Abstract
Propionic acidemia (PA) is a metabolic disorder that causes mental retardation and that can be fatal if untreated. PA is inherited in an autosomal recessive fashion involving mutations in PCCA or PCCB encoding the α and β subunits of propionyl-CoA carboxylase (PCC). Current treatment is based on dietary restriction of substrate amino acids, which attenuates symptoms. However, patients still experience episodes of hyperammonemia that can cause progressive neurologic damage. In this paper, we have tested gene therapy approaches to PA in a stringent mouse model of PCCA deficiency, in which homozygous knockout mice are born but die within 36 hr. In this work, we have delivered first-generation and helper-dependent adenovirus serotype 5 (Ad5) vectors expressing the human PCCA cDNA by intraperitoneal injection into newborn mice. Unmodified Ad5 vectors mediated extensive transduction of the peritoneum with weak liver transduction as determined by luciferase imaging and dsRed expression. In contrast, modification of Ad5 with polyethylene glycol detargeted the virus from the peritoneum and retargeted it for transduction in the liver. When vectors expressing PCCA were injected, significant increases in life span were observed for both the unmodified and polyethylene glycol (PEG)-modified Ad5 vectors. However, this rescue was transient. Similarly, adeno-associated virus serotype 8-mediated transduction also produced only transient rescue. These data show first proof of principle for gene therapy of PA and demonstrate the potential utility of PEG to modify viral tropism in an actual gene therapy application.
Overview Summary
Propionic acidemia (PA) is a metabolic genetic disease likely requiring correction of 10 to 50% of gene function to rescue the phenotype in the absence of other treatments. PCCA−/− mice are born with liver damage and die within 36 hr of birth, and represent a stringent but simple model with which to test the potential of gene therapy for PA. Adenovirus is one of the few gene therapy vectors that can achieve this level of correction in animal models. This work shows that injection of adenovirus by the simple intraperitoneal route can double animal life span and provides proof of principle for the application of gene therapy for the treatment of humans with less severe phenotypes. This work also shows that PEGylation of adenovirus effectively detargets the virus from peritoneal transduction after intraperitoneal injection and increases the level of liver transduction by the vector. This and other work suggests that PEG can be used not just to reduce innate and adaptive immune responses to Ad, but to detarget and retarget Ad vectors for in vivo gene therapy.
Introduction
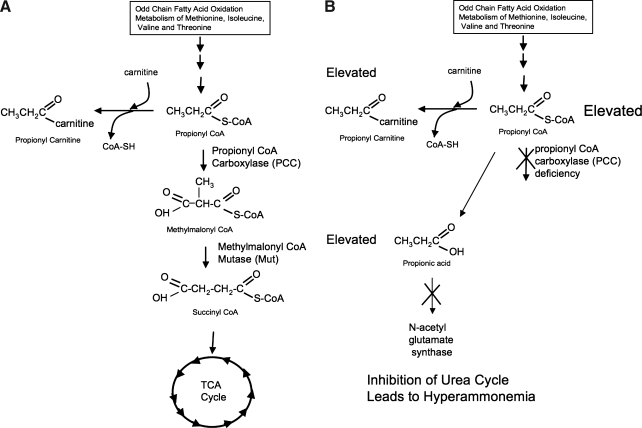
Propionic acidemia (PA) is a metabolic disorder first described in a patient by Childs and colleagues in 1961. PA affects approximately 1 in 30,000 live births worldwide, and in areas of high prevalence, such as Saudi Arabia, the disorder can affect as few as 1 in 2000 births (reviewed in Al Essa et al., 1998; Ugarte et al., 1999). Propionic acidemia is caused by defects in propionyl-CoA carboxylase (PCC) (Hsia et al., 1971). PCC is a biotin-dependent mitochondrial enzyme involved in the metabolism of odd-chain fatty acids and the amino acids methionine, threonine, isoleucine, and valine by converting propionyl-CoA to methylmalonyl-CoA (Fig. 1). PCC is a dodecamer consisting of six α and six β subunits encoded by the PCCA and PCCB genes on chromosomes 11 and 3 (Lamhonwah et al., 1986). Mutations in either gene can cause PA with a spectrum of phenotypes (Campeau et al., 2001).
FIG. 1.
Metabolic pathway for propionyl-CoA carboxylase (PCC). The pathways in a normal patient (A) and in a propionic acidemia patient (B).
In severe cases, patients cease feeding and become wasted within days of birth. They are found to be hyperammonemic with elevated levels of propionyl carnitine in the blood. If diagnosed early and a protein-restricted diet is applied, patients can be rescued, but they will likely still undergo severe mental retardation and recurring episodes of hyperammonemia that can lead to death. Long-term survival for early-onset patients under treatment and monitoring is approximately 67% (Barshes et al., 2006). In less severe cases, PA can be diagnosed on the basis of bouts of hyperammonemia in persons aged 3 months to 20 years or older.
