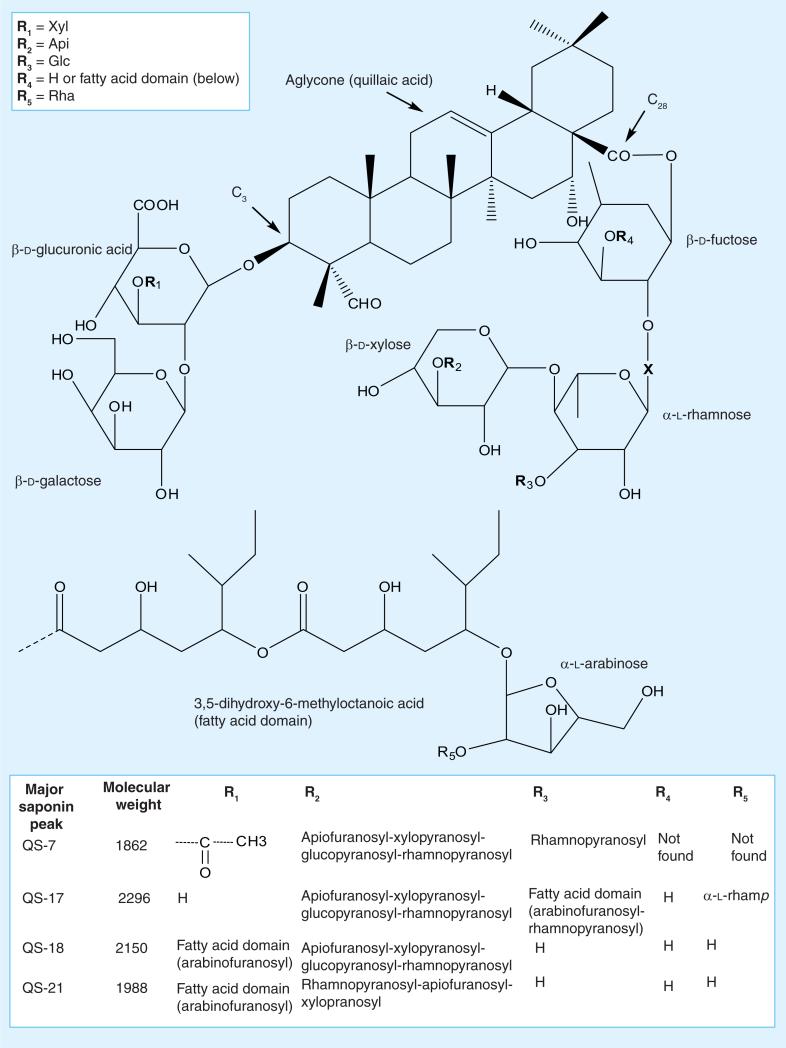
Figure 2. Major saponins from Quillaja saponaria Molina.
Api: Arabinose; Glc: Glucose; Rha: Rhamnose; Xyl: Xylose. Adapted with permission from [38,40,42,43,49,51–55].
An additional extract was also tested, Vax Sap. This material is a further purification of the original Ultra Dry 100-Q material with a saponin content of greater than 90% and approval for use as an adjuvant in human vaccines.
The existence of many different saponins, which vary in their chemical or biological activities, makes the characterization of Quillaja extracts difficult. The variable content of the individual saponins in the extracts also contributes to the difficulty of characterizing them. Additional characterization requires identification of individual saponins to assess the quality, purity and toxicity of extract, a process we are now engaged in. For most studies, researchers have relied on the identification and quantification of the major saponins, QS-7, QS-17, QS-18 and QS-21, because they represent up to 90% of total saponin content in most extract preparations. The saponin content and identification of unpurified, semi-purified and highly purified Quillaja extracts can be performed by reverse-phase HPLC; we have included the profiles from the extracts we used in this study in Figures 1 & 2. At least 22 peaks (denominated QS-1 to QS-22) are separable. The individual components can be identified by retention time on a Vydac C4 HPLC column; we have compiled data from a number of researchers and included this in Figure 2 [38,40,42,43,49,51–55].
■ Quillaja extract cytotoxicity
Quillaja extract cytotoxicity was determined by plating the indicated cells at a concentration of 5 × 105 cells per well (six-well plate) in MEM with 10% FBS and incubating the plates at 37°C in 5% CO2 for approximately 24 h, or until the cells divided to yield 1 × 106 cells per well. At this time, the medium was removed and replaced with MEM with 10% FBS, the indicated Quillaja extract concentration and the cells incubated for 96 h in the presence of the extract. Cytotoxicity was measured microscopically after 96 h, by counting the cells in three individual wells of a six-well plate, 500 cells/well and using the trypan blue-dye exclusion procedure [56]. Assays were performed in triplicate to generate nine measurements per time point. The results are presented as the concentration of the candidate drug that results in the death of 50% of the host cells. This value is commonly referred to as the median cellular cytotoxicity concentration and is identified by the initializations CCIC50.
■ Direct virucidal effect of Quillaja extract
The ability of the Quillaja extract to directly inactivate reovirus or rhesus rotavirus was examined by a standard plaque-reduction assay. Briefly, 1 × 106 plaque-forming units (PFUs) of each virus were incubated in the presence or absence of Quillaja extract ranging from 0 to 10 mg/ml. Viruses were suspended in 200 μl of Quillaja extract-containing MEM with 1% fetal bovine serum for 1 h at 37°C. Virus was pelleted at 100,000 × g for 15 min, re-suspended in fresh MEM with 1% FBS, repelleted and the process repeated three times to remove any remaining Quillaja extract. After final centrifugation the virus was resuspended in 200 μl MEM with 1% FBS and virus infectivity assayed by plaque assay or infectious units as described. Each assay was performed in triplicate.
■ Virus attachment to cells
The ability of these viruses to attach to their host cells was determined by adding 1 × 106 PFUs to 1 × 106 of the appropriate host cells in six-well plates in MEM with 10% FBS. After 5, 10, 15, 20, 30, 45 or 60 min the virus-containing medium was removed and the cells washed three times with 1 ml fresh MEM with 10% FBS. The three washes were combined and the ‘free’ virus (i.e., virus that did not remain attached to the host cells) was determined by plaque assay or infectious unit assay. The assays were repeated using three individual wells of a six-well plate. Assays were preformed in triplicate to generate nine measurements per time point. We selected this experimental design because it permitted us to follow the role of both unattached virus and virus able to attach to cells in the presence of Quillaja extract. Virus that was unable to attach was assayed for infectivity to examine the possibility that the Quillaja extract inactivated the free virus rather than prevented it from attaching to the cell monolayers as we had hypothesized. For virus that did attach, we examined the cells to see if the attached virus was still able to initiate an active infection or was destroyed and/or released but damaged, and was subsequently not detected in the assays for infectious virus that we carried out on the supernatant and cell washes. This assay mimics conditions that would exist when viruses are ingested, inhaled, injected or otherwise introduced into the human body, conditions under which saponins could be used to protect various body surfaces.
To validate this method we also used the standard technique of adding radioisotope-labeled virus (35S-methionine/cysteine) to cells and measuring cell-associated radioactivity. Briefly, radioisotope-labeled virus was prepared, infecting the indicated host cell line at a multiplicity of infection of 20 in the presence of 35S-methionine/cysteine (20 uCi/ml) for 48 h and the viruses extracted from cell debris as described [57]. The ability of each virus to attach to their host cells was determined by adding 1 × 106 CPM of each virus to 1 × 106 of the appropriate host cells in six-well plates in MEM with 10% FBS. After 5, 10, 15, 20, 30, 45 or 60 min the virus-containing media was removed and the cells washed three times with 1 ml of fresh MEM with 10% FBS. The cells were then harvested and the radioactivity (bound virus) determined by liquid scintillation counting of triplicate samples. The amount of virus bound is expressed as a percentage of the total input 1 × 106 CPM.
