Abstract
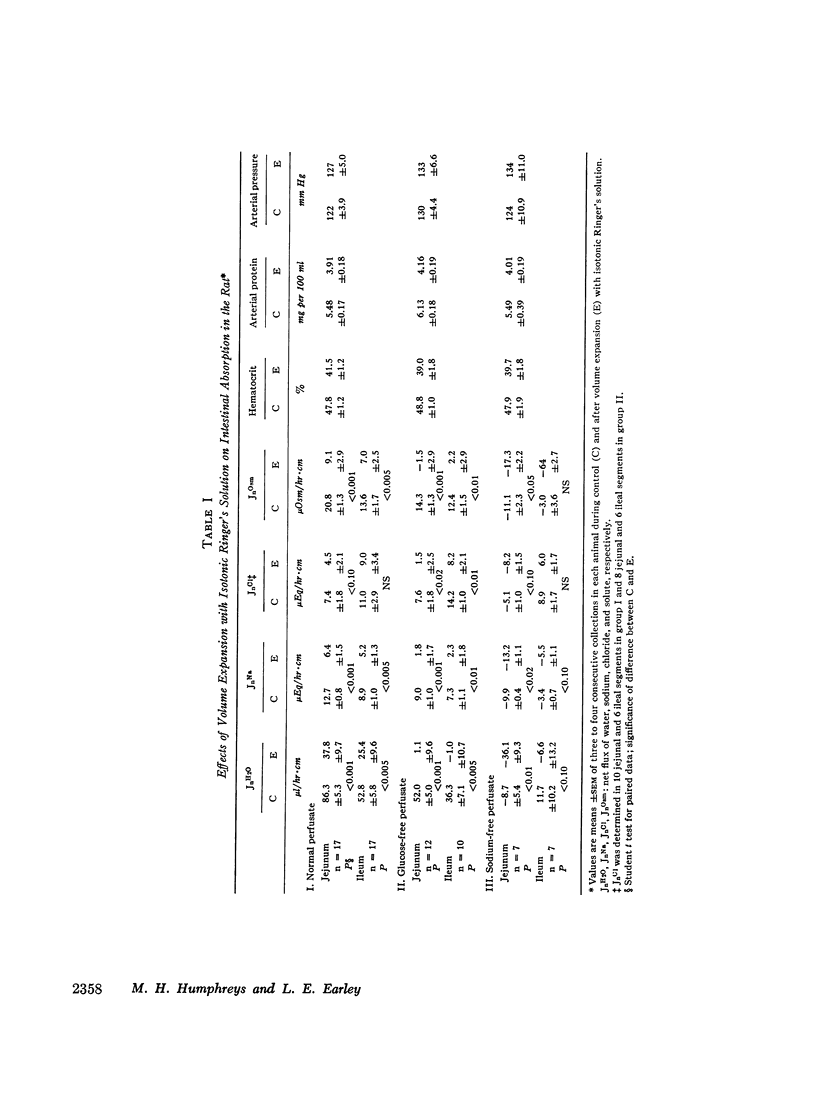
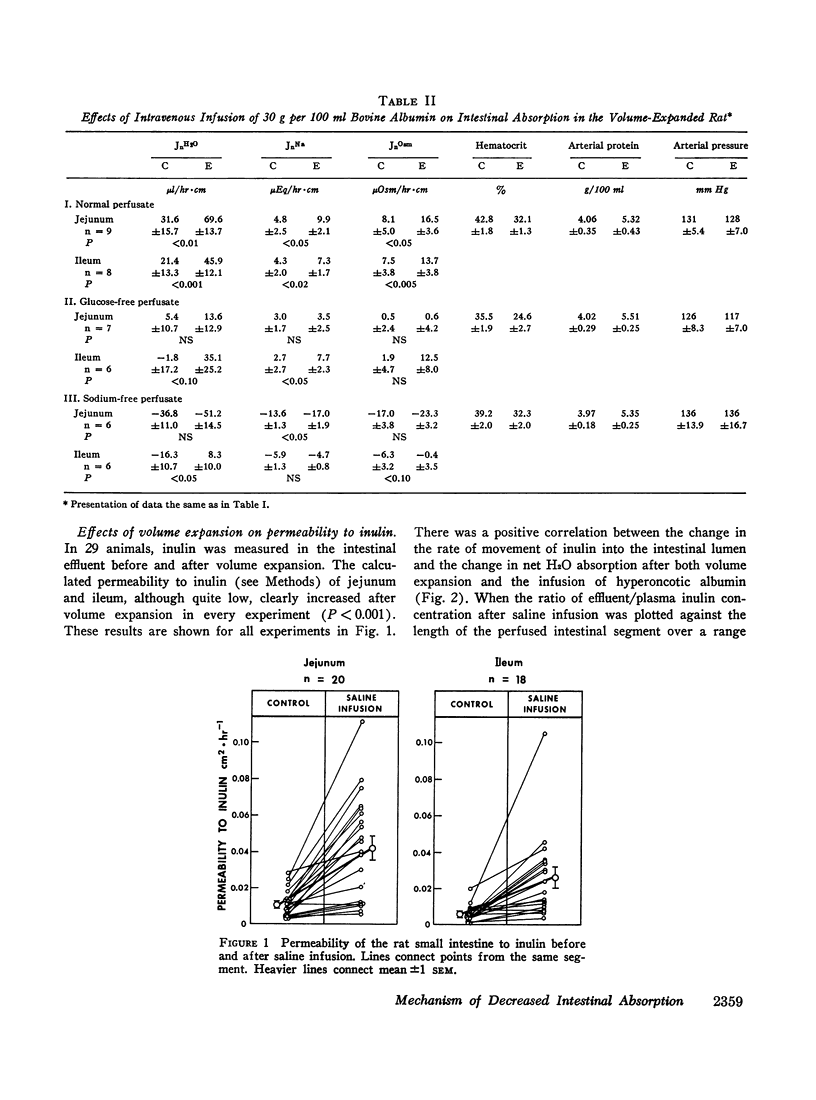
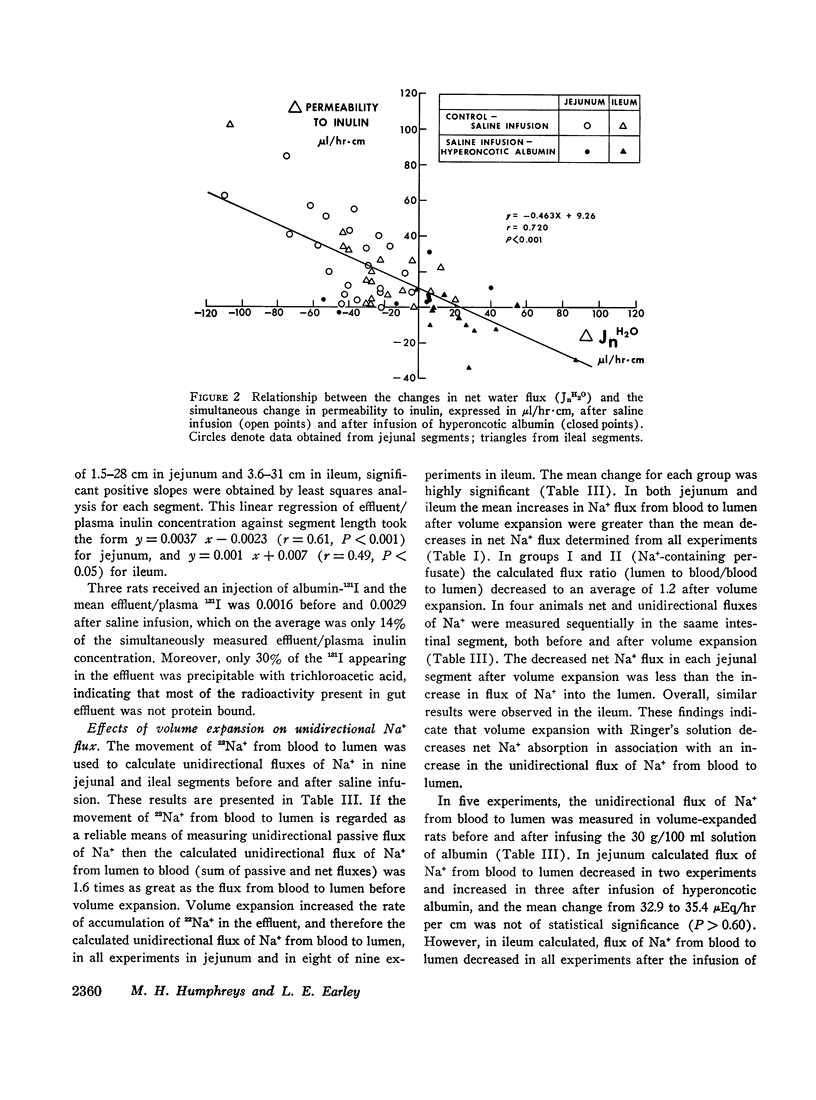
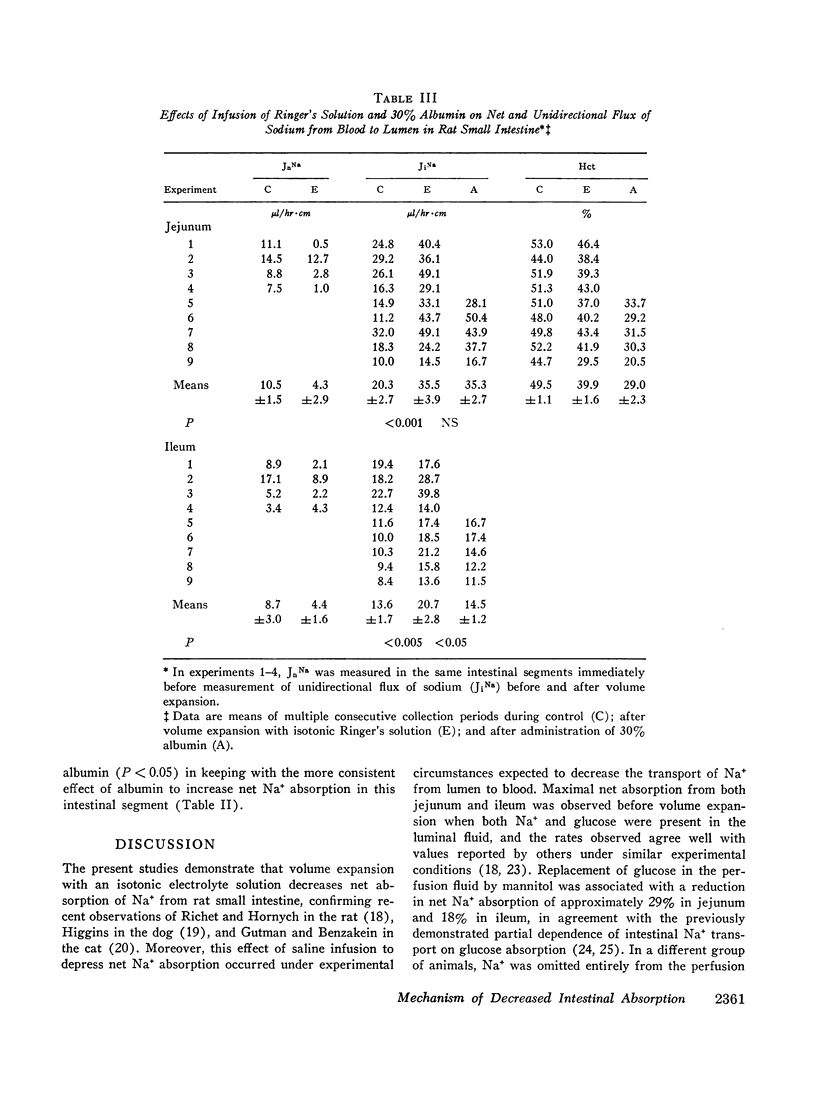
Studies were performed in rat small intestine in vivo to determine the effect of saline infusion on intestinal transport of Na+ and H2O. Saline infusion decreased net Na+ flux (JnNa) from 12.7 ±0.8 to 6.4 ±1.5 μEq/hr per cm in the jejunum when the intestinal perfusate contained both Na+ and glucose. A similar fall in JnNa occurred in ileum. When mannitol was substituted for glucose in the perfusate, control absorption decreased 29% in jejunum and 18% in ileum, but saline infusion still caused a decrease in JnNa quantitatively similar to that seen when glucose was present. When choline was substituted for Na+ in the perfusate, there was net movement of Na+ from blood to lumen during control and this net secretion was increased further after saline infusion. These observations suggest that saline infusion has a similar effect to decrease intestinal JnNa under three widely different conditions of basal sodium transport. Permeability of intestinal mucosa to inulin was very low under basal conditions but increased fivefold after saline infusion, and the unidirectional flux of Na+ from blood to lumen doubled. This increase in unidirectional flux of Na+ was greater than the observed decrease in JnNa.
