Abstract
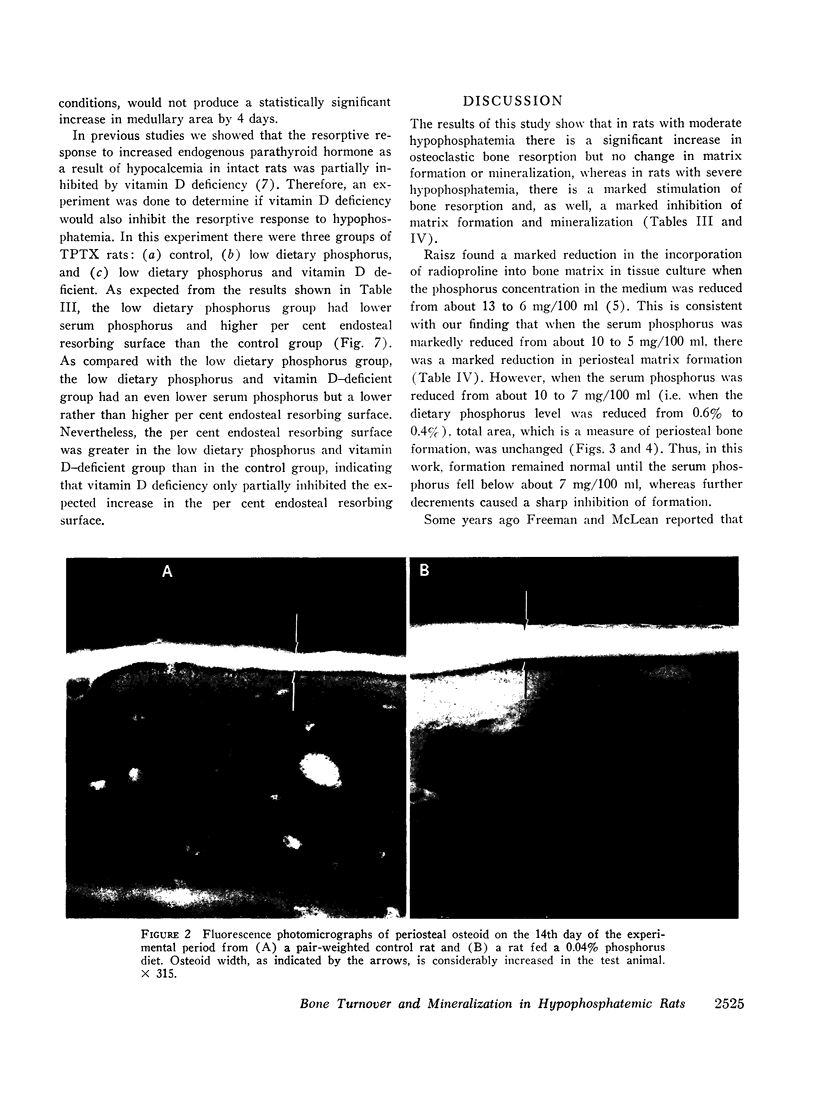
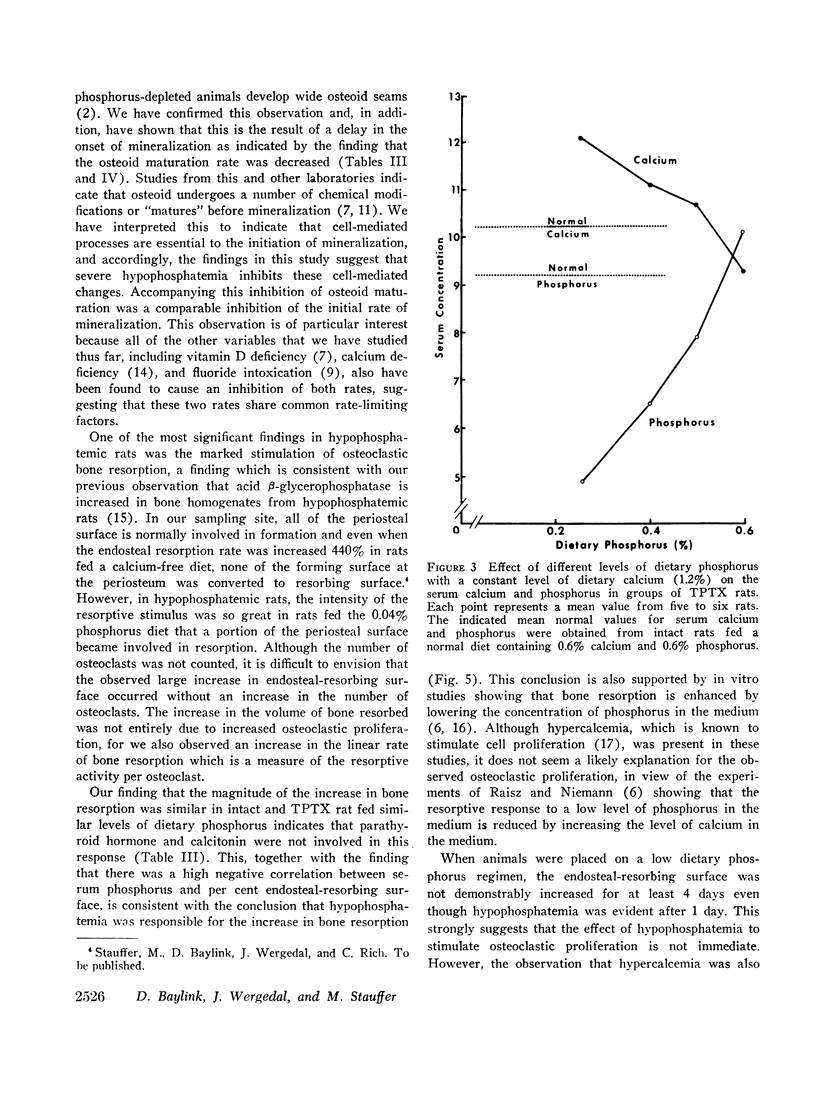
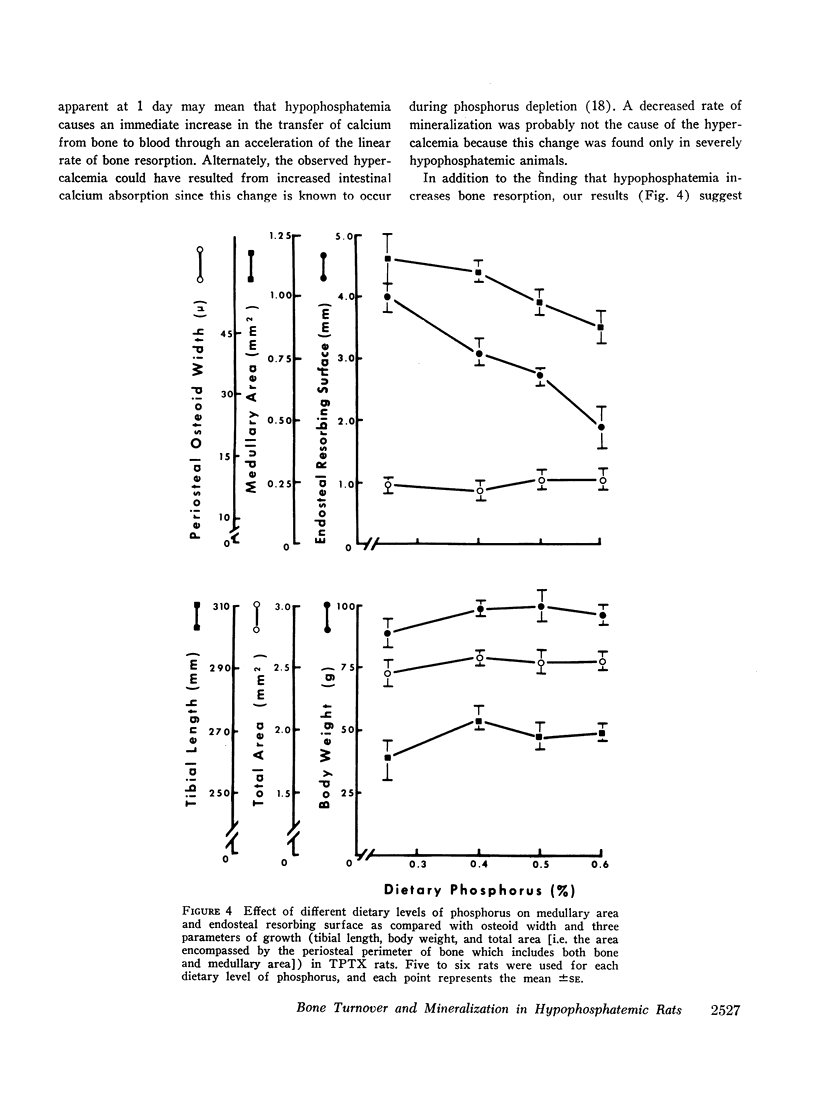
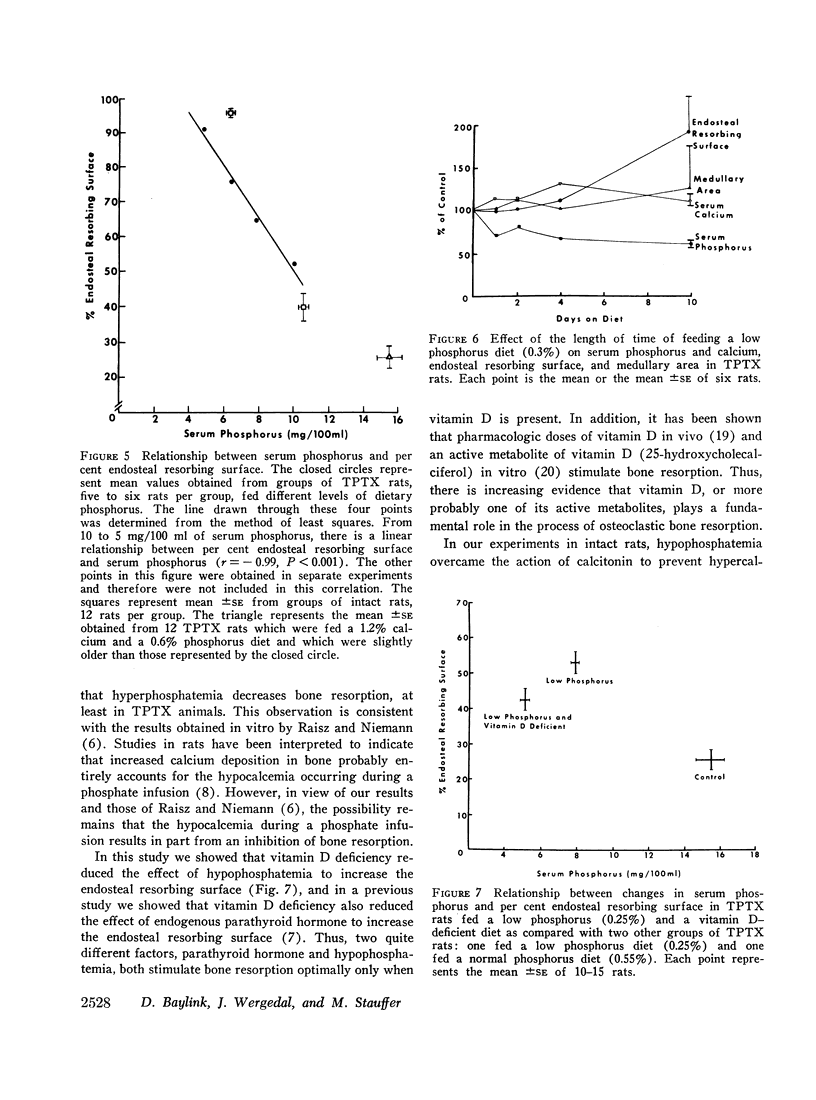
Quantitative morphologic methods were used to measure the effects of feeding a low phosphorus diet to intact and thyroparathyroidectomized rats on several processes of bone mineralization and turnover. In severely hypophosphatemic animals, the matrix formation rate was decreased, the osteoid maturation rate was decreased, which indicated a delay in the onset of mineralization, the initial rate of mineralization was decreased, and the endosteal osteoclastic bone resorption rate was increased. In moderately hypophosphatemic animals, there was a substantial increase in bone resorption but no change in formation or in mineralization. The increase in endosteal bone resorption was due to an increase in the linear rate of bone resorption and particularly to an increase in the length of the endosteal resorbing surface. The magnitude of the increase in bone resorption was similar in thyroparathyroidectomized and intact rats indicating that neither parathyroid hormone nor calcitonin is involved in this change. This, together with the finding that there was a