■ Infectious center assay to measure cells infected following treatment with Quillaja extract
The ability of each virus to infect its host cells following Quillaja extract treatment was determined by incubating 1 × 106 of the appropriate host cells in six-well plates in MEM with 10% FBS supplemented with the indicated concentration ofQuillaja extract. Following incubation for 1 h at 37°C, 5% CO2, the Quillaja extract-containing medium was removed, the cells washed three times with MEM without extract, and the cells infected by adding 1 × 106 PFUs of the indicated virus to duplicate wells of the appropriate host cells in six-well plates in MEM with 10% FBS. After 5, 10, 15, 20, 30, 45 or 60 min the virus-containing medium was removed and the cells washed with 1 ml fresh MEM with 10% FBS. After washing three times to remove unbound virus the cells were harvested into 1 ml of MEM without serum, pipetted forcefully to generate a single cell suspension and serial tenfold dilutions prepared. The diluted cell suspensions were plated onto cell monolayers as described for the standard viral plaque assays. Cells infected in the presence of the Quillaja extract that are able to support virus replication and produce infectious progeny virus, will release this new virus which will infect cells of the cell monolayers and generate plaques. Assays were preformed in triplicate to generate six measurements per time point. These assays examined the ability of these viruses to establish a productive infection in Quillaja extract treated cells and for the infection to spread to adjacent cells to produce a visible ‘plaque’.
■ Infectious center assay to measure the lasting effect of Quillaja extract treatment on cells
The lasting effects of Quillaja extract treatment were examined using a standard infectious center assay as described earlier. Cells (1 × 106) were treated with Quillaja extract as previously described for 1 h. The Quillaja extract-containing medium was removed, the cells washed three times and fresh MEM with 10% FBS added. Immediately (time 0) or after 1, 2, 4, 8, 12, 16 or 24 h, the cells were infected by adding 1 × 106 PFUs of the indicated virus to duplicate wells of the appropriate host cells in six-well plates in MEM with 10% FBS. After an additional 60 min the virus-containing medium was removed and the cells washed with 1 ml of fresh MEM with 10% FBS. The remaining procedure was identical as described for the infectious center assay previous.
■ Reovirus & rhesus rotavirus plaque assays
L929 or MA-104 cells were grown to confluency in six-well plates in MEM with 10% FBS. The medium was removed and the cells infected with either reovirus or rhesus rotavirus at serial tenfold dilutions ranging from 1 × 106 to 10 PFUs per well in 250 μl of MEM without serum. After 60 min the medium was removed and replaced with 2 ml of MEM with 5% FBS and noble agar 1%. After incubation at 37°C in 5% CO2 for a total of 96 h the cells were stained with neutral red, 0.1%, and the plaques counted [58]. Assays were preformed in triplicate.
Results & discussion
■ Highest concentration of Quillaja extract tolerated by cell cultures
As summarized in Table 1, the growth of L929 and MA-104 cells was unaffected by concentrations of 0.1 mg/ml for the Ultra Dry 100 Q and 1 mg/ml for the VaxSap extract in MEM with 10% FBS. The CCIC50 values for these cells were tenfold higher at 1 mg/ml for the Ultra Dry 100 Q and 9.5 mg/ml for the Vax Sap extract. Cells maintained in this Quillaja extract-containing medium continued to divide during the 96 h of treatment and when the Quillaja extract was removed and replaced with Quillaja extract-free medium the cells suffered no long-term effects and were maintained in the laboratory for at least 3 months. Based on these results, we tested for antiviral activity at Quillaja extract concentrations of 1.0 and 9.5 mg/ml. We have previously demonstrated the activity of Ultra Dry 100 Q against reovirus [46] and we have included reovirus in this study to permit comparisons to be made to the newly reported antiviral activity against a related virus, rotavirus. This study presents new findings and evaluates the antiviral activity of the highly purified saponin extract, VaxSap, against both of these viruses.
Table 1.
Cytotoxicity of extracts and direct inactivation of viruses.
| Cells | CCIC50† |
Virus | ED50‡ |
||
|---|---|---|---|---|---|
| Ultra Dry 100 Q extract (mg/ml) | Vax Sap extract (mg/ml) | Ultra Dry 100 Q extract (mg/ml) | Vax Sap extract (mg/ml) | ||
| L929 | 1.0 +/- 0.1 | 9.5 +/- 0.1 | Reovirus | >1.0 +/- 0.1 | >9.5 +/- 0.1 |
| | |||||
| MA-104 | 1.0 +/- 0.1 | 9.5 +/- 0.5 | Rhesus rotavirus | >1.0 +/- 0.1 | >9.5 +/- 0.5 |
The results are presented as means of four experiments using triplicate samples in each experiment. See materials and methods for conditions for the assays. These data from the treatment of reovirus with Ultra Dry 100 Q are new but generated by repeating experiments previously published [47]. The experiments were repeated and the data are included to permit a direct comparison to the new data presented for reovirus treated with Vax Sap and rotavirus treated with either Ultra Dry 100 Q or Vax Sap.
Loss of cell viability of 50% of treated cells after 96 h.
Inactivation of 50% of virus within 1 h.
■ Direct inactivation of viruses by Quillaja extracts
Also summarized in Table 1 are the results of tests to examine the ability of Quillaja extract to directly inactivate both of these viruses at concentrations from 0 to 9.5 mg/ml. The results are presented as the ED50s, the dose of a drug that is effective at inactivating 50% of the treated virus within 1 h at 37°C. After incubation for 1 h at 37°C at Quillaja extract concentrations of 1.0 mg/ml or less we measured no reduction in virus infectivity. If the Quillaja extract does demonstrate direct antiviral activity it would be only at concentrations higher than those cytotoxic for the cell lines themselves and, thus, would prevent treatment at such a high concentration.
■ Can Quillaja extracts be used to treat cells & reduce infection efficiency?