There is currently no cure for PA. Administration of carnitine can attenuate symptoms, because carnitine can act as a carrier to remove propionyl-CoA from cells (Roe et al., 1984). In addition, N-carbamylglutamate administration can reduce hyperammonemia by bypassing propionyl-CoA inhibition of N-acetylglutamate synthetase (O'Connor et al., 1989). In some cases, children with PA who experienced frequent metabolic decompensation have been treated by liver transplantation (Yorifuji et al., 2000; Kayler et al., 2002). Although liver transplantation had only minimal effects on the levels of propionyl-CoA metabolites in the blood, hyperammonemia was corrected and metabolic decompensation was controlled (Yorifuji et al., 2000). More importantly, the growth rate and mental development of these children also improved significantly after transplantation. Although liver transplantation helped attenuate the symptoms of PA, this intervention is dangerous and invasive and requires life-long immune suppression. There is therefore a need for alternative treatments for PA.
Miyazaki and colleagues engineered a PCCA knockout mouse, which serves as a model for PA. These mice live 24–36 hr, show extremely high levels of propionyl carnitine, and display classic wasting seen in severe early-onset PA patients (Miyazaki et al., 2001). Importantly, this group also showed that these mice could be rescued for various amounts of time when their systemic mutation was covered by transgenesis with liver-specific PCCA expression. These data in mice and transplantation data in humans provide a rationale for testing the feasibility of performing systemic or liver-specific gene therapy for PA. The PCCA knockout and covering transgene study also provides some guidance on the level of genetic correction that might be needed for effective treatment. In this case, long-term liver-specific expression of PCCA at 10–20% of wild-type levels was insufficient to rescue the animals (Miyazaki et al., 2001). In contrast, mice heterozygous for PCCA deficiency, with 50% expression of the gene in the liver, were reportedly phenotypically normal. Therefore, one likely needs from 20 to 50% of liver correction for survival in the mouse model where protein restriction and other treatments are not feasible. Considering that parents of children with PA do not experience overt effects of carrying only one copy of the gene, this also suggests 50% correction may be the minimum needed for liver-directed therapy.
Adenoviral (Ad) vectors have been widely used as to deliver genes in vivo. Adenoviral vectors are a good option for delivery of the corrective transgene because of the high infectivity of dividing and nondividing cells with extremely high transduction to the liver (Tang et al., 1994; Huard et al., 1995). In particular for PA gene therapy, few other vectors have been demonstrated to mediate the same robust level of liver transgene expression as adenoviral vectors. For example, a single adenovirus serotype 5 (Ad5) liver transduction can produce supraphysiologic levels of 6 mg/ml of α1-antitrypsin in the blood of mice (Morral et al., 1998). In addition, Ad5 vectors are nonintegrating, and therefore reduce the chance of oncogenesis (Desviat et al., 2004, 2006) and chromosomal rearrangements. Ad5 vectors have been injected into prenatal mice to treat hemophilia A in a factor VIII knockout demonstrating a short-term therapeutic level of correction (Lipshutz et al., 1999) In addition, neonatal adenoviral gene therapy has been effective in treating the twitcher mouse model of Krabbe disease by intraventricular injection (Shen et al., 2001), and in a neonatal lethal mouse model of citrullinemia by retro-orbital injection (Ye et al., 2000).
Although adenoviral vectors are robust, they are also immunogenic. To reduce adenoviral immunogenicity, polyethylene glycol (Lorimer et al., 1996) has been used to coat Ad5 vectors by covalent attachment. Adenoviral PEGylation has been shown to block vector neutralization by antibodies, reduce T cell responses against transduced cells, reduce innate immune responses, and blunt thrombocytopenia induced by the vector (Chillon et al., 1998; O'Riordan et al., 1999; Croyle et al., 2001b; Mok et al., 2005). In addition, PEG also has the interesting effect of blocking Ad5 interactions with coxsackievirus–adenovirus receptor (CAR) in vitro (Ogawara et al., 2004; Mok et al., 2005) without disrupting liver transduction in vivo after intravenous injection (Mok et al., 2005). These data suggest that adenoviral PEGylation can increase the safety profile of the vector, but may also have utility in detargeting the vector from CAR interactions in vivo.
In this work, we have tested first-generation and helper-dependent Ad5 vectors and AAV8 vectors for their ability to deliver the PCCA transgene into PCCA−/− newborn mice to rescue this lethal phenotype. We have also tested the effects of PEGylation on the tissue tropism of Ad5 after intraperitoneal injection. We show that adenoviral vectors are able to extend the life span of PCCA−/− mice, but that this protection is transient. We also show, in agreement with previously published work in adult mice, that in neonatal mice, PEG can detarget adenovirus from peritoneal transduction and retarget the vector to the liver from this site.
Materials and Methods
Mice
All animals used for the experiments described in this paper were treated according to the provisions of the Animal Welfare Act, PHS Animal Welfare Policy, the principles of the NIH Guide for the Care and Use of Laboratory Animals, and the policies and procedures of Baylor College of Medicine (Houston, TX) and the Mayo Clinic (Rochester, MN). Outbred ICR mice were purchased from Harlan (Indianapolis, IN).