strong negative correlation (r = -0.99) between the per cent endosteal resorbing surface and the serum phosphorus, supports the view that the increased resorption was due to hypophosphatemia. This inverse relationship between endosteal resorbing surface and serum phosphorus appeared to hold for values of serum phosphorus above normal. The resorptive response to hypophosphatemia, as previously shown for the resorptive response to excess endogenous parathyroid hormone, was partially inhibited by vitamin D deficiency. Increased resorption occurred at levels of serum phosphorus where no changes were observed in bone formation, mineralization, or growth, suggesting that this resorptive response functions as a homeostatic mechanism to maintain serum and intracellular phosphorus concentrations.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylink D., Morey E., Rich C. Effect of calcitonin on the rates of bone formation and resorption in the rat. Endocrinology. 1969 Feb;84(2):261–269. doi: 10.1210/endo-84-2-261. [DOI] [PubMed] [Google Scholar]
- Baylink D., Stauffer M., Wergedal J., Rich C. Formation, mineralization, and resorption of bone in vitamin D-deficient rats. J Clin Invest. 1970 Jun;49(6):1122–1134. doi: 10.1172/JCI106328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. J., Jowsey J., Riggs B. L., Elveback L. R. Relationship between serum phosphate concentration and bone resorption in osteoporosis. J Lab Clin Med. 1967 Jan;69(1):110–115. [PubMed] [Google Scholar]
- Lotz M., Zisman E., Bartter F. C. Evidence for a phosphorus-depletion syndrome in man. N Engl J Med. 1968 Feb 22;278(8):409–415. doi: 10.1056/NEJM196802222780802. [DOI] [PubMed] [Google Scholar]
- Moore E. W. Ionized calcium in normal serum, ultrafiltrates, and whole blood determined by ion-exchange electrodes. J Clin Invest. 1970 Feb;49(2):318–334. doi: 10.1172/JCI106241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagant de Deuxchaisnes C., Krane S. M. The treatment of adult phosphate diabetes and Fanconi syndrome with neutral sodium phosphate. Am J Med. 1967 Oct;43(4):508–543. doi: 10.1016/0002-9343(67)90177-5. [DOI] [PubMed] [Google Scholar]
- Nichols G., Jr, Flanagan B., Veer J. van der S. Distortions of bone cell metabolism in uremia and their cause. Arch Intern Med. 1969 Nov;124(5):530–538. [PubMed] [Google Scholar]
- Raisz L. G., Niemann I. Effect of phosphate, calcium and magnesium on bone resorption and hormonal responses in tissue culture. Endocrinology. 1969 Sep;85(3):446–452. doi: 10.1210/endo-85-3-446. [DOI] [PubMed] [Google Scholar]