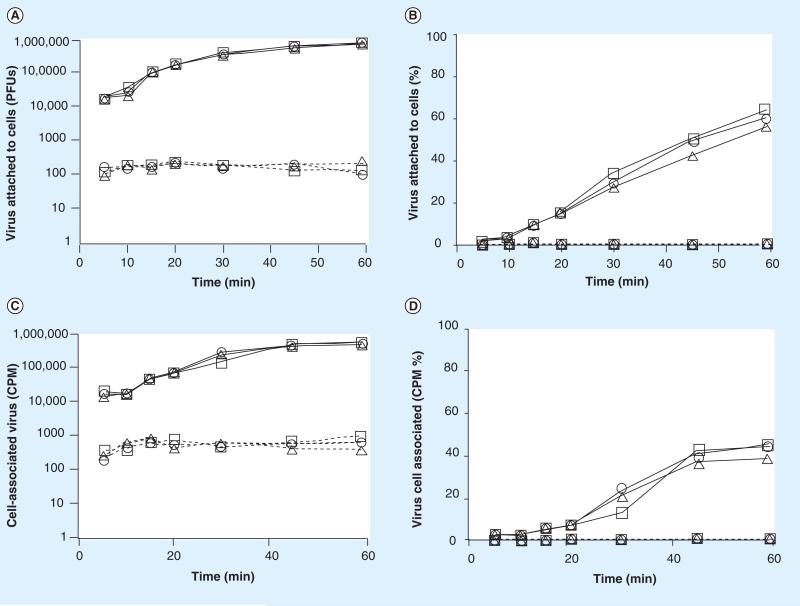
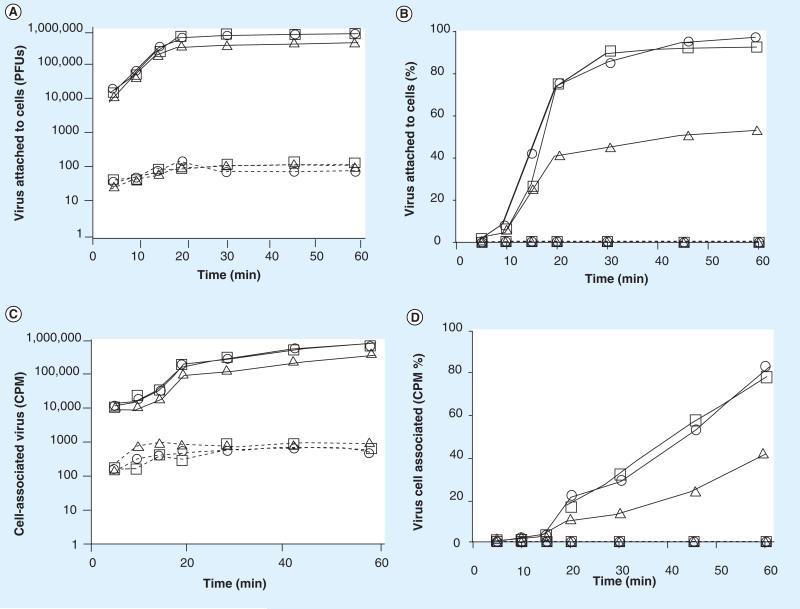
To determine if Quillaja extracts treated cells were now resistant to virus attachment, we treated cells with Quillaja extracts, Ultra Dry 100 Q or Vax Sap for 1 h, replaced the media with extract-free media and then measured the amount of virus that could bind to treated or untreated cells within an additional 60 min. We tested both Quillaja extracts at concentrations ranging from the highest that demonstrated no cell cytotoxicity (0.1 mg/ml) across a 4-log reduction to 0.00001 mg/ml. The ability of each virus to attach to its host cells following treatment of the cells was determined as described. We measured virus binding using two methods, the first an assay for infectious virus, the second an assay measuring binding of radiolabeled virus to the treated cells. The infectious assay measures the removal (binding to cells) of virus from the media during the 5– 60 min test period. Summarized in Figures 3–6, panels A and B are the results of the infectious virus assay, with panels C and D showing the binding of radiolabeled virus. The data demonstrate that treatment of cells with Quillaja extract renders them resistant to virus infection even in the absence of continuous Quillaja extract in the media. As demonstrated for reovirus and rotavirus, Ultra Dry 100 Q extract at concentrations as low as 0.001 mg/ml and as low as 0.0001 mg/ml for the Vax Sap extract were able to completely block virus binding as measured using both the infectious virus and radiolabeled virus binding assays.
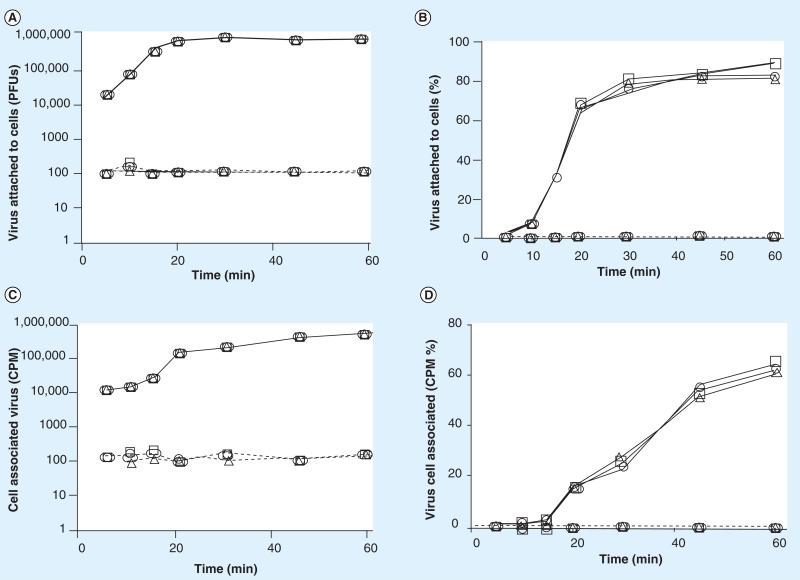
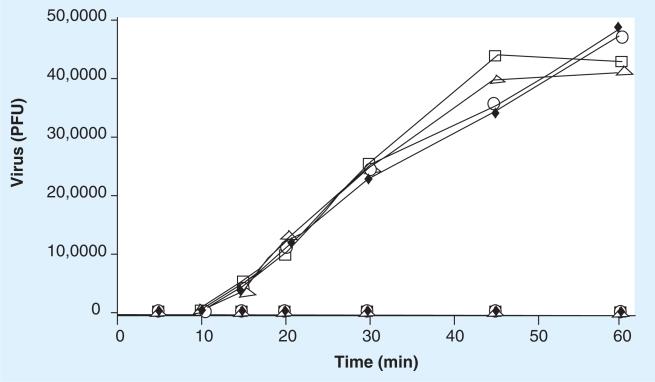
Figure 3. Treatment of cells with Ultra Dry 100 Q extract blocks reovirus cell attachment.
The ability of reovirus to attach to cells pretreated with Ultra Dry 100 Q extract was tested. The results are represented as (A) the number of virus PFUs, (B) percentage of total added virus, (C) the amount of radioisotope-labeled virus and (D) the percentage of radiolabeled virus bound to the cell monolayers (1 × 106 cells) within 1 h. See materials and methods for details. The data are plotted as —□— (Ultra Dry 100 Q extract = 0 mg/ml), ----△---- (Ultra Dry 100 Q extract = 0.1 mg/ml), ---○--- (Ultra Dry 100 Q extract = 0.01 mg/ml), ---□---- (Ultra Dry 100 Q extract = 0.001 mg/ml), —△— (Ultra Dry 100 Q extract = 0.0001 mg/ml), —○— (Ultra Dry 100 Q extract = 0.00001 mg/ml). These data are new but generated by repeating experiments previously published [47]. The experiments were repeated and the data are included to permit a direct comparison to the new data presented for both reovirus and rotavirus in Figures 3 -7. PFU: Plaque-forming unit.
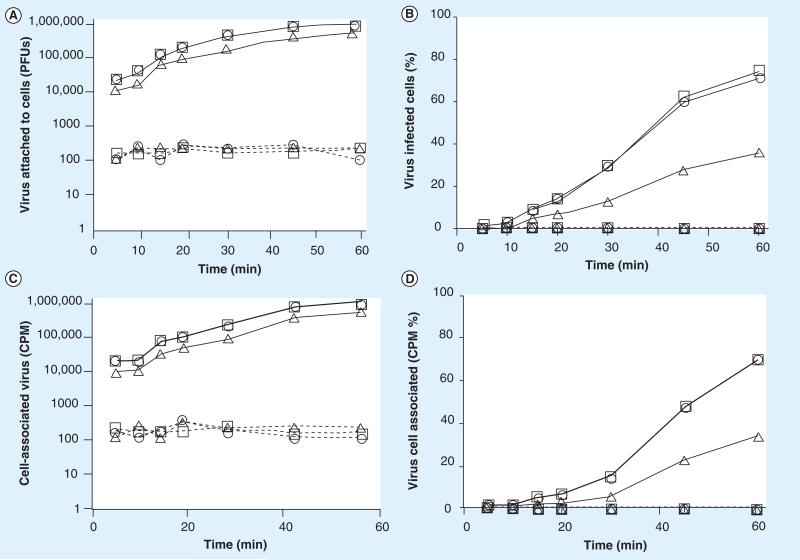
Figure 6. Treatment of cells with Vax Sap blocks rotavirus cell attachment.
The ability of rotavirus to attach to cells pretreated with Vax Sap was tested. The results are represented as (A) the number of virus PFUs, (B) percentage of total added virus, (C) the amount of radioisotope labeled virus and (D) the percentage of radiolabeled virus bound to the cell monolayers (1 × 106 cells) within 1 h. See materials and methods for details. The data are plotted as —□— (Vax Sap extract = 0 mg/ml), ----△---- (Vax Sap extract = 0.1 mg/ml), ---○--- (Vax Sap extract = 0.01 mg/ml), ----□---- (Vax Sap extract = 0.001 mg/ml), —△— (Vax Sap extract = 0.0001 mg/ml), —○— (Vax Sap extract = 0.00001 mg/ml). PFU: Plaque-forming unit.
