Abstract
The renal handling of phosphorus was evaluated in rats with acute renal failure (ARF) induced by injection of mercuric chloride (HgCl2). Clearances of endogenous creatinine (Ccr) and of phosphorus (Cp) were measured in the following groups: 1. Intact animals (control); 2. Parathyroidectomized rats (PTX) with normal kidney function (PTX control); 3. Animals with mercury-induced acute renal failure (Hg-ARF); 4. PTX rats with Hg-ARF; 5. Rats with Hg-ARF maintained normophosphatemic with dietary phosphate restriction; 6. Animals with oliguric ARF following renal artery constriction; 7. Rats with unilateral Hg-ARF. In addition, radioinulin clearances were measured in 6 normal and in 14 azotemic animals and correlated with simultaneously recorded endogenous Ccr. Radioinulin clearance was also used as an estimate of GFR (glomerular filtration rate) in the animals of group 7.
The Cp/GFR in the intact animals (group 1) was 0.25 ±0.06 (mean ±SD). PTX (group 2) caused a subsequent decrease in Cp/GFR to 0.11 ±0.04 P < 0.0005. The ARF animals in group 3 were classified either as oliguric (Uvol [urine volume] <2 ml/24 hr, Ccr 0.008 ±0.005 ml/min) or nonoliguric (Vvol >2 ml/24 hr, Ccr 0.136 ±0.12). The Cp/GFR in the oliguric animals (0.16 ±0.09) was lower than that in group 1, P < 0.0005, and failed to increase following administration of exogenous parathyroid hormone. The Cp/GFR in the oliguric animals in groups 5 and 7 was also lower than the clearance ratio in group 1, 0.030 ±0.08 and 0.077 ±0.006, respectively. In the nonoliguric ARF animals of group 3 the Cp/GFR (0.94 ±0.29) was higher than that in group 1, P < 0.0005. In the nonoliguric ARF animals of group 4 the Cp/GFR 0.27 ±0.08 did not differ from the clearance ratio in group 1, however it was higher than that in the PTX animals (group 2) P < 0.0005. Cp/GFR in the nonoliguric animals of group 5 was not different from that in the nonoliguric rats of group 3. In the animals with nonoliguric unilateral Hg-ARF