- Raisz L. G. The pharmacology of bone. Introduction. Fed Proc. 1970 May-Jun;29(3):1176–1178. [PubMed] [Google Scholar]
- Rasmussen H., Feinblatt J., Nagata N., Pechet M. Effect of ions upon bone cell function. Fed Proc. 1970 May-Jun;29(3):1190–1197. [PubMed] [Google Scholar]
- Rixon R. H. Mitotic activity in the bone marrow of rats and its relation to the level of plasma calcium. Curr Mod Biol. 1968 May-Jun;2(2):68–74. doi: 10.1016/0303-2647(68)90010-5. [DOI] [PubMed] [Google Scholar]
- Trummel C. L., Raisz L. G., Blunt J. W., Deluca H. F. 25-Hydroxycholecalciferol: stimulation of bone resorption in tissue culture. Science. 1969 Mar 28;163(3874):1450–1451. doi: 10.1126/science.163.3874.1450. [DOI] [PubMed] [Google Scholar]
- Wergedal J. E., Baylink D. J. Factors affecting bone enzymatic activity in vitamin D-deficient rats. Am J Physiol. 1971 Feb;220(2):406–409. doi: 10.1152/ajplegacy.1971.220.2.406. [DOI] [PubMed] [Google Scholar]
- Wergedal J. E. Enzymes of protein and phosphate catabolism in rat bone. I. Enzyme properties in normal rats. Calcif Tissue Res. 1969;3(1):55–66. doi: 10.1007/BF02058645. [DOI] [PubMed] [Google Scholar]