PCCA+/− heterozygous mice (Miyazaki et al., 2001) were generously provided by T. Miyazaki (University of Texas Southwestern Medical Center, Dallas, TX). These mice were rederived into the specific pathogen-free animal facility by crossing with C57BL/6 mice to ensure the animals were pathogen free. The PCCA−/− mice were reported to have knockout of a 2-kbp region encompassing exons 1 through 4 of the mouse PCCA gene (Miyazaki et al., 2001). Because exons 1–4 span a substantially larger size than 2 kbp, polymerase chain reaction (PCR) analysis was performed to reconcile this discrepancy. This PCR analysis determined the knockout region to span a 2-kbp region of exons 4 and its intron, rather than exons 1 through 4 (data not shown). With this information, genotyping was performed as follows. Animals with a knocked-out PCCA allele were identified by PCR for the inserted neomycin resistance gene (neo) with a primer specific to neo (TGCTCCTGCCGAGAAAGTATCCATCATGGC) and a primer specific to the PCCA gene upstream to the knocked-out exons (GGC AGC AAA GAT GGT CTC AGG C). To identify the endogenous PCCA allele (designated PCCA+), primers (from Miyazaki and colleagues, 2001) that were specific for introns 2 and 3 (CTA GAA AGT AAA TGT TTA CCA GAA) and exon 4 (AGA ACT GGC ATC AAC ATC ACT GTG) were used, because these will amplify the intact gene, but fail with the knocked-out PCCA allele because exon 4 is partially deleted. These primers were used to genotype the animals and the mice were back-crossed with C57BL/6 mice for five generations to homogenize their genetic background 97.5% to reduce experimental and metabolic variation due to mixed genetic background. PCCA−/− mice were generated by crossing PCCA+/− heterozygous parents, because PCCA−/− mice die within 36 hr of birth (Miyazaki et al., 2001).
Virus production
All first-generation viruses used for this study are first-generation adenoviral vectors made with the AdEasy system from Stratagene (La Jolla, CA). The cDNA for the human PCCA was purchased from Invitrogen (Carlsbad, CA). This cDNA was amplified by PCR, sequenced, and cloned into the pShuttle-IRES-hrGFP plasmid from Stratagene. This cassette was recombined into the AdEasy plasmid to generate the full Ad5 vector, using BJ5183 bacterial cells as described by Parrott and colleagues (2003). After recombination, positive clones were sequenced and wild-type clones were used. Ad5 virus was generated by digesting the plasmids with PacI and purified linear DNA was transfected in 293 cells, using Polyfect (Qiagen, Valencia, CA). Once the Ad5 vector expressing the human PCCA cDNA (Ad-hPCCA) was rescued, it was amplified and purified on CsCl gradients as in Campos and colleagues (2004). Once purified, the virus was desalted, and the CsCl buffer was exchanged for KPBS buffer (10 mM K2PO4, 150 mM NaCl, 1 mM MgCl2, pH 7.8) supplemented with 0.5 M sucrose for stability at −80°C (Croyle et al., 2001a). Tris buffer was avoided, because it has amines that will react and quench reactions with the succinimide group on PEG. Helper-dependent adenoviral (HD-Ad) vectors expressing hPCCA were generated in the HDΔ28E4 and HDΔ24E4 backbones generously provided by B. Lee (Baylor College of Medicine) and were rescued as described previously (Palmer and Ng, 2003). HD-Ad vectors were engineered to express the same CMV-hPCCA-SV40polyA cassette as in the first-generation AdEasy vectors or were engineered for liver-specific expression, using the phosphoenolpyruvate carboxykinase (PEPCK) promoter and woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) cassette (Mian et al., 2004) supplied by B. Lee (Baylor College of Medicine).
PEGylation of adenoviral vectors
PEGylation was performed as in Hofherr and colleagues (2007), Briefly, 1×1012 VP were incubated at room temperature for 1 hr in a 10-mg/ml concentration of the 5-kDa PEG ME-050HS (NOF, Tokyo, Japan) in KPBS–0.5 M sucrose with constant mixing on a rotating wheel. The reaction was stopped by addition of 10 mM Tris and excess PEG was removed by gel filtration on Sephadex G50 columns (GE Healthcare Life Sciences, Piscataway, NJ). The KPBS–0.5 M sucrose buffer was exchanged for A195 buffer (Evans et al., 2004). Viral concentrations were determined by absorbance at 260 nm and confirmed by quantitative PCR as described in Hofherr and colleagues (2007). Historically, the degree of PEGylation is approximately 65% by 3-(4-carboxybenzoyl)quinoline-2-carboxaldehyde (CBQCA) assay for free amines.
