Abstract
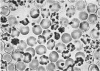
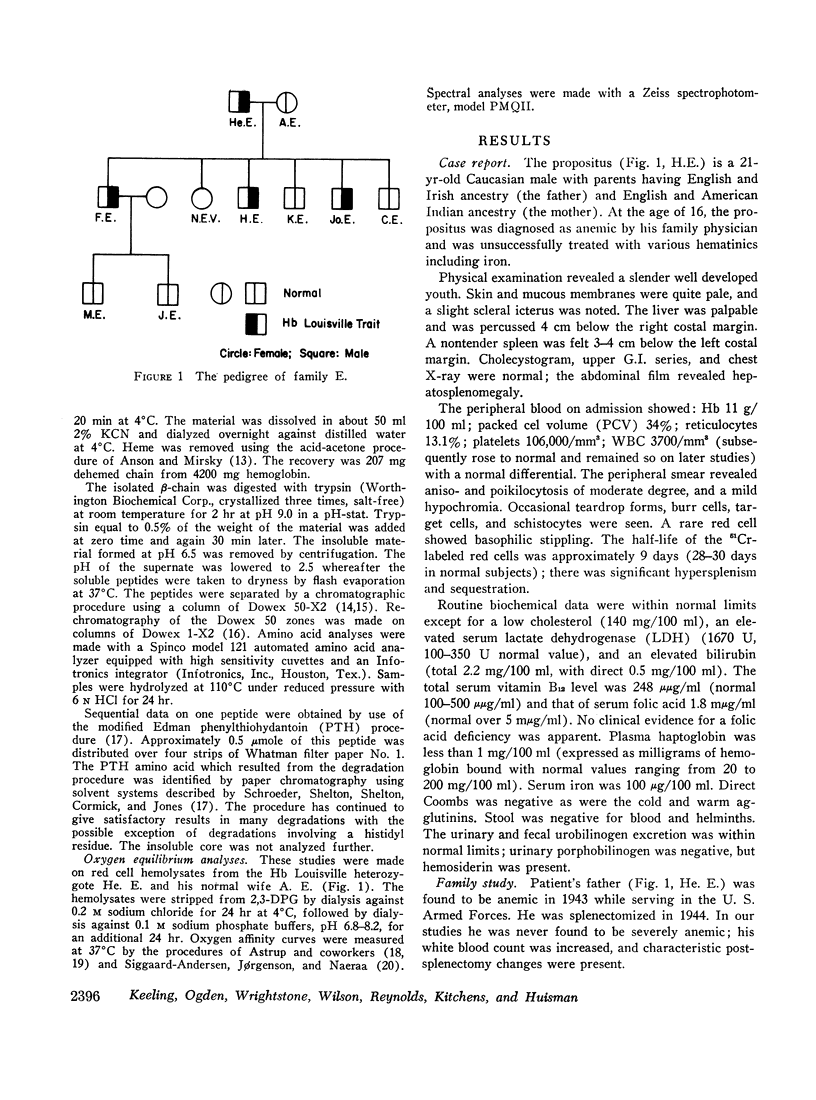
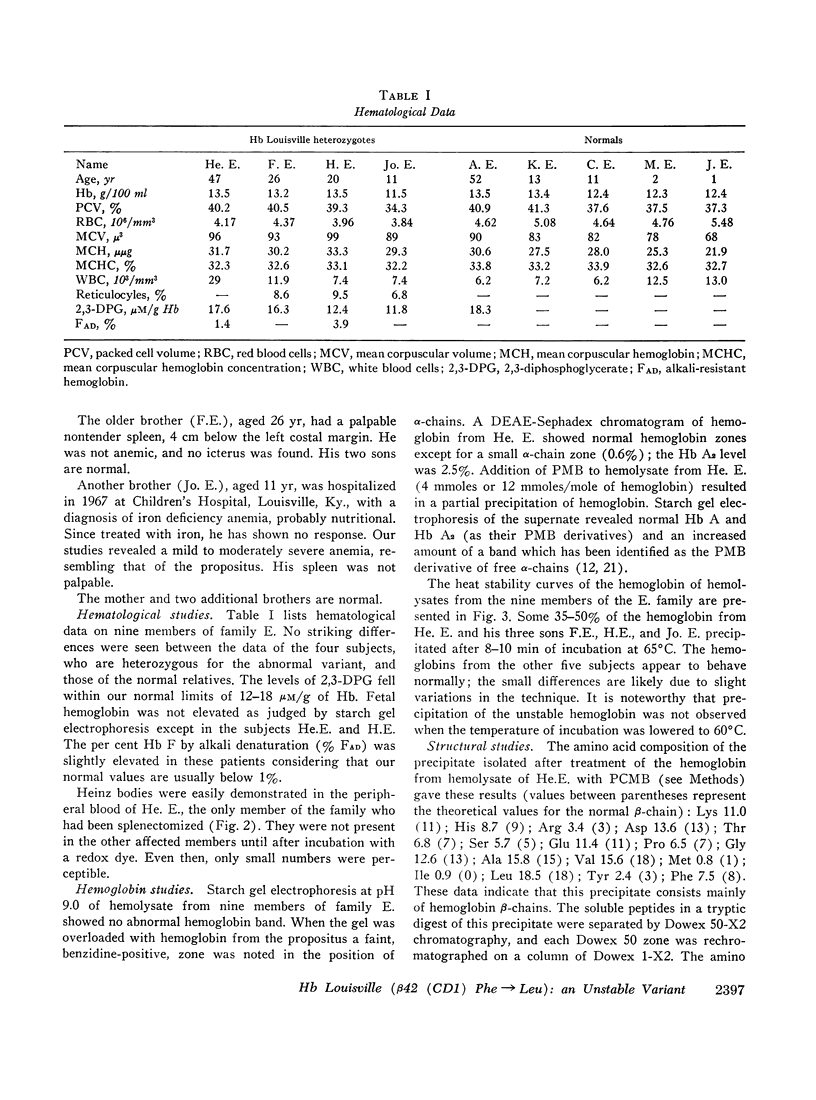
An unstable hemoglobin variant termed Hb Louisville, was found in four members of a Caucasian family, who were suffering from a mild hemolytic anemia. The variant showed a decreased stability upon warming at 65°C and an increased tendency to dissociate in the presence of sulfhydryl group-blocking agents. The structural abnormality was identified as a replacement of phenylalanyl residue in position 42 (CD1) by a leucyl residue. Substitution of this phenylalanyl residue, which participates in the contact with heme, by a nonpolar leucyl residue has apparently less severe consequences than a replacement of the same residue by a polar seryl residue as in Hb Hammersmith.
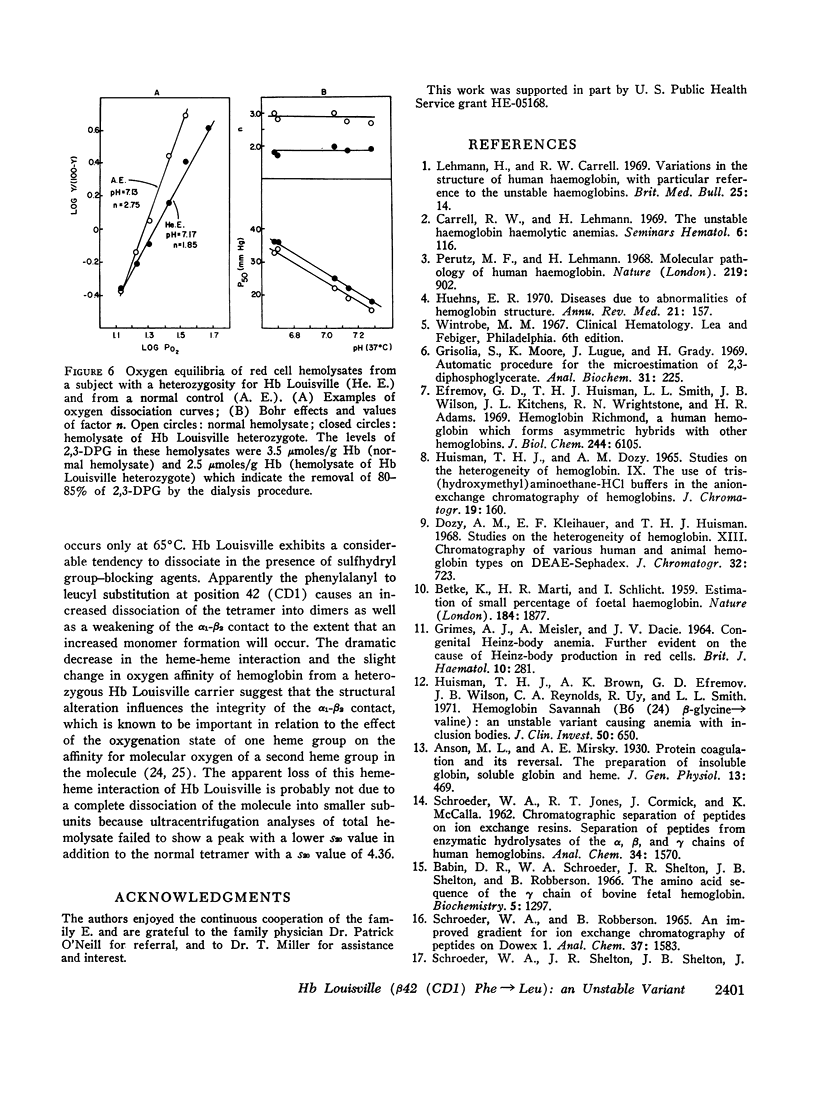
Oxygen equilibrium studies of total hemolysate from one Hb Louisville heterozygote indicated a decreased oxygen affinity, a marked decrease in heme-heme interaction, and a normal Bohr effect. Studies with isolated Hb Louisville were not made because it was not possible to separate the variant from normal Hb A.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN O. S., JORGENSEN K., NAERAA N. Spectrophotometric determination of oxygen saturation in capillary blood. Scand J Clin Lab Invest. 1962;14:298–302. [PubMed] [Google Scholar]
- Astrup P., Engel K., Severinghaus J. W., Munson E. The influence of temperature and pH on the dissociation curve of oxyhemoglobin of human blood. Scand J Clin Lab Invest. 1965;17(6):515–523. doi: 10.1080/00365516509083359. [DOI] [PubMed] [Google Scholar]