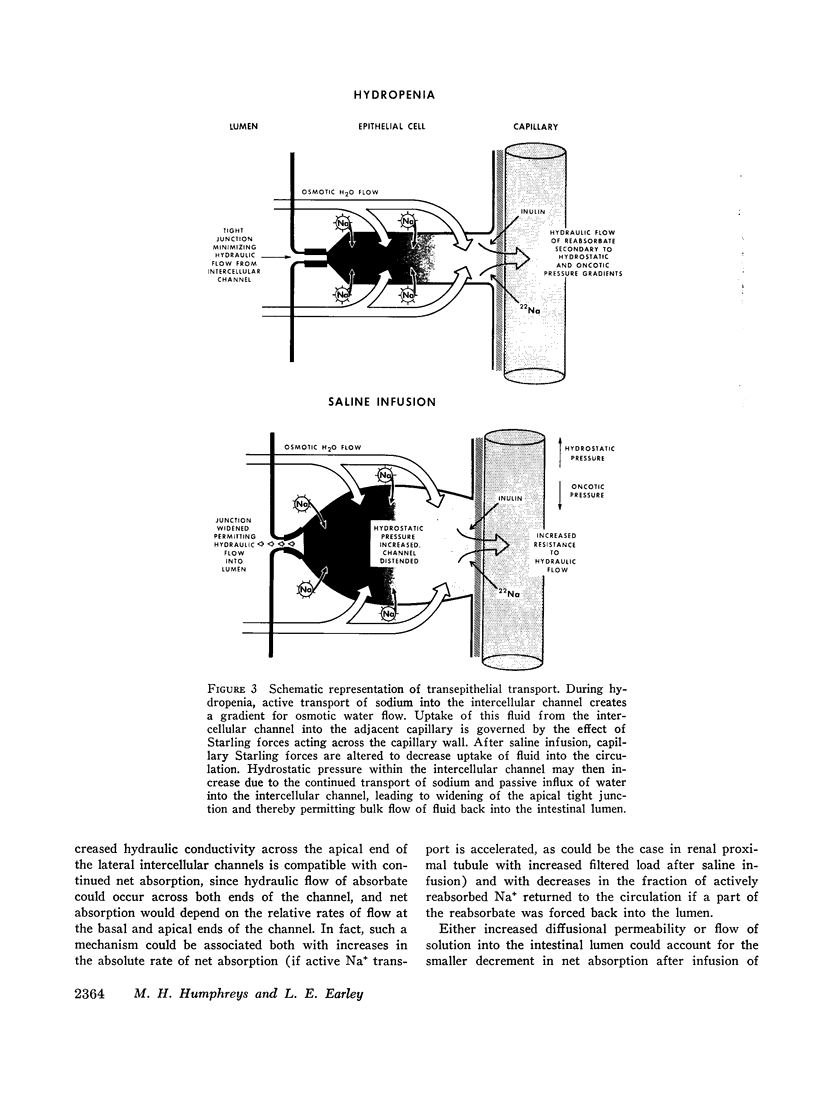
Thus, saline infusion decreased net absorption of Na+ and H2O from small intestine through mechanisms which did not appear to be dependent upon the rate of Na+ flux from lumen to blood, and in association with an increased flux of inulin and Na+ into the intestinal lumen. The data suggest that the effect of saline infusion to decrease net absorption from the intestine could be due either to an increase in passive permeability of the epithelium which could disrupt solute gradients within the membrane or to an increase in flow of solution into the intestinal lumen.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY B. A., MATTHEWS J., SMYTH D. H. Transfer of glucose and fluid by different parts of the small intestine of the rat. J Physiol. 1961 Jul;157:279–288. doi: 10.1113/jphysiol.1961.sp006721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank N., Koch K. M., Aynedjian H. S., Aras M. Effect of changes in renal perfusion pressure on the suppression of proximal tubular sodium reabsorption due to saline loading. J Clin Invest. 1969 Feb;48(2):271–283. doi: 10.1172/JCI105983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank N., Yarger W. E., Aynedjian H. S. A microperfusion study of sucrose movement across the rat proximal tubule during renal vein constriction. J Clin Invest. 1971 Feb;50(2):294–302. doi: 10.1172/JCI106494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder H. J., Katz L. A., Spencer R. P., Spiro H. M. The effect of inhibitors of renal transport on the small intestine. J Clin Invest. 1966 Dec;45(12):1854–1858. doi: 10.1172/JCI105489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L. Postglomerular vascular protein concentration: evidence for a causal role in governing fluid reabsorption and glomerulotublar balance by the renal proximal tubule. J Clin Invest. 1971 Feb;50(2):336–349. doi: 10.1172/JCI106501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAULFIELD J. B., TRUMP B. F. Correlation of ultrastructure with function in the rat kidney. Am J Pathol. 1962 Feb;40:199–218. [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., MACINTOSH J. R. A model system for biological water transport. Nature. 1962 Jan 27;193:347–348. doi: 10.1038/193347a0. [DOI] [PubMed] [Google Scholar]
- CURRAN P. F., SOLOMON A. K. Ion and water fluxes in the ileum of rats. J Gen Physiol. 1957 Sep 20;41(1):143–168. doi: 10.1085/jgp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. M., Miller M., Shields R. Intestinal transport of sodium, potassium, and water in the dog during sodium depletion. Gastroenterology. 1967 May;52(5):846–858. [PubMed] [Google Scholar]
- Cortney M. A., Mylle M., Lassiter W. E., Gottschalk C. W. Renal tubular transport of water, solute, and PAH in rats loaded with isotonic saline. Am J Physiol. 1965 Dec;209(6):1199–1205. doi: 10.1152/ajplegacy.1965.209.6.1199. [DOI] [PubMed] [Google Scholar]