Injection of adenovirus into newborn mice
PCCA+/− mice were crossed to generate litters of mice with PCCA−/− mice. Given that their genotypes would be determined only postmortem, all pups in the litters were injected with the indicated vectors within the first 6 hr of birth. Injections were administered intraperitoneally at a dose of 2.5×109 to 5×1010 VP per neonate (VP/pup), as used by Shen and colleagues (2001), in a volume of 20 μl, using a Hamilton gastight 25-μl syringe and a 30-gauge needle. Mice were monitored every 6 hr and as pups died they were removed from the cage and their tails were snipped for genotyping. The remaining pups in the litter were killed at 7 days and their tails were also snipped for genotyping. The genomic DNA was isolated with a Qiagen DNeasy kit. PCRs were then performed to detect the endogenous PCCA allele and the PCCA knockout allele. Alternatively, the neonatal mice were injected, with the same instruments and at the same time points, via the intrahepatic route (Chandler and Venditti, 2008).
Imaging gene delivery in neonates
Pregnant outbred Cr:NIH(S) (NIH Swiss) mice were ordered from the NCI-Frederick Animal Production Area (Frederick, MD) to arrive on day 12 of gestation (12 days after the appearance of a vaginal plug). The resulting litters were injected with 2.5×109 VP of Ad as described previously. The pups were injected with 20 μl of d-luciferin (20 mg/ml in PBS) and were placed in a Lumazone imager (MAG Biosystems/Photometrics, Tucson, AZ) for imaging 24, 48, and 72 hr after injection. All images were taken at a 1-min exposure and 1×1 binning.
Confocal microscopy for dsRed2 expression
Newborn ICR pups were injected with first-generation AddsRed2 or PEG-Ad-dsRed2 at a dose of 2.5×109 genomes per pup on day 0. These pups were killed 72 hr postinjection and frozen. To facilitate sectioning, the frozen animals were cut in half with a razor on the sagittal plane and were then embedded in O.C.T. cryostat embedding medium (Sakura Finetek USA, Torrance, CA) and sectioned in a conventional cryostat at a thickness of 20 μm. Sections were mounted in VECTASHIELD medium with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA) and immediately observed with a Zeiss Axiovert 100 M confocal microscope. Images were acquired and analyzed with LSM 510 version 4.0 software.
Graphing and statistics
Graphing, Kaplan-Meier survival, and statistical analysis was performed with Prism statistical and graphing software (GraphPad Software, San Diego, CA).
Results
Tissue tropism of unmodified and PEGylated adenoviral vectors after intraperitoneal injection
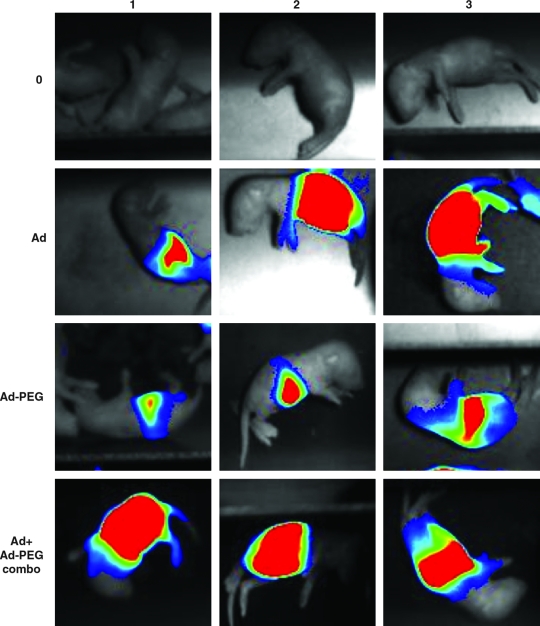
PCCA−/− mice are born alive, but die within 36 hr. Given their small size, intraperitoneal injection is the simplest route of vector administration. However, this route may not efficiently transduce the liver, which is generally thought to be the optimal target for gene therapy of metabolic diseases. We showed that PEGylation of Ad5 vectors retargets these vectors from the peritoneum to the liver after intraperitoneal injection in adult mice (Hofherr et al., 2008). On the basis of this, we tested whether the same strategy could be used to enable simple intraperitoneal injection of newborn mice to apply liver-directed gene therapy. When newborn ICR mice were injected with 2.5×109 VP of luciferase-expressing vectors, the pattern of expression was similar to that observed in adults. Unmodified Ad5 produced peritoneal expression whereas the PEGylated vector retargeted expression from the peritoneum to the liver as suggested by more restricted expression near the forelimbs (Fig. 2). A mixture of 1.25×109 VP of Ad-CMV-hPCCA and 1.25×109 VP of PEGylated Ad-CMV-hPCCA generated an expression pattern throughout the abdomen. Real-time PCR for viral genomes in the livers of the pups was not feasible, because of the fragility of the livers of the animals. Given similar levels of vector delivery to the liver by intravenous and intraperitoneal routes in adults (Hofherr et al., 2008), these data suggest that intraperitoneal injection into the newborn pups would likely deliver similar amounts of adenoviral vector to the liver compared with the more technically demanding intravenous injection route.
FIG. 2.