- Astrup P., Hellung-Larsen P., Kjeldsen K., Mellemgaard K. The effect of tobacco smoking on the dissociation curve of oxyhemoglobin. Investigations in patients with occlusive arterial diseases and in normal subjects. Scand J Clin Lab Invest. 1966;18(4):450–457. doi: 10.3109/00365516609113166. [DOI] [PubMed] [Google Scholar]
- BALDRIDGE R. C., LEWIS H. B. Diet and the ergothioneine content of blood. J Biol Chem. 1953 May;202(1):169–176. [PubMed] [Google Scholar]
- BETKE K., MARTI H. R., SCHLICHT I. Estimation of small percentages of foetal haemoglobin. Nature. 1959 Dec 12;184(Suppl 24):1877–1878. doi: 10.1038/1841877a0. [DOI] [PubMed] [Google Scholar]
- Babin D. R., Schroeder W. A., Shelton J. R., Shelton J. B., Robberson B. The amino acid sequence of the gamma chain of bovine fetal hemoglobin. Biochemistry. 1966 Apr;5(4):1297–1310. doi: 10.1021/bi00868a025. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Lehmann H. The unstable haemoglobin haemolytic anaemias. Semin Hematol. 1969 Apr;6(2):116–132. [PubMed] [Google Scholar]
- Dacie J. V., Shinton N. K., Gaffney P. J., Jr, Lehmann H. Haemoglobin Hammersmith (beta-42 (CDI) Phe replaced by ser). Nature. 1967 Nov 18;216(5116):663–665. doi: 10.1038/216663a0. [DOI] [PubMed] [Google Scholar]
- Dozy A. M., Kleihauer E. F., Huisman T. H. Studies on the heterogeneity of hemoglobin. 13. Chromatography of various human and animal hemoglobin types on DEAE-Sephadex. J Chromatogr. 1968 Feb 20;32(4):723–727. doi: 10.1016/s0021-9673(01)80551-3. [DOI] [PubMed] [Google Scholar]