Cp/GFR on the affected side 0.51 ±0.16 was higher than that on the control (contralateral) side, 0.23 ±0.07, P < 0.0005. These results indicate that the low Cp/GFR observed in the oliguric ARF animals was not related to the level of circulating parathyroid hormone nor to the presence or absence of azotemia but probably was due to a reduced renal cortical perfusion. The high Cp/GFR in the nonoliguric ARF animals could be explained by secondary hyperparathyroidism and impaired phosphorus reabsorption due to tubular injury.
Full text
PDF

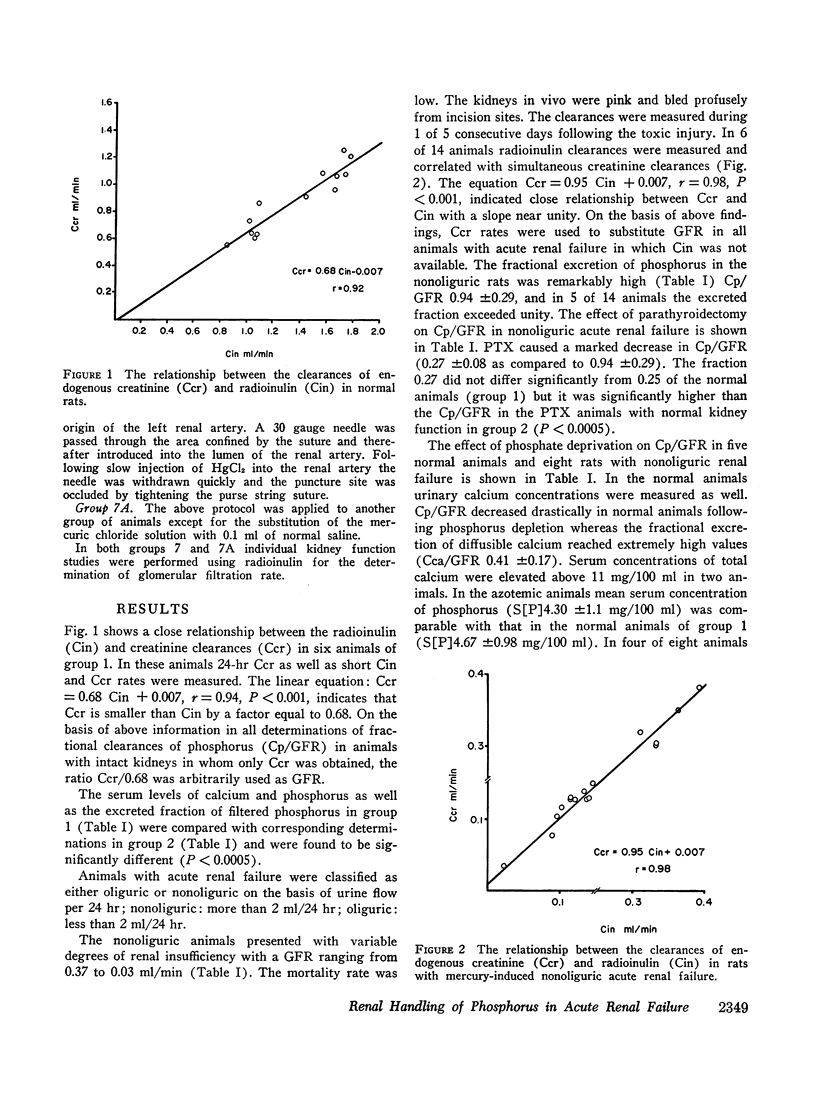

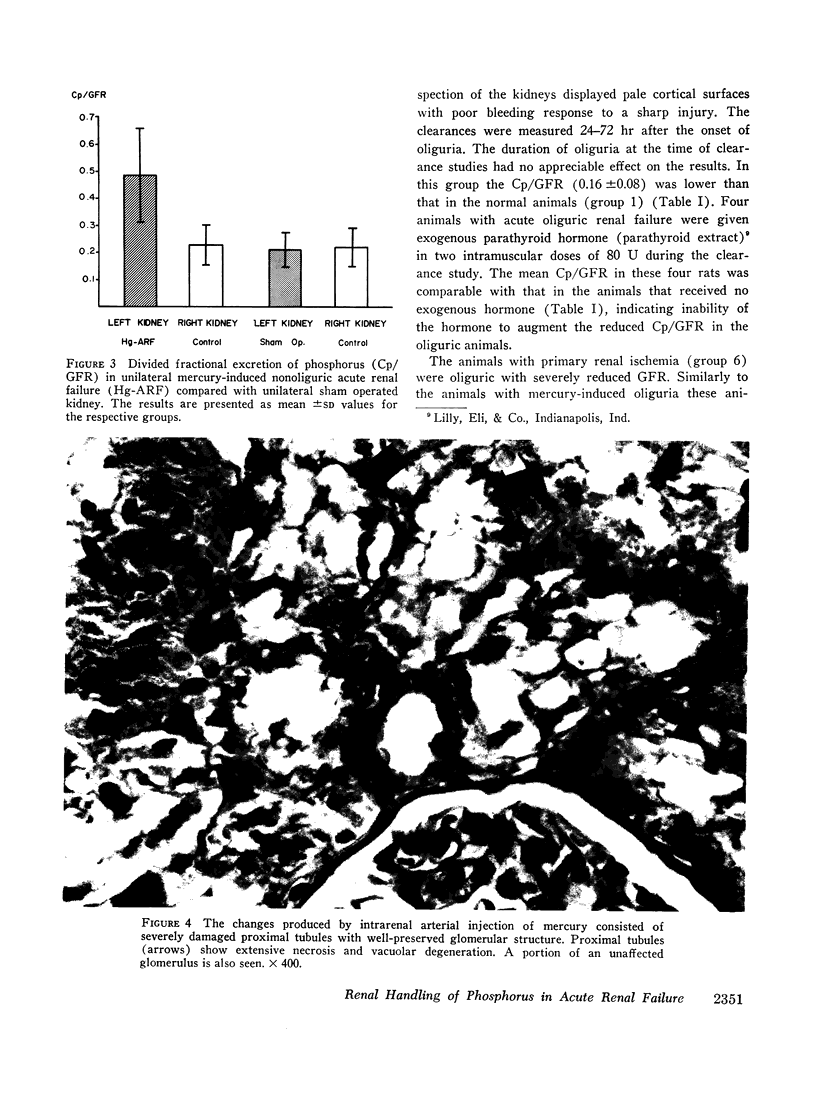
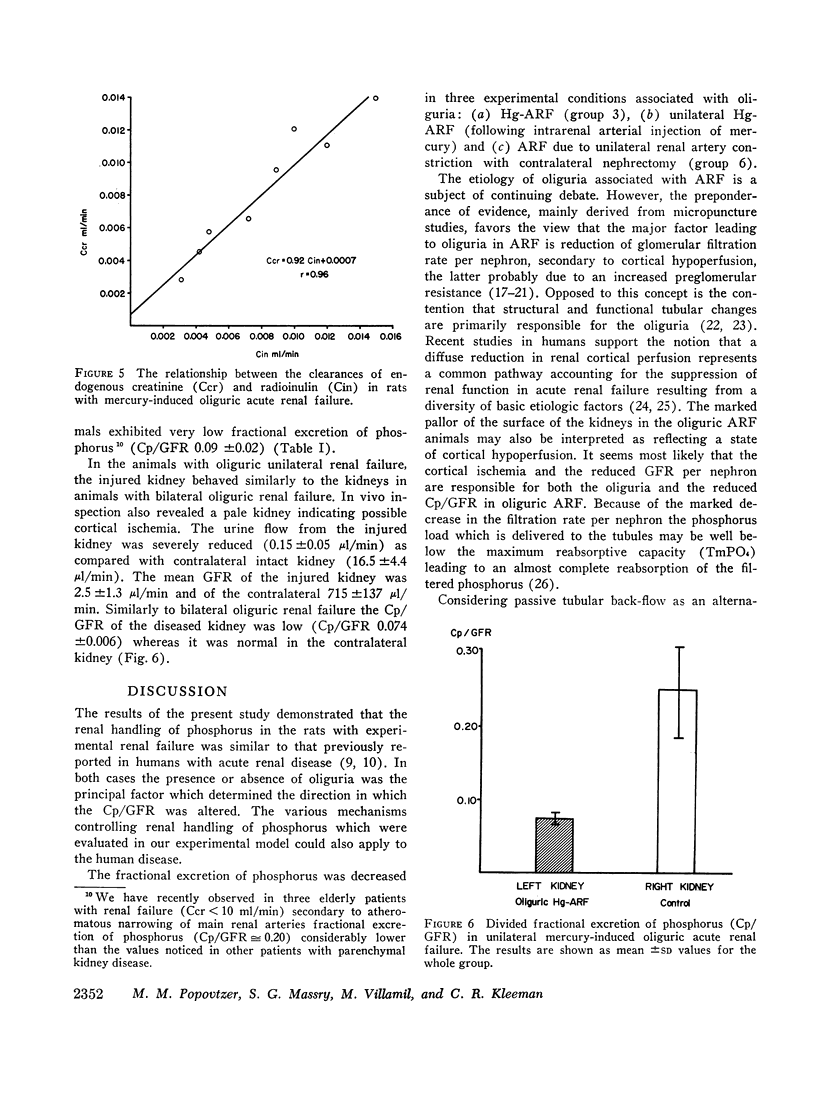


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRICKER N. S., KLAHR S., LUBOWITZ H., RIESELBACH R. E. RENAL FUNCTION IN CHRONIC RENAL DISEASE. Medicine (Baltimore) 1965 Jul;44:263–288. doi: 10.1097/00005792-196507000-00001. [DOI] [PubMed] [Google Scholar]
- BRICKER N. S., MORRIN P. A., KIME S. W., Jr The pathologic physiology of chronic Bright's disease. An exposition of the "intact nephron hypothesis". Am J Med. 1960 Jan;28:77–98. doi: 10.1016/0002-9343(60)90225-4. [DOI] [PubMed] [Google Scholar]
- Bank N., Mutz B. F., Aynedjian H. S. The role of "leakage" of tubular fluid in anuria due to mercury poisoning. J Clin Invest. 1967 May;46(5):695–704. doi: 10.1172/JCI105570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenberg R. L., Solomon S., Papper S., Anderson R. Clearance and micropuncture study of renal function in mercuric chloride treated rats. J Lab Clin Med. 1968 Sep;72(3):473–484. [PubMed] [Google Scholar]
- Better O. S., Kleeman C. R., Gonick H. C., Varrady P. D., Maxwell M. H. Renal handling of calcium, magnesium and inorganic phosphate in chronic renal failure. Isr J Med Sci. 1967 Jan-Feb;3(1):60–79. [PubMed] [Google Scholar]
- Biber T. U., Mylle M., Baines A. D., Gottschalk C. W., Oliver J. R., MacDowell M. C. A study by micropuncture and microdissection of acute renal damage in rats. Am J Med. 1968 May;44(5):664–705. doi: 10.1016/0002-9343(68)90251-9. [DOI] [PubMed] [Google Scholar]