Luciferase imaging of gene delivery in neonatal mice. ICR mice were injected within 6 hr of birth intraperitoneally with 2.5×109 VP of unmodified or PEGylated AdLucIREShrGFP and luciferase activity was imaged 24, 48, and 72 hr after injection, using a Lumazone imager. Shown are images of a representative pup with luciferase expression in pseudo-color overlaid on the white light image “0” represents uninjected mice.
Microscopy examination of transduction after intraperitoneal injection
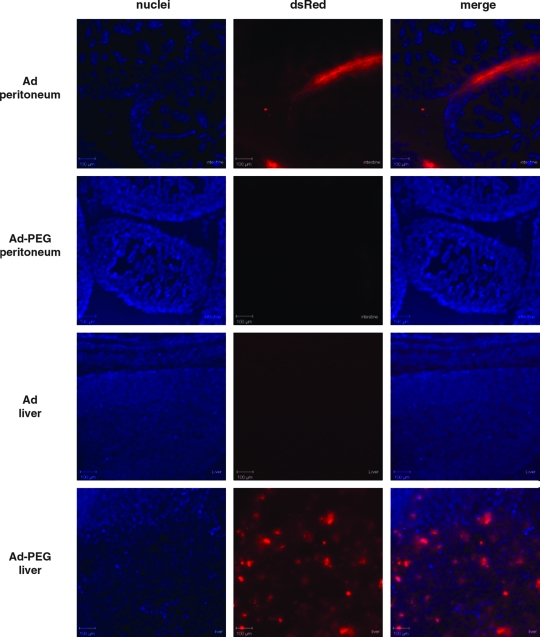
The luciferase imaging data suggested that unmodified adenovirus transduces primarily in the peritoneum and that PEGylation detargets this peritoneal transduction, allowing the virus to more effectively transduce the liver. To more closely examine the effects of PEGylation on tropism, neonatal ICR mice were injected intraperitoneally with 2.5×109 VP of Ad-CMV-dsRed2 red fluorescent protein-expressing vectors. Seventy-two hours after injection, the animals were killed and the intact pups were frozen and then sectioned in the sagittal plane. Confocal microscopy of the animals demonstrated that unmodified Ad-dsRed generated bright dsRed expression on the surfaces inside the peritoneum including segments of the bowel, but gave undetectable transduction of liver (Fig. 3 and data not shown). This was consistent with previous studies that showed that adenovirus transduces the mesothelium lining the organs of the peritoneum (Akiyama et al., 2004). In contrast, PEGylated Ad-dsRed gave no observable red fluorescence in the peritoneum, but generated detectable red fluorescence in the liver parenchyma (Fig. 3). These data suggest that conjugation of 5-kDa PEG detargets adenovirus from transduction in the peritoneum, allowing the virus to transduce the parenchyma of the liver.
FIG. 3.
DsRed microscopy of gene delivery in neonatal mice. ICR mice were injected intraperitoneally within 6 hr of birth with 2.5×109 VP of unmodified or PEGylated Ad-DsRed. Seventy-two hours after injection the pups were frozen embedded in O.C.T. and sectioned (thickness of sections, 20 μm). Confocal images were taken after DAPI staining. Blue represents DAPI-stained nuclei and red represents DsRed fluorescence.
Extension of life span of PCCA knockout mice with Ad5
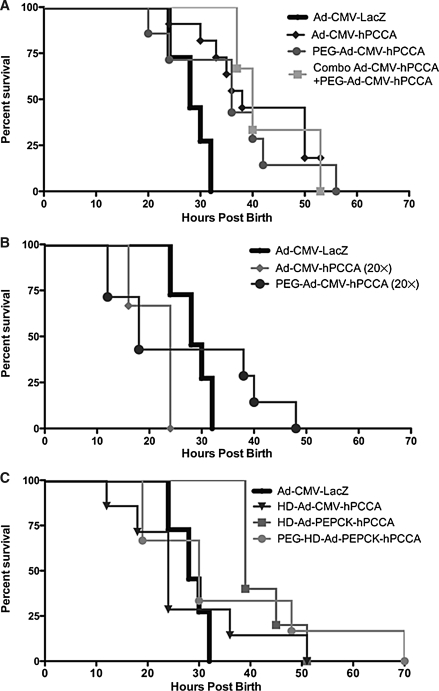
We hypothesized that injection of first-generation adenovirus expressing human PCCA cDNA under the control of the CMV promoter (Ad-CMV-hPCCA) might extend survival of PCCA−/− mice by expressing the gene in the peritoneum or liver. To test this, 2.5×109 VP of Ad-CMV-hPCCA or Ad-CMV-LacZ was injected intraperitoneally within 6 hr of birth into litters from PCCA+/− ×PCCA+/− parents. Injection of either vector into PCCA+/+ or PCCA+/− mice had no discernable effect on their survival, because they normally survive (data not shown). Control PCCA−/− mice receiving Ad-CMV-LacZ died within 32 hr of birth (Fig. 4A). In contrast, PCCA−/− pups receiving Ad-CMV-hPCCA lived nearly two times longer and their survival was significantly improved as compared with Ad-CMV-LacZ-injected PCCA−/− mice (p = 0.0002).