- Efremov G. D., Huisman T. H., Smith L. L., Wilson J. B., Kitchens J. L., Wrightstone R. N., Adams H. R. Hemoglobin Richmond, a human hemoglobin which forms asymmetric hybrids with other hemoglobins. J Biol Chem. 1969 Nov 25;244(22):6105–6116. [PubMed] [Google Scholar]
- GRIMES A. J., MEISLER A., DACIE J. V. CONGENITAL HEINZ-BODY ANAEMIA. FURTHER EVIDENCE ON THE CAUSE OF HEINZ-BODY PRODUCTION IN RED CELLS. Br J Haematol. 1964 Jul;10:281–290. doi: 10.1111/j.1365-2141.1964.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Huehns E. R., Bellingham A. J. Diseases of function and stability of haemoglobin. Br J Haematol. 1969 Jul;17(1):1–10. doi: 10.1111/j.1365-2141.1969.tb05659.x. [DOI] [PubMed] [Google Scholar]
- Huehns E. R. Diseases due to abnormalities of hemoglobin structure. Annu Rev Med. 1970;21:157–178. doi: 10.1146/annurev.me.21.020170.001105. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Brown A. K., Efremov G. D., Wilson J. B., Reynolds C. A., Uy R., Smith L. L. Hemoglobin Savannah (B6(24) beta-glycine is greater than valine): an unstable variant causing anemia with inclusion bodies. J Clin Invest. 1971 Mar;50(3):650–659. doi: 10.1172/JCI106535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Schroeder W. A. New aspects of the structure, function, and synthesis of hemoglobins. CRC Crit Rev Clin Lab Sci. 1970 Jul;1(3):471–472. doi: 10.3109/10408367009027952. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Winterhalter K. H. The role of hemoglobin heme loss in Heinz body formation: studies with a partially heme-deficient hemoglobin and with genetically unstable hemoglobins. J Clin Invest. 