As demonstrated in Figures 3–6, within 45 min after virus addition to untreated cells, more than 95% of each of the two viruses tested attached to their host cells (were removed from the test media) in the absence of either Quillaja extract. Following treatment with noncytotoxic amounts of either Quillaja extract (0.001–0.1 mg/ml), less than 0.25% of each of the two viruses were able to attach to their host cells after 60 min and remained free and infectious in the cell culture media. The treated cells demonstrated no cytotoxicity, yet remained highly resistant to virus binding during both of the virus binding assays. Greater than 99.75% of the original 1 × 106 PFUs of added infectious virus remained free and infectious in the supernatant and less than 1% of the radiolabeled virus was able to bind to these cells. It should be noted that the infectious assay measures ‘loss’ of virus from the test media during the incubation period. This is because the test medium is removed, the cells washed to remove unbound virus and the combined rinse media tested for infectious virus. If any residual Quillaja extract is acting on the viruses directly and rendering them noninfectious, then they would not be detected in the plaque assay of the media preformed at the end of the treatment period. This is not what we found; viruses added to cells treated with either Quillaja extract do not absorb to cells but remain free in the supernatant and infectious during the 60 min test period. In separate experiments we have demonstrated that the concentrations of Quillaja extract that we are using have no direct effect on virus infectivity. The results using the radiolabeled virus assay in combination with the infectious virus assay support our hypothesis that the Quillaja extracts prevent virus binding/association to treated cells.
■ Quillaja extract treated cells do not demonstrate active viral infection
We were also interested in the fate of the few cells that appeared to be binding virus following treatment with Quillaja extract. To pursue this we examined Quillaja extract-treated and infected cells using an infectious center assay. This method, as described in the materials and methods, involves recovering treated and potentially infected cells and plating them onto new cell monolayers to assay for infectious virus produced and released by these treated cells. This assay permits us to examine the fate of the adsorbed virus during the 60 min absorption period following treatment of the cells with Quillaja extract. If the viruses were able to attach, infect the cells and initiate an infection, then the progeny virus would infect cells of the new monolayer and generate a plaque. As shown in Figure 7, allowing virus to attach to untreated cells for 60 min followed by incubation for an additional 6 h, results in both virus/host cell systems demonstrating active viral infections in 70–90% of the cells. Following infection of cells pretreated with the lowest active amounts of Quillaja extract (0.001 or 0.0001 mg/ml), less than 0.005% of each of the virus/host cells displayed active viral infections. Quillaja extract treatment appears to alter the cells and suppress virus replication even for the rare event when the viruses were able to attach to and infect the cells. Quillaja extract has not been shown to induce an interferon response, but we were unable to detect a response in our treated cells (data not shown). We demonstrated that only 0.25% of the virus added was able to attach to Quillaja extract treated cells. This low percentage should result in at least 0.125% of the cells displaying active viral infections based on our results with untreated cells. We found that less than 0.005% of the cells produce infectious virus.
Figure 7. Pretreatment of cells with Ultra Dry 100 Q or Vax Sap extract blocks virus infection of these cells.
Infectious center assays on extract-treated cells demonstrating active viral infection. The ability of reovirus or rotavirus to infect cells pretreated with either Quillaja extract was tested. The results are represented as the number of cells demonstrating active viral infection (plaques) when infected within 1 h by immediately plating the infected cells onto uninfected cell monolayers. See materials and methods for details. The data are plotted as reovirus/L929 —□— (Ultra Dry 100 Q = 0 mg/ml), reovirus/L929 - - - □- - - (Ultra Dry 100 Q = 0.001 mg/ml), reovirus/L929 —△— (Vax Sap = 0 mg/ml), reovirus/L929 - - - △- - - (Vax Sap = 0.0001 mg/ml), rotavirus/MA104 —◆— (Ultra Dry 100 Q = 0 mg/ml), rotavirus/MA104 - - - ◆- - - (Ultra Dry 100 Q = 0.001 mg/ml), rotavirus/MA104 —0— (Vax Sap = 0 mg/ml), rotavirus/MA104 - - -0- - - (Vax Sap = 0.0001 mg/ml). These data from the treatment of reovirus with Ultra Dry 100 Q are new but generated by repeating experiments previously published [47]. The experiments were repeated and the data are included to permit a direct comparison to the new data presented for reovirus treated with Vax Sap and rotavirus treated with either Ultra Dry 100 Q or Vax Sap. PFU: Plaque-forming unit.
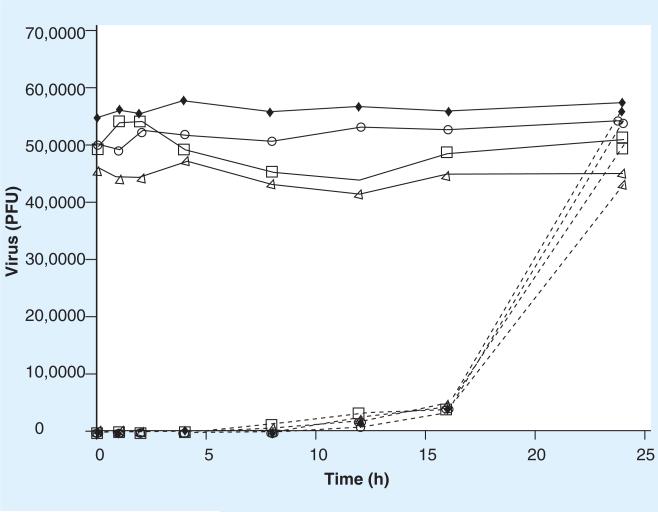
■ Maintenance of virus protection following Quillaja extract removal
We were also interested in the length of time cells would remain resistant to virus attachment/infection after the Quillaja extract was removed. To examine this, cells (1 × 106) were treated with Quillaja extract as previously indicated for 60 min. The Quillaja extract-containing medium was removed, the monolayers washed three times and fresh MEM with 10% FBS was added. Immediately (time 0), or after 1, 2, 4, 8, 12, 16 or 24 h, 1 × 106 PFUs or IUs of each virus were added to the appropriate cell line. As described previously, the virus inoculum was removed after 1 h, and 6 h later cells harvested and plated onto new cell monolayers as described for the infectious center assay. As demonstrated in Figure 8, cells treated with the lowest amounts of either Quillaja extract (0.001 or 0.0001 mg/ml) remain resistant to infection by each of the viruses tested for at least 16 h after the Quillaja extract is removed. A total of 24 h after treatment with Quillaja extract, the two cell lines tested returned to near normal with regard to virus susceptibility and virus infection were rates nearly identical to that of untreated cells indicating no long term alteration of the cells following Quillaja extract treatment.
Figure 8. Loss of virus protection following Ultra Dry 100 Q or Vax Sap extract removal.
Infectious center assays on demonstrating the loss of virus protection following Quillaja extract removal. The ability of reovirus or rotavirus to infect cells pretreated with Quillaja extract was tested. Cells were treated with Quillaja extract for 1 h and then the monolayers washed and fresh media added without Quillaja extract. This represents time 0. After 1, 2, 4, 8, 12, 16 and 24 h virus was added to these cells. The results are represented as the number of cells demonstrating active viral infection (plaques) when plated onto uninfected cell monolayers 1 h after virus addition. See materials and methods for details. These data are plotted as reovirus/L929 —□— (Ultra Dry 100 Q = 0 mg/ml), reovirus/L929 - - - □- - - (Ultra Dry 100 Q = 0.001 mg/ml), reovirus/L929 —△— (Vax Sap = 0 mg/ml), reovirus/L929 - - - △- - - (Vax Sap = 0.0001 mg/ml), rotavirus/MA104 —◆— (Ultra Dry 100 Q = 0 mg/ml), rotavirus/MA104 - - - ◆- - -(Ultra Dry 100 Q = 0.001 mg/ml), rotavirus/MA104 —0— (Vax Sap = 0 mg/ml), rotavirus/MA104 - - -0- - - (Vax Sap = 0.0001 mg/ml). These data from the treatment of reovirus with Ultra Dry 100 Q are new but generated by repeating experiments previously published [47]. The experiments were repeated and the data is included to permit a direct comparison to the new data presented for reovirus treated with Vax Sap and rotavirus treated with either Ultra Dry 100 Q or Vax Sap. PFU: Plaque-forming unit.