- DE WARDENER H. E., MILLS I. H., CLAPHAM W. F., HAYTER C. J. Studies on the efferent mechanism of the sodium diuresis which follows the administration of intravenous saline in the dog. Clin Sci. 1961 Oct;21:249–258. [PubMed] [Google Scholar]
- DIAMOND J. M. THE MECHANISM OF ISOTONIC WATER TRANSPORT. J Gen Physiol. 1964 Sep;48:15–42. doi: 10.1085/jgp.48.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIRKS J. H., CIRKSENA W. J., BERLINER R. W. THE EFFECTS OF SALINE INFUSION ON SODIUM REABSORPTION BY THE PROXIMAL TUBULE OF THE DOG. J Clin Invest. 1965 Jul;44:1160–1170. doi: 10.1172/JCI105223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Tormey J. M. Role of long extracellular channels in fluid transport across epithelia. Nature. 1966 May 21;210(5038):817–820. doi: 10.1038/210817a0. [DOI] [PubMed] [Google Scholar]
- Earley L. E., Friedler R. M. Studies on the mechanism of natriuresis accompanying increased renal blood flow and its role in the renal response to extracellular volume expansion. J Clin Invest. 1965 Nov;44(11):1857–1865. doi: 10.1172/JCI105293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley L. E., Martino J. A., Friedler R. M. Factors affecting sodium reabsorption by the proximal tubule as determined during blockade of distal sodium reabsorption. J Clin Invest. 1966 Nov;45(11):1668–1684. doi: 10.1172/JCI105474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. I. Identification and isolation of the pathogenetic antigen. J Exp Med. 1968 Mar 1;127(3):555–572. doi: 10.1084/jem.127.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Jr, Carter N. W. The mechanisms of sodium absorption in the human small intestine. J Clin Invest. 1968 Apr;47(4):884–900. doi: 10.1172/JCI105781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman Y., Benzakein F. Effect of saline loading on absorption from the cat ileum in vivo. Isr J Med Sci. 1970 Mar-Apr;6(2):195–200. [PubMed] [Google Scholar]
- Hakim A. A., Lifson N. Effects of pressure on water and solute transport by dog intestinal mucosa in vitro. Am J Physiol. 1969 Feb;216(2):276–284. doi: 10.1152/ajplegacy.1969.216.2.276. [DOI] [PubMed] [Google Scholar]
- Howards S. S., Davis B. B., Knox F. G., Wright F. S., Berliner R. W. Depression of fractional sodium reabsorption by the proximal tubule of the dog without sodium diuresis. J Clin Invest. 1968 Jul;47(7):1561–1572. doi: 10.1172/JCI105848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye G. I., Wheeler H. O., Whitlock R. T., Lane N. Fluid transport in the rabbit gallbladder. A combined physiological and electron microscopic study. J Cell Biol. 1966 Aug;30(2):237–268. doi: 10.1083/jcb.30.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCHI R., GASBARRINI G. CONTRIBUTO ALLO STUDIO DELL'ASSORBIMENTO INTESTINALE MEDIANTE LA MICROSCOPIA ELETTRONICA. Sperimentale. 1963 Sep-Oct;113:239–250. [PubMed] [Google Scholar]
- Loeschke K., Bentzel C. J., Csáky T. Z. Asymmetry of osmotic flow in frog intestine: functional and structural correlation. Am J Physiol. 1970 Jun;218(6):1723–1731. doi: 10.1152/ajplegacy.1970.218.6.1723. [DOI] [PubMed] [Google Scholar]