- FLANIGAN W. J., OKEN D. E. RENAL MICROPUNCTURE STUDY OF THE DEVELOPMENT OF ANURIA IN THE RAT WITH MERCURY-INDUCED ACUTE RENAL FAILURE. J Clin Invest. 1965 Mar;44:449–457. doi: 10.1172/JCI105158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN R., BASSETT S. H. Phosphorus excretion in renal failure. J Clin Invest. 1954 Dec;33(12):1623–1628. doi: 10.1172/JCI103042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg N. K., Adams D. F., Oken D. E., Abrams H. L., Merrill J. P. Acute renal failure due to nephrotoxins. N Engl J Med. 1970 Jun 11;282(24):1329–1334. doi: 10.1056/NEJM197006112822401. [DOI] [PubMed] [Google Scholar]
- Hollenberg N. K., Epstein M., Rosen S. M., Basch R. I., Oken D. E., Merrill J. P. Acute oliguric renal failure in man: evidence for preferential renal cortical ischemia. Medicine (Baltimore) 1968 Nov;47(6):455–474. doi: 10.1097/00005792-196811000-00001. [DOI] [PubMed] [Google Scholar]
- Jaenike J. R. The renal lesion associated with hemoglobinemia: a study of the pathogenesis of the excretory defect in the rat. J Clin Invest. 1967 Mar;46(3):378–387. doi: 10.1172/JCI105539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastak J. T., Morrison A. B., Raisz L. G. Effects of renal insufficiency on the parathyroid gland and calcium homeostasis. Am J Physiol. 1968 Jul;215(1):84–89. doi: 10.1152/ajplegacy.1968.215.1.84. [DOI] [PubMed] [Google Scholar]
- KLEEMAN C. R., COOKE R. E. The acute effects of parathyroid hormone on the metabolism of endogenous phosphate. J Lab Clin Med. 1951 Jul;38(1):112–127. [PubMed] [Google Scholar]
- Massry S. G., Coburn J. W., Chapman L. W., Kleeman C. R. Effect of NaCl infusion on urinary Ca++ and Mg++ during reduction in their filtered loads. Am J Physiol. 1967 Nov;213(5):1218–1224. doi: 10.1152/ajplegacy.1967.213.5.1218. [DOI] [PubMed] [Google Scholar]