Other saponin extracts have demonstrated similar activity. The antiviral activity of a triterpene saponin isolated from Anagallis arvensis, Primulaceae, was studied in vitro against several viruses including herpes simplex virus type 1 and poliovirus type 2. The authors demonstrated that the antiviral activity was not due to a virucidal effect but appeared to involve inhibition of virus–host cell attachment [59]. The antiviral effects of triterpene glycosides and monoterpene glycosides were demonstrated by their ability to prevent viral activation of Raji cells by Epstein-Barr virus binding [60].
Conclusion
Reovirus is a double stranded RNA(dsRNA), nonenveloped virus that utilizes cellular receptors containing sialic acid, and normally uses endocytosis into vesicles to gain access to the cell cytoplasm. Within the cytoplasm the infecting particle is not broken down but is activated as a transcription machine producing large amounts of mRNA in the infected cells [61]. Rotavirus is also a dsRNA virus but, unlike its mostly benign cousin, reovirus, is a virus that when ingested via contaminated water results in diarrhea and approximately 300,000 deaths worldwide each year [62–64].
We hypothesized that natural, aqueous extracts of Quillaja Saponaria Molina, the Chilean soap bark tree, contain a number of physiologically active triterpenoid saponins that have antiviral activity. Our results demonstrate that a Quillaja extract does not disrupt viral envelopes and capsid proteins at concentrations of ten- (Ultra Dry 100 Q) to 100-fold (Vax-Sap) below cytotoxic levels. Additionally, following treatment of cells with this extract a ‘block’ prevents virus attachment and reduces virus spread to uninfected cells from cells that do manage to become infected, at concentrations 100- to 10,000-fold below the cytotoxic levels. Quillaja Saponaria Molina, which we are currently using, is only cytotoxic at high concentrations (>1 mg/ml), and is currently approved for use in food and beverages by the US FDA (under CFR 172.510, FEMA number 2973 NON-GMO) and is allowed for use in organic foods (under N.O.P 205.605).
One question for which a simple answer does not yet exist surrounds the mechanism by which saponins might ‘coat’ cells to prevent virus attachment and subsequent infection. We demonstrated that two viruses that use different viral attachment proteins are both blocked from binding to saponin-treated cells. Reovirus uses the junction adhesion molecule [65] for attachment to cells and rota-virus uses a generalized sialic acid or possibly the α2β1 or αVβ3 integrins [66]. A mixture of saponin in water or cell culture media forms a colloidal emulsion, which, when applied to cells, reduces the surface tension. Saponins are also known to interact with cell membranes due to the affinity of the aglycone moiety for membrane sterols, particularly cholesterol [67]. Since removal of cholesterol leads to an increase in membrane fluidity, conformational changes that ATPases undergo during their transport cycle may be facilitated. Membrane fluidity controls the enzyme activity of biological membranes and plays an important role in ion transport, therefore the ability of saponins to affect this parameter may explain their impact on the availability of functional receptors for virus binding. Additional impacts on cell membranes include the ability to open large Ca-dependent conductance channels causing membrane hyperpolarization [67], suppression of electrical activity and relaxation of smooth muscle [68]. On the other hand, there are also reports of the ability of saponins to block membrane ion channels on neurons and human neutrophils [69]. These interactions are complex and may involve different mechanisms, but together suggest major yet reversible changes to the cell membranes of saponin-treated cells, which would result in reduced protein–protein interactions at the membrane surface that would likely reduce the ability of a virus to dock and attach to these cells.
The ability to open large Ca-dependent conductance channels causing membrane hyperpolarization, suppression of electrical activity and relaxation of smooth muscle. On the other hand, there are also reports of the ability of saponins to block membrane ion channels on neurons and human neutrophils. The interactions are therefore complex and may involve different mechanisms.
Future perspective
At extract concentrations only sufficient to protect cells from virus infection and not inactivate the viruses, the interaction between the virus and the cell is the most likely site of inhibition. We postulate that the Quillaja extract modifies the cells, preventing the virus from attaching to these cells. Any virus that does manage to attach to cells and is internalized is reduced in its capacity to initiate and maintain an infection. This may be due to a reversible modification of the cell membrane or to modification of the cellular endocytosis process. We demonstrate the Quillaja extract-treated cells remain modified for 16 h and by 24 h they return to normal and are readily infected at rates equivalent to pretreatment levels. Saponins have been reported to produce a marked decrease of microsomal enzyme activities [70–72]. Future research will address these questions.
Yucca and Quillaja extracts are natural phytochemicals currently approved by the FDA for use in humans and that are present in a number of foods, beverages and herbal products. In addition to the novel antiviral activity we report here, research from has suggested that the consumption of saponin-rich herbs by the Masai tribe's people in East Africa may be responsible for their very low serum cholesterol levels. These people consume a diet very high in animal products, cholesterol and saturated fat yet maintain very low serum cholesterol levels.
Yucca schidigera and Quillaja saponaria are packed with phytochemicals that have the potential to improve human health, nutrition and battle infectious diseases. Researchers are just beginning to explore and gain an understanding of the many biological effects of steroidal and triterpenoid saponins, and their potentials for improving human health. The future medical applications of saponins remain to be discovered.
Executive summary.
Antiviral activity of Ultra Dry 100 Q
■ We demonstrate that a water extract from the soap bark tree (Quillaja saponaria Molina) is equally effective at preventing rotavirus infection of cells in culture as we have previously demonstrated for reovirus. This has major implications for human health as rotavirus, unlike reovirus, infects the human bowels and is the most common cause of severe diarrhea among infants and children throughout the world, being responsible for the death of approximately 300,000 children worldwide annually.
■ The Ultra Dry 100 Q extract demonstrated low cytotoxicity (0.1 mg/ml) against cell lines used to support the replication of both rotavirus and reovirus.
■ The presence of the Ultra Dry 100 Q extract in cell culture media was very effective at preventing both rotavirus and reovirus from attaching to cells, thereby blocking infection.
■ The presence of the Ultra Dry 100 Q extract in cell culture media was also very effective at reducing the spread of both rotavirus and from any infected cell to the surrounding cells.
■ A single exposure to the Ultra Dry 100 Q extract in cell culture media was very effective at preventing both rotavirus and reovirus from attaching to cells for at least 16 h.
Antiviral activity of Vax Sap
■ In preparation for testing in animals and eventually in humans, a purified preparation derived from the Ultra Dry 100 Q, Vax Sap was tested for activity against both rotavirus and reovirus in vitro. As demonstrated by the HPLC profiles in Figure 1, the Vax Sap material contains the same saponins found in the parent Ultra Dry 100 Q but greatly reduced amounts of the contaminants present in the early elution times.
■ The Vax Sap only 10% as toxic and was active at concentrations approximately tenfold lower than the Ultra Dry 100 Q.
Antiviral properties of saponin containing extracts of Quillaja saponaria Molina
■ The extracts do not have direct virucidal activity against nonenveloped viruses.
■ The extracts can prevent virus binding to cells.
■ The extracts can prevent virus spread from infected to uninfected cells.
■ The ‘protective coating’ of cells by the extract lasts for at least 16 h.
Figure 4. Treatment of cells with Ultra Dry 100 Q blocks rotavirus cell attachment.