FIG. 4.
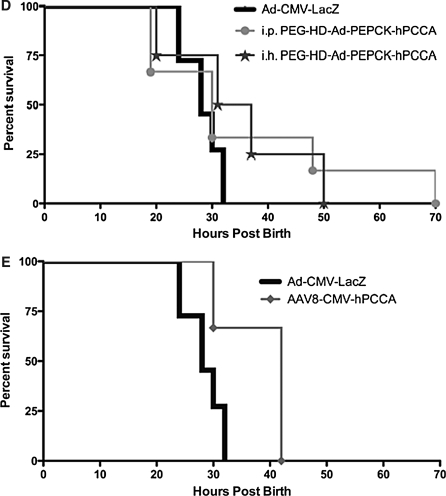
Survival of PCCA−/− neonatal mice. The litters from PCCA+/− crosses were injected with the indicated vectors within 6 hr of birth and they were monitored for survival time. At 1 week, the remaining pups were killed and all were genotyped. Kaplan–Meier survival curves are shown only for PCCA−/− mice, because PCCA+/+ and PCCA+/− mice all survived. (Note: Uninjected animals survived at a rate indistinguishable from that of Ad-LacZ-injected mice and are not shown on the graph for clarity.) (A) Survival of mice receiving unmodified, PEGylated, and 50% unmodified plus 50% PEGylated first-generation (Ad-CMV-hPCCA) vector at a total vector dose of 2.5×109 viral particles (VP) injected intraperitoneally. (B) Survival of mice receiving unmodified and PEGylated first-generation (Ad-CMV-hPCCA) vector at a vector dose of 5×1010 VP injected intraperitoneally. (C) Survival of mice receiving unmodified helper-dependent vectors expressing the hPCCA transgene under the control of the CMV promoter (HD-Ad-CMV-hPCCA) or the PEPCK promoter (HD-Ad-CMV-hPCCA), and PEGylated HD-Ad-PEPCK-hPCCA (PEG-HD-Ad-PEPCK-hPCCA), all injected intraperitoneally at a dose of 2.5×109 VP. (D) Survival of mice injected with PEG-HD-Ad-PEPCK-hPCCA either intraperitoneally (i.p.) or intrahepatically (i.h.) at a dose of 2.5×109 VP. (E) Survival of mice receiving adeno-associated virus serotype 8 (AAV8-hPCCA) intraperitoneally injected at a vector dose of 1.2×1010 VP. (A) through (E) all present the same group intraperitoneally injected with 2.5×109 VP of Ad-CMV-LacZ, which serves as a nontherapeutic control vector. This group was indistinguishable in terms of survival from the uninjected group (data not shown).
Effects of PEGylated Ad5 on the life span of PCCA knockout mice
Given that adenoviral PEGylation retargeted the vector to the liver, we hypothesized that this modification might improve the rescue of PCCA−/− mice by delivering the PCCA gene to the site most relevant to metabolism. To test this, 2.5×109 VP of PEGylated Ad-CMV-hPCCA was injected intraperitoneally into litters from PCCA+/− crosses and survival was assessed (Fig. 4A). In this case, PEGylated Ad-CMV-hPCCA extended the life span of the PCCA−/− mice compared with the Ad-CMV-LacZ control (Table 1). There was, however, not a significant difference between the life span of PCCA−/− mice injected with unmodified or PEGylated Ad-hPCCA (Table 1). Given the nonoverlapping expression patterns of adenovirus and PEGylated adenovirus, we next tested whether mixing equal amounts of the two vectors had any benefit. Injection of a mixture of 1.25×109 VP of Ad-CMV-hPCCA and 1.25×109 VP of PEGylated Ad-CMV-hPCCA generated a survival curve that was similar to that generated by treatment with the two vectors alone (Fig. 4A). These data suggest that genetic correction with adenoviral vectors in either the peritoneum or the liver can temper some of the toxicity associated with PCCA deficiency, but that both are insufficient for long-term rescue of neonatal animals.
Table 1.
Survival Curve Detailsa
| Group | Dose (×109VP) | Route of injection | Number of knockouts per group | Maximal Life Span | p Value of group vs. Ad-CMV-LacZ (log rank) |
|---|---|---|---|---|---|
| Ad-CMV-LacZ | 2.5 | Intraperitoneal | 11 | 32 | NA |
| Ad-CMV-hPCCA | 2.5 | Intraperitoneal | 11 | 53 | 0.0002 |
| PEG-Ad-CMV-hPCCA | 2.5 | Intraperitoneal | 7 | 56 | 0.0158 |
| Combo Ad-CMV-hPCCA | 1.25 | Intraperitoneal | 3 | 53 | 0.0051 |
| +PEG-Ad-CMV-hPCCA | 1.25 | ||||
| Ad-CMV-hPCCA (20×) | 50 | Intraperitoneal | 6 | 24 | 0.0018 |
| PEG-Ad-CMV-hPCCA (20×) | 50 | Intraperitoneal | 7 | 48 | 0.4900 |
| HD-Ad-CMV-hPCCA | 2.5 | Intraperitoneal | 7 | 51 | 0.9940 |
| HD-Ad-PEPCK-hPCCA | 2.5 | Intraperitoneal | 5 | 70 | 0.0006 |
| PEG-HD-Ad-PEPCK-hPCCA | 2.5 | Intraperitoneal | 6 | 51 | 0.3436 |
| IH PEG-HD-Ad-PEPCK-hPCCA | 2.5 | Intrahepatic | 4 | 50 | 0.1339 |
| AAV8-CMV-hPCCA | 12 | Intraperitoneal | 3 | 42 | 0.0360 |
Abbreviation: NA, not applicable.