- MCCARTHY C. F., BORLAND J. L., Jr, LYNCH H. J., Jr, OWEN E. E., TYOR M. P. DEFECTIVE UPTAKE OF BASIC AMINO ACIDS AND L-CYSTINE BY INTESTINAL MUCOSA OF PATIENTS WITH CYSTINURIA. J Clin Invest. 1964 Aug;43:1518–1524. doi: 10.1172/JCI105028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILNE M. D., CRAWFORD M. A., GIRAO C. B., LOUGHRIDGE L. W. The metabolic disorder in Hartnup disease. Q J Med. 1960 Jul;29:407–421. [PubMed] [Google Scholar]
- Martino J. A., Earley L. E. Demonstraton of a role of physical factors as determinants of the natriuretic response to volume expansion. J Clin Invest. 1967 Dec;46(12):1963–1978. doi: 10.1172/JCI105686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J. A., Earley L. E. Relationship between intrarenal hydrostatic pressure and hemodynamically induced changes in sodium excretion. Circ Res. 1968 Sep;23(3):371–386. doi: 10.1161/01.res.23.3.371. [DOI] [PubMed] [Google Scholar]
- McLeod G. M., French A. B., Good C. J., Wright F. S. Gastrointestinal absorption and biliary excretion of phenolsulfonphthalein (phenol red) in man. J Lab Clin Med. 1968 Feb;71(2):192–200. [PubMed] [Google Scholar]
- PARSONS B. J., SMYTH D. H., TAYLOR C. B. The action of phlorrhizin on the intestinal transfer of glucose and water in vitro. J Physiol. 1958 Dec 30;144(3):387–402. doi: 10.1113/jphysiol.1958.sp006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richet G., Hornych A. The effect of an expansion of extracellular fluids on net Na flux in the jejunum of rats. An experimental model for the study of the third factor. Nephron. 1969;6(3):365–378. doi: 10.1159/000179739. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. II. THE INTERACTION BETWEEN ACTIVE SODIUM AND ACTIVE SUGAR TRANSPORT. J Gen Physiol. 1964 Jul;47:1043–1059. doi: 10.1085/jgp.47.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. W., Earley L. E. Effects of hematocrit on renal hemodynamics and sodium excretion in hydropenic and volume-expanded dogs. J Clin Invest. 1970 Sep;49(9):1656–1667. doi: 10.1172/JCI106383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey J. E., Kirshman J. D., Laragh J. H. Natriuretic activity in plasma and urine of salt-loaded man and sheep. J Clin Invest. 1969 Dec;48(12):2210–2224. doi: 10.1172/JCI106187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer A., Windhager E. E. Effect of peritubular oncotic pressure changes on proximal tubular fluid reabsorption. Am J Physiol. 1970 Apr;218(4):1188–1193. doi: 10.1152/ajplegacy.1970.218.4.1188. [DOI] [PubMed] [Google Scholar]
- THIER S. O., SEGAL S., FOX M., BLAIR A., ROSENBERG L. E. CYSTINURIA: DEFECTIVE INTESTINAL TRANSPORT OF DIBASIC AMINO ACIDS AND CYSTINE. J Clin Invest. 1965 Mar;44:442–448. doi: 10.1172/JCI105157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS A. W. Electron microscopic changes associated with water absorption in the jejunum. Gut. 1963 Mar;4:1–7. doi: 10.1136/gut.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]