The ability of rotavirus to attach to cells pretreated with Vax Sap was tested. The results are represented as (A) the number of virus PFUs, (B) percentage of total added virus, (C) the amount of radioisotope labeled virus and (D) the percentage of radiolabeled virus bound to the cell monolayers (1 × 106 cells) within 1 h. See materials and methods for details. The data are plotted as —□— (Vax Sap extract = 0 mg/ml), ----△---- (Vax Sap extract = 0.1 mg/ml), ---○--- (Vax Sap extract = 0.01 mg/ml), ----□---- (Vax Sap extract = 0.001 mg/ml), —△— (Vax Sap extract = 0.0001 mg/ml), —○— (Vax Sap extract = 0.00001 mg/ml). PFU: Plaque-forming unit.
Figure 5. Treatment of cells with Vax Sap blocks reovirus cell attachment.
The ability rotavirus to attach to cells pretreated with Vax Sap was tested. The results are represented as the (A) number of virus PFUs, (B) percentage of total added virus, (C) the amount of radioisotope labeled virus and (D) the percentage of radiolabeled virus bound to the cell monolayers (1 × 106 cells) within 1 h. See materials and methods for details. The data are plotted as —□— (Vax Sap extract = 0 mg/ml), ----△---- (Vax Sap extract = 0.1 mg/ml), ---○--- (Vax Sap extract = 0.01 mg/ml), ----□---- (Vax Sap extract = 0.001 mg/ml), —△— (Vax Sap extract = 0.0001 mg/ml), —○— (Vax Sap extract = 0.00001 mg/ml). PFU: Plaque-forming unit.
Acknowledgments
Financial & competing interests disclosure
This work was supported by grant 1R15AT004267–01 to MRR from The National Center for Complementary and Alternative Medicine. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert t estimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
- 1.Acebes B, Bernabe M, Diaz-Lanza AM, Bartolome C. Two new sulfated saponins from the roots of Gypsophila bermejoi. J. Nat. Prod. 1998;61(12):1557–1559. doi: 10.1021/np9705221. [DOI] [PubMed] [Google Scholar]
- 2.Capiod T, Combettes L, Noel J, Claret M. Evidence for bile acid-evoked oscillations of Ca2(+)-dependent K+ permeability unrelated to a d-myo-inositol 1,4,5-trisphosphate effect in isolated guinea pig liver cells. J. Biol. Chem. 1991;266(1):268–273. [PubMed] [Google Scholar]
- 3.Lamri Y, Roda A, Dumont M, Feldmann G, Erlinger S. Immunoperoxidase localization of bile salts in rat liver cells. Evidence for a role of the Golgi apparatus in bile salt transport. J. Clin. Invest. 1988;82(4):1173–1182. doi: 10.1172/JCI113714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4 ■.Williams JR, Gong H. Isolation and synthesis of shark-repelling saponins. Lipids. 2004;39(8):795–799. doi: 10.1007/s11745-004-1298-z. [Unexpected applications of saponins.] [DOI] [PubMed] [Google Scholar]
- 5.Kersten GF, Spiekstra A, Beuvery EC, Crommelin DJ. On the structure of immune-stimulating saponin–lipid complexes (iscoms). Biochim. Biophys. Acta. 1991;1062(2):165–171. doi: 10.1016/0005-2736(91)90388-o. [DOI] [PubMed] [Google Scholar]
- 6.Schnurr M, Chen Q, Shin A, et al. Tumor antigen processing and presentation depend critically on dendritic cell type and the mode of antigen delivery. Blood. 2005;105(6):2465–2472. doi: 10.1182/blood-2004-08-3105. [DOI] [PubMed] [Google Scholar]
- 7.Taverna E, Saba E, Rowe J, Francolini M, Clementi F, Rosa P. Role of lipid microdomains in P/Q-type calcium channel (Cav 2.1) clustering and function in presynaptic membranes. J. Biol. Chem. 2004;279(7):5127–5134. doi: 10.1074/jbc.M308798200. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JW, Major AW. Pulses and lipaemia, short- and long-term effect: potential in the prevention of cardiovascular disease. Br. J. Nutr. 2002;88(Suppl. 3):S263–S271. doi: 10.1079/BJN2002716. [DOI] [PubMed] [Google Scholar]
- 9.Escudero NL, de Arellano ML, Luco JM, Gimenez MS, Mucciarelli SI. Comparison of the chemical composition and nutritional value of Amaranthus cruentus flour and its protein concentrate. Plant Foods Hum. Nutr. 2004;59(1):15–21. doi: 10.1007/s11130-004-0033-3. [DOI] [PubMed] [Google Scholar]
- 10.Harwood HJ, Jr, Chandler CE, Pellarin LD, et al. Pharmacologic consequences of cholesterol absorption inhibition: alteration in cholesterol metabolism and reduction in plasma cholesterol concentration induced by the synthetic saponin β-tigogenin cellobioside (CP-88818; tiqueside). J. Lipid Res. 1993;34(3):377–395. [PubMed] [Google Scholar]
- 11.Malinow MR, McLaughlin P, Stafford C, Livingston AL, Kohler GO. Alfalfa saponins and alfalfa seeds. Dietary effects in cholesterolfed rabbits. Atherosclerosis. 1980;37(3):433–438. doi: 10.1016/0021-9150(80)90148-3. [DOI] [PubMed] [Google Scholar]
- 12.Oakenfull DG, Fenwick DE. Adsorption of bile salts from aqueous solution by plant fibre and cholestyramine. Br. J. Nutr. 1978;40(2):299–309. doi: 10.1079/bjn19780126. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Wu T, He K, Fu ZG. Effect of aerobic exercise and ginsenosides on lipid metabolism in diet-induced hyperlipidemia mice. Zhongguo Yao Li Xue Bao. 1999;20(6):563–565. [PubMed] [Google Scholar]
- 14.Abreu A, Carulla JE, Lascano CE, Diaz TE, Kreuzer M, Hess HD. Effects of Sapindus saponaria fruits on ruminal fermentation and duodenal nitrogen flow of sheep fed a tropical grass diet with and without legume. J. Anim. Sci. 2004;82(5):1392–1400. doi: 10.2527/2004.8251392x. [DOI] [PubMed] [Google Scholar]
- 15.Fievez V, Dragomir C, Mbanzamihigo L, Demeyer D. Clover saponins as methane inhibitors and their effect on rumen n utilisation efficiency as studied in vitro and in vivo. Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet. 2001;66(4):299–304. [PubMed] [Google Scholar]
- 16.Hu W, Liu J, Wu Y, Guo Y, Ye J. Effects of tea saponins on in vitro ruminal fermentation and growth performance in growing Boer goat. Arch. Anim. Nutr. 2006;60(1):89–97. doi: 10.1080/17450390500353119. [DOI] [PubMed] [Google Scholar]
- 17.Hu WL, Wu YM, Liu JX, Guo YQ, Ye JA. Tea saponins affect in vitro fermentation and methanogenesis in faunated and defaunated rumen fluid. J. Zhejian. Univ. Sci. B. 2005;6(8):787–792. doi: 10.1631/jzus.2005.B0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newbold CJ, el Hassan SM, Wang J, Ortega ME, Wallace RJ. Influence of foliage from African multipurpose trees on activity of rumen protozoa and bacteria. Br. J. Nutr. 1997;78(2):237–249. doi: 10.1079/bjn19970143. [DOI] [PubMed] [Google Scholar]
- 19.Sliwinski BJ, Kreuzer M, Wettstein HR, Machmuller A. Rumen fermentation and nitrogen balance of lambs fed diets containing plant extracts rich in tannins and saponins, and associated emissions of nitrogen and methane. Arch. Tierernahr. 2002;56(6):379–392. doi: 10.1080/00039420215633. [DOI] [PubMed] [Google Scholar]