1970 Nov;49(11):2008–2016. doi: 10.1172/JCI106421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H., Carrell R. W. Variations in the structure of human haemoglobin. With particular reference to the unstable haemoglobins. Br Med Bull. 1969 Jan;25(1):14–23. doi: 10.1093/oxfordjournals.bmb.a070664. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Rieder R. F., Oski F. A., Clegg J. B. Hemoglobin Philly (beta 35 tyrosine phenylalanine): studies in the molecular pathology of hemoglobin. J Clin Invest. 1969 Sep;48(9):1627–1642. doi: 10.1172/JCI106128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemeyer M. A., Huehns E. R. On the mechanism of the dissociation of haemoglobin. J Mol Biol. 1967 Apr 28;25(2):253–273. doi: 10.1016/0022-2836(67)90141-6. [DOI] [PubMed] [Google Scholar]
- SCHROEDER W. A., SHELTON J. R., SHELTON J. B., CORMICK J., JONES R. T. THE AMINO ACID SEQUENCE OF THE GAMMA CHAIN OF HUMAN FETAL HEMOGLOBIN. Biochemistry. 1963 Sep-Oct;2:992–1008. doi: 10.1021/bi00905a016. [DOI] [PubMed] [Google Scholar]
- Schroeder W. A., Robberson B. An improved gradient for ion exchange chromatography of peptides on Dowex-1. Anal Chem. 1965 Nov;37(12):1583–1585. doi: 10.1021/ac60231a036. [DOI] [PubMed] [Google Scholar]
- Shinton N. K., Williams H. P., Thursby-Pelham D. C. Congenital Heinz-body haemolytic anaemia due to haemoglobin Hammersmith. Postgrad Med J. 1969 Sep;45(527):629–632. doi: 10.1136/pgmj.45.527.629. [DOI] [PMC free article] [PubMed] [Google Scholar]