- Oken D. E., DiBona G. F., McDonald F. D. Micropuncture studies of the recovery phase of myohemoglobinuric acute renal failure in the rat. J Clin Invest. 1970 Apr;49(4):730–737. doi: 10.1172/JCI106285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovtzer M. M., Massry S. G., Makoff D. L., Maxwell M. H., Kleeman C. R. Renal handling of phosphate in patients with chronic renal failure. The role of variations in serum phosphorus and parathyroid activity. Isr J Med Sci. 1969 Sep-Oct;5(5):1018–1023. [PubMed] [Google Scholar]
- Popovtzer M. M., Schainuck L. I., Massry S. G., Kleeman C. R. Divalent ion excretion in chronic kidney disease: relation to degree of renal insufficiency. Clin Sci. 1970 Mar;38(3):297–307. doi: 10.1042/cs0380297. [DOI] [PubMed] [Google Scholar]
- REISS E., BRICKER N. S., KIME S. W., Jr, MORRIN P. A. Observations on phosphate transport in experimental renal disease. J Clin Invest. 1961 Jan;40:165–170. doi: 10.1172/JCI104231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Guiñazú A., Coelho J. B., Paz R. A. Methemoglobin-induced acute renal failure in the rat. In vivo observation, histology and micropuncture measurements of intratubular and postglomerular vascular pressures. Nephron. 1967;4(5):257–275. doi: 10.1159/000179587. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E., Gradowska L., Kashemsant C., Keltner R., Manley C., Bricker N. S. The control of phosphate excretion in uremia. J Clin Invest. 1966 May;45(5):672–677. doi: 10.1172/JCI105382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatopolsky E., Robson A. M., Elkan I., Bricker N. S. Control of phosphate excretion in uremic man. J Clin Invest. 1968 Aug;47(8):1865–1874. doi: 10.1172/JCI105877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhausen M., Eisenbach G. M., Helmstädter V. Concentration of Lissamine green in proximal tubules of antidiuretic and mercury poisoned rats and the permeability of these tubules. Pflugers Arch. 1969;311(1):1–15. doi: 10.1007/BF00588058. [DOI] [PubMed] [Google Scholar]