- 20.Wallace RJ. Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 2004;63(4):621–629. doi: 10.1079/pns2004393. [DOI] [PubMed] [Google Scholar]
- 21.Wallace RJ, Arthaud L, Newbold CJ. Influence of Yucca shidigera extract on ruminal ammonia concentrations and ruminal microorganisms. Appl. Environ. Microbiol. 1994;60(6):1762–1767. doi: 10.1128/aem.60.6.1762-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22 ■.Wina E, Muetzel S, Becker K. The impact of saponins or saponin-containing plant materials on ruminant production – a review. J. Agric. Food Chem. 2005;53(21):8093–8105. doi: 10.1021/jf048053d. [Review of saponins in animal feed.] [DOI] [PubMed] [Google Scholar]
- 23.McAllister TA, Annett CB, Cockwill CL, Olson ME, Wang Y, Cheeke PR. Studies on the use of Yucca schidigera to control giardiosis. Vet. Parasitol. 2001;97(2):85–99. doi: 10.1016/s0304-4017(01)00394-6. [DOI] [PubMed] [Google Scholar]
- 24.Waterhouse AL. Wine phenolics. Ann. N.Y. Acad. Sci. 2002;957:21–36. doi: 10.1111/j.1749-6632.2002.tb02903.x. [DOI] [PubMed] [Google Scholar]
- 25 ■.Spanghero M, Salem AZM, Robinson PH. Chemical composition, including secondary metabolites, and rumen fermentability of seeds and pulp of Californian (USA) and Italian grape pomaces. Anim. Feed Sci. Tech. 2009;152(3–4):243–255. [Saponins in wines.] [Google Scholar]
- 26.Achike FI, Kwan CY. Nitric oxide, human diseases and the herbal products that affect the nitric oxide signalling pathway. Clin. Exp. Pharmacol. Physiol. 2003;30(9):605–615. doi: 10.1046/j.1440-1681.2003.03885.x. [DOI] [PubMed] [Google Scholar]
- 27.Steinkamp-Fenske K, Bollinger L, Xu H, et al. Reciprocal regulation of endothelial nitric-oxide synthase and NADPH oxidase by betulinic acid in human endothelial cells. J. Pharmacol. Exp. Ther. 2007;322(2):836–842. doi: 10.1124/jpet.107.123356. [DOI] [PubMed] [Google Scholar]
- 28.Johns T, Mahunnah RLA, Sanaya P, Chapman L, Ticktin T. Saponins and phenolic content in plant dietary additives of a traditional subsistence community, the Batemi of Ngorongoro District, Tanzania. J.Ethnopharmacol. 1999;66(1):1–10. doi: 10.1016/s0378-8741(98)00179-2. [DOI] [PubMed] [Google Scholar]
- 29.Arstila P. Characteristics of Vesicular Stomatitis virus envelopes released with saponin. J. Gen. Virol. 1974;24(2):319–326. doi: 10.1099/0022-1317-24-2-319. [DOI] [PubMed] [Google Scholar]
- 30.Abe H, Konishi H, Komiya H, Arichi S. Effects of saikosaponins on biological membranes. Planta Med. 1981;42(8):356–363. doi: 10.1055/s-2007-971655. [DOI] [PubMed] [Google Scholar]
- 31.Labrecque J, Anastassov V, Lau G, Darkes M, Mosi R, Fricker SP. The development of an europium-GTP assay to quantitate chemokine antagonist interactions for CXCR4 and CCR5. Assay Drug Dev. Technol. 2005;3(6):637–648. doi: 10.1089/adt.2005.3.637. [DOI] [PubMed] [Google Scholar]
- 32 ■.Sjolander S, Hansen JE, Lovgren-Bengtsson K, Akerblom L, Morein B. Induction of homologous virus neutralizing antibodies in guinea-pigs immunized with two human immunodeficiency virus type 1 glycoprotein gp120-iscom preparations. A comparison with other adjuvant systems. Vaccine. 1996;14(4):344–352. doi: 10.1016/0264-410x(95)00163-u. [Review-type article on the use of saponins as adjuvants.] [DOI] [PubMed] [Google Scholar]
- 33.Britt W, Fay J, Seals J, Kensil C. Formulation of an immunogenic human cytomegalovirus vaccine: responses in mice. J. Infect. Dis. 1995;171(1):18–25. doi: 10.1093/infdis/171.1.18. [DOI] [PubMed] [Google Scholar]
- 34.Lipford GB, Wagner H, Heeg K. Vaccination with immunodominant peptides encapsulated in Quil A-containing liposomes induces peptide-specific primary CD8+ cytotoxic T cells. Vaccine. 1994;12(1):73–80. doi: 10.1016/0264-410x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 35.Imagawa T, Nakai J, Takeshima H, Nakasaki Y, Shigekawa M. Expression of Ca(2+)-induced Ca2+ release channel activity from cardiac ryanodine receptor cDNA in Chinese hamster ovary cells. J. Biochem. 1992;112(4):508–513. doi: 10.1093/oxfordjournals.jbchem.a123930. [DOI] [PubMed] [Google Scholar]
- 36.Oosterlaken TA, Brandenburg A, Schielen P, Fransen R, Kraaijeveld CA, Snippe H. Efficient induction of Semliki Forest virus and mumps virus neutralizing anti-anti-idiotypic antibodies using Quil A as adjuvant. J. Immunol. Methods. 1991;136(2):169–175. doi: 10.1016/0022-1759(91)90003-x. [DOI] [PubMed] [Google Scholar]
- 37.Guo S, Kenne L. Characterization of some O-acetylated saponins from Quillaja saponaria Molina. Phytochemistry. 2000;54(6):615–623. doi: 10.1016/s0031-9422(00)00161-8. [DOI] [PubMed] [Google Scholar]
- 38.Dalsgaard K. Saponin adjuvants. 3. Isolation of a substance from Quillaja saponaria Molina with adjuvant activity in food-and-mouth disease vaccines. Arch Gesamte Virusforsch. 1974;44(3):243–254. [PubMed] [Google Scholar]
- 39.Wu JY, Gardner BH, Murphy CI, et al. Saponin adjuvant enhancement of antigen-specific immune responses to an experimental HIV-1 vaccine. J. Immunol. 1992;148(5):1519–1525. [PubMed] [Google Scholar]
- 40.Cleland JL, Kensil CR, Lim A, et al. Isomerization and formulation stability of the vaccine adjuvant QS-21. J. Pharm. Sci. 1996;85(1):22–28. doi: 10.1021/js9503136. [DOI] [PubMed] [Google Scholar]
- 41.Jacobsen NE, Fairbrother WJ, Kensil CR, Lim A, Wheeler DA, Powell MF. Structure of the saponin adjuvant QS-21 and its base-catalyzed isomerization product by 1H and natural abundance 13C NMR spectroscopy. Carbohydr. Res. 1996;280(1):1–14. doi: 10.1016/0008-6215(95)00278-2. [DOI] [PubMed] [Google Scholar]
- 42 ■.Kensil CR. Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 1996;13(1–2):1–55. [Strong review discussing the use of saponins as adjuvants.] [PubMed] [Google Scholar]
- 43.Kensil CR, Soltysik S, Wheeler DA, Wu JY. Structure/function studies on QS-21, a unique immunological adjuvant from Quillaja saponaria. Adv. Exp. Med. Biol. 1996;404:165–172. doi: 10.1007/978-1-4899-1367-8_15. [DOI] [PubMed] [Google Scholar]
- 44.Pillion DJ, Amsden JA, Kensil CR, Recchia J. Structure–function relationship among Quillaja saponins serving as excipients for nasal and ocular delivery of insulin. J. Pharm. Sci. 1996;85(5):518–524. doi: 10.1021/js9504651. [DOI] [PubMed] [Google Scholar]
- 45.Apers S, Baronikova S, Sindambiwe JB, et al. Antiviral, haemolytic and molluscicidal activities of triterpenoid saponins from Maesa lanceolata: establishment of structure–activity relationships. Planta Med. 2001;67(6):528–532. doi: 10.1055/s-2001-16489. [DOI] [PubMed] [Google Scholar]