Listed are group numbers, maximal life spans, and p values for log-rank statistical analysis when each curve is compared with Ad-LacZ injected intraperitoneally at a dose of 2.5 × 109 VP/pup.
Effects of higher doses of adenovirus on PCCA−/− neonate survival
We next tested whether an increased dose of adenoviral vector would be more effective. Surprisingly, intraperitoneal injection of 5×1010 VP of Ad-CMV-hPCCA actually accelerated death among the PCCA−/− mice relative to mice injected with 2.5×109 VP of Ad-CMV-LacZ (Fig. 4B). Given that PEGylation of adenovirus markedly reduces many of the dangerous side effects of the virus (thrombocytopenia, innate immune responses) (Chillon et al., 1998; O'Riordan et al., 1999; Croyle et al., 2001b; Mok et al., 2005), we tested whether PEGylation could mitigate the toxicity associated with injection of larger amounts of the virus. Injection of 5×1010 VP of PEGylated Ad-CMV-hPCCA produced a somewhat intermediate effect in the pups. Approximately one-half of the injected PCCA−/− neonates appeared to die more rapidly, whereas the other half appeared to have somewhat extended survival (Fig. 4B). Although the survival curves were somewhat different in appearance, the curves were not significantly different from each other by log-rank test (Table 1). In contrast, the high-dose adenovirus injection had no negative effects on the survival of PCCA+/− and PCCA+/+ mice (data not shown), suggesting that the high-dose vector injection was exacerbating PA toxicity.
Testing helper-dependent adenovirus for PCCA−/− rescue
HD-Ad vectors have been shown to mediate substantially longer genetic correction in the livers of adult mice in a number of models. Given this, we next tested two HD-Ad vectors expressing hPCCA, one with ubiquitous expression from the same CMV cassette used in the first-generation adenoviral vector Ad-CMV-hPCCA and a second expressing hPCCA under the control of the liver-specific promoter from the PEPCK promoter cassette (Mian et al., 2004). Injection of 2.5×109 VP of HD-Ad-CMV-hPCCA generated survival no better than that of LacZ-injected mice (Fig. 4C). In contrast, injection of HD-Ad-PEPCK-hPCCA extended survival significantly (Table 1), but almost all pups still died within 52 hr of birth (Fig. 4C).
Testing PEGylated helper-dependent adenovirus for PCCA−/− rescue
Given that the PEPCK promoter restricts PCCA expression mostly to the liver (Mian et al., 2004), these data could suggest that the survival effects produced by unmodified adenovirus after intraperitoneal injection are mediated more by the small amount of virus that is delivered to the liver than it is by the bulk of transduction that occurs in the peritoneum. However, the PEPCK promoter is not absolutely specific for liver; it is also expressed in the kidney, adipose tissue, small intestine, and mammary gland (McGrane et al., 1990). It is therefore possible that some level of rescue could be mediated by PCCA expression in the intestine or other sites after intraperitoneal injection of unmodified HD-Ad-PEPCK-hPCCA. To test this, neonatal mice were injected with PEGylated HD-Ad-PEPCK-hPCCA, which should apply both physical and transcriptional targeting of PCCA expression to the liver. Under these conditions, survival was similar to that of mice receiving other treatments (Fig. 4C). However, PEGylated HD-Ad-PEPCK-hPCCA did mediate the longest survival of any mice, with mice surviving out to 70 hr. These data suggest that the combination of PEGylation and PEPCK transcriptional targeting may mediate better targeting of PCCA expression in the liver.
Testing intrahepatic injection of PEGylated helper-dependent adenovirus for PCCA−/− rescue
One study reported that intrahepatic injection of a first-generation Ad5 vector expressing the mut transgene was able to rescue Mut−/− mice up to weaning (Chandler and Venditti, 2008). These Mut−/− mice are commonly used as a neonatal lethal methylmalonic acidemia (MMA) model. Because propionic acidemia and MMA are diseases of the same metabolic pathway (Fig. 1), it is possible that this same technique could improve the efficacy of our intraperitoneally administered therapy. However, when we administered our PEGylated helper-dependent adenovirus HD-Ad-PEPCK-hPCCA intrahepatically, we saw no increase in efficacy of our treatment (Fig. 4D). In fact, the mice died within 50 hr. These data suggest that this mouse model of PA may be more severe, or more difficult to treat, than the MMA mouse model.