- 46 ■.Roner MR, Sprayberry J, Spinks M, Dhanji S. Antiviral activity obtained from aqueous extracts of the Chilean soap bark tree (Quillaja saponaria Molina). J. Gen. Virol. 2007;88:275–285. doi: 10.1099/vir.0.82321-0. [First report on antiviral activity present in saponin extracts from Quillaja saponaria Molina.] [DOI] [PubMed] [Google Scholar]
- 47.Ramos-Alvarez M, Sabin AB. Enteropathogenic viruses and bacteria; role in summer diarrheal diseases of infancy and early childhood. J. Am. Med. Assoc. 1958;167(2):147–156. doi: 10.1001/jama.1958.02990190001001. [DOI] [PubMed] [Google Scholar]
- 48.van Setten DC, ten Hove GJ, Wiertz EJ, Kamerling JP, van de Werken G. Multiple-stage tandem mass spectrometry for structural characterization of saponins. Anal. Chem. 1998;70(20):4401–4409. doi: 10.1021/ac980365q. [DOI] [PubMed] [Google Scholar]
- 49 ■.van Setten DC, van de Werken G. Molecular structures of saponins from Quillaja saponaria Molina. Adv. Exp. Med. Biol. 1996;404:185–193. doi: 10.1007/978-1-4899-1367-8_17. [Report on the structures of saponins from Quillaja saponaria Molina.] [DOI] [PubMed] [Google Scholar]
- 50.van Setten DC, van de Werken G, Zomer G, Kersten GF. Glycosyl compositions and structural characteristics of the potential immuno-adjuvant active saponins in the Quillaja saponaria Molina extract quil A. Rapid Commun. Mass Spectrom. 1995;9(8):660–666. doi: 10.1002/rcm.1290090808. [DOI] [PubMed] [Google Scholar]
- 51.Higuchi R, Komori T. Structures of compounds derived from the acyl moieties of Quillaja saponin. Phytochemistry. 1987;26(8):2357–2360. [Google Scholar]
- 52.Higuchi R, Tokimitsu Y, Komori T. An acylated triterpenoid saponin from Quillaja saponaria. Phytochemistry. 1988;27(4):1165–1168. [Google Scholar]
- 53 ■.Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 1991;146(2):431–437. [Report on the structures of saponins from Quillaja saponaria Molina.] [PubMed] [Google Scholar]
- 54.Kensil CR, Wu JY, Anderson CA, Wheeler DA, Amsden J. QS-21 and QS-7: purified saponin adjuvants. Dev. Biol. Stand. 1998;92:41–47. [PubMed] [Google Scholar]
- 55.So HS, Yoon HS, Choi DY, et al. Effect of a novel saponin adjuvant derived from Quillaja saponaria on the immune response to recombinant hepatitis B surface antigen. Mol. Cells. 1997;7(2):178–186. [PubMed] [Google Scholar]
- 56.Bjorkerud S, Bondjers G. Endothelial integrity and viability in the aorta of the normal rabbit and rat as evaluated with dye exclusion tests and interference contrast microscopy. Atherosclerosis. 1972;15(3):285–300. doi: 10.1016/0021-9150(72)90019-6. [DOI] [PubMed] [Google Scholar]
- 57.Balazs I, Caldarella J. Retrovirus gene expression during the cell cycle. I. Virus production, synthesis, and expression of viral proteins in Rauscher murine leukemia virus-infected mouse cells. J. Virol. 1981;39(3):792–799. doi: 10.1128/jvi.39.3.792-799.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roner MR, Joklik WK. Reovirus reverse genetics: incorporation of the CAT gene into the reovirus genome. Proc. Natl Acad. Sci. USA. 2001;98(14):8036–8041. doi: 10.1073/pnas.131203198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amoros M, Fauconnier B, Girre RL. In vitro antiviral activity of a saponin from Anagallis arvensis, Primulaceae, against herpes simplex virus and poliovirus. Antiviral Res. 1987;8(1):13–25. doi: 10.1016/0166-3542(87)90084-2. [DOI] [PubMed] [Google Scholar]
- 60.Tokuda H, Konoshima T, Kozuka M, Kimura T. Inhibitory effects of 12-O-tetradecanoylphorbol-13-acetate and teleocidin B induced Epstein-Barr virus by saponin and its related compounds. Cancer Lett. 1988;40(3):309–317. doi: 10.1016/0304-3835(88)90090-0. [DOI] [PubMed] [Google Scholar]
- 61 ■.Tyler KL, Clarke P, DeBiasi RL, Kominsky D, Poggioli GJ. Reoviruses and the host cell. Trends Microbiol. 2001;9(11):560–564. doi: 10.1016/s0966-842x(01)02103-5. [Strong review on reovirus attachment to host cells.] [DOI] [PubMed] [Google Scholar]
- 62.Amador JJ, Vasquez J, Orozco M, et al. Rotavirus disease burden, Nicaragua 2001–2005: defining the potential impact of a rotavirus vaccination program. Int. J. Infect. Dis. 2010;14(7):e592–e595. doi: 10.1016/j.ijid.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 63.Flem ET, Latipov R, Nurmatov ZS, Xue Y, Kasymbekova KT, Rheingans RD. Costs of diarrheal disease and the cost-effectiveness of a rotavirus vaccination program in Kyrgyzstan. J. Infect. Dis. 2009;200(Suppl 1):S195–S202. doi: 10.1086/605040. [DOI] [PubMed] [Google Scholar]
- 64.Esparza-Aguilar M, Bautista-Marquez A, Gonzalez-Andrade Mdel C, Richardson-Lopez-Collada VL. Analysis of the mortality due to diarrhea in younger children, before and after the introduction of rotavirus vaccine. Salud Publica Mex. 2009;51(4):285–290. doi: 10.1590/s0036-36342009000400004. [DOI] [PubMed] [Google Scholar]
- 65.Barton ES, Forrest JC, Connolly JL, et al. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104(3):441–451. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 66 ■.Arias CF, Isa P, Guerrero CA, et al. Molecular biology of rotavirus cell entry. Arch. Med. Res. 2002;33(4):356–361. doi: 10.1016/s0188-4409(02)00374-0. [Strong review discussing rotavirus attachment to host cells.] [DOI] [PubMed] [Google Scholar]
- 67 ■.Bangham AD, Horne RW, Glauert AM, Dingle JT, Lucy JA. Action of saponin on biological cell membranes. Nature. 1962;196:952–955. doi: 10.1038/196952a0. [Strong review discussing the impact of saponins on cell membranes.] [DOI] [PubMed] [Google Scholar]
- 68.McManus OB, Harris GH, Giangiacomo KM, et al. An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry. 1993;32(24):6128–6133. doi: 10.1021/bi00075a002. [DOI] [PubMed] [Google Scholar]
- 69.Bei L, Hu T, Qian ZM, Shen X. Extracellular Ca2+ regulates the respiratory burst of human neutrophils. Biochim. Biophys. Acta. 1998;1404(3):475–483. doi: 10.1016/s0167-4889(98)00081-0. [DOI] [PubMed] [Google Scholar]
- 70.Chang PL, Ameen M, Lafferty KI, Varey PA, Davidson AR, Davidson RG. Action of surface-active agents on arylsulfatase-C of human cultured fibroblasts. Anal. Biochem. 1985;144(2):362–370. doi: 10.1016/0003-2697(85)90129-0. [DOI] [PubMed] [Google Scholar]
- 71.Choi J, Huh K, Kim SH, Lee KT, Lee HK, Park HJ. Kalopanaxsaponin A from Kalopanax pictus, a potent antioxidant in the rheumatoidal rat treated with Freund's complete adjuvant reagent. J. Ethnopharmacol. 2002;79(1):113–118. doi: 10.1016/s0378-8741(01)00382-8. [DOI] [PubMed] [Google Scholar]
- 72.Larocca JN, Ledeen RW. Hydrolysis of inositol trisphosphate by purified rat brain myelin. J. Neurochem. 1993;60(5):1864–1869. doi: 10.1111/j.1471-4159.1993.tb13413.x. [DOI] [PubMed] [Google Scholar]
- 101 ■.ScienceDaily: New cholesterol fighter found in red wine. 2003 September 9; [Discusses cholesterol fighting saponins in wines.]