Discussion
This work tested proof of principle for gene therapy of propionic acidemia (PA). In this study, we have demonstrated the feasibility of treatment in the stringent PCCA knockout mouse model, using first-generation and helper-dependent adenoviral vectors. These data suggest that similar gene therapy approaches may have merit in humans as an alternative to liver transplantation.
Although some rescue was observed, we were unable to extend the life spans of newborn pups beyond 70 hr with these adenoviral vectors alone. We hypothesize that this short-term rescue could be caused by several factors. The first factor may simply be that peritoneal transduction by unmodified adenovirus and liver transduction by PEGylated adenovirus simply may not produce sufficient levels of transgene products to rescue the animals. PCCA−/− mice with a liver-specific PCCA covering transgene expressing 10–20% of wild-type levels die before reaching 3 weeks of age (Miyazaki et al., 2001). In contrast, PCCA−/− mice with 50% of wild-type liver-specific PCCA survive. These data suggest that any therapy for PA may need to establish greater than 20% of wild-type expression to rescue the deficiency.
Another factor that may make full rescue difficult is the effect the mutation has on liver function and liver damage. The predicted need for a 20 to 50% level of correction is based on animals that are born with this level of PCCA expression. In contrast, the PCCA−/− pups are born without any covering transgene. At birth, PCCA−/− mice have a 500% increase in serum propionyl-carnitine, indicating that they are born with a burgeoning metabolic cascade (Miyazaki et al., 2001). Once they nurse, catabolism of milk proteins produces substrates for PCC that have no outlet. These substrates produce acidosis and ketonuria within 10 hr of birth, causing the pups to stop feeding and to become dehydrated. Therefore, the genetic correction we aim to apply essentially must “chase” an already deteriorating metabolic catastrophe that is well underway at birth.
Given that adenovirus is known to cause liver damage, it is possible that the existing liver damage is exacerbated by adenoviral liver damage, and this may temper the ability to extend survival beyond 70 hr. This speculation is consistent with our observation that high-dose Ad-hPCCA killed PCCA−/− mice faster than even uninjected controls. This is also consistent with work in rats with cirrhotic liver damage, where adenovirus injection increased toxicity (Smith et al., 2004 a,b).
In human patients, PA symptoms can be mitigated to some degree by restricting protein intake and treating with drugs such as carnitine to liberate CoA from propionyl-CoA to improve metabolism. Ironically, testing these approaches in neonatal mice is more difficult than in humans. For example, it is challenging to attempt to restrict protein in the diet of a neonatal mouse that can normally be fed only by nursing from its mother. In one preliminary test, we treated the mothers of PCCA mice with l-carnitine. However, this did not improve survival alone or in combination with Ad-hPCCA (data not shown). Another agent under consideration is Carbaglu (N-carbamylglutamate), which blocks hyperammonemia. Preliminary tests of intraperitoneal injection of carnitine, glucose, and N-carbamylglutamate (to mitigate hyperammonemia) into PCCA−/− pups at birth produced only modest increases in survival (data not shown). These data suggest that this PCCA−/− model is a quite stringent model for testing gene or drug therapies for PA.
A second goal of this study was to determine whether PEGylation of adenoviral vectors could assist in retargeting the vector to the liver after intraperitoneal injection in neonatal mice. This was based on prior work from our group (Hofherr et al., 2008) showing that intraperitoneal injection of adenoviral vectors ablated for CAR binding detargeted the virus from the peritoneum and increased liver transduction. This, coupled with our work and that of other showing that PEGylation blocks CAR-mediated transduction in vitro (Ogawara et al., 2004; Mok et al., 2005), suggested that PEGylation should retarget intraperitoneally injected virus. This hypothesis appears to be accurate, because we observed that PEGylated adenovirus mediated substantially reduced expression in the peritoneum, and substantially increased expression in the liver as was observed in adult mice. Therefore, PEG can be used to detarget adenovirus, at least from CAR binding. It should be noted that in this study, we used small (5 kDa) PEG for shielding. It is likely that the use of larger or more complex PEG molecules may also be able to detarget the virus from other sites such as the liver. If this is the case, the question will be whether PEG detargeting can then overcome specific targeting.
Although PEG was able to retarget adenovirus to the liver from the peritoneum, this retargeting did not mediate any better protection than that produced by peritoneal transduction by unmodified adenovirus. This was surprising, because the liver is the presumptive best site for gene therapy of a metabolic disease such as PA. This lack of improvement may again be explained by the problems of chasing an already decaying metabolic catastrophe in the mice. Alternatively, rescue by liver transduction by the PEGylated vector may be hampered by the slower ramp-up of transgene expression mediated by the modified vector. If this is the case, a combination of adenovirus and PEGylated adenovirus may actually mediate better rescue by providing rapid phenotypic “coverage” by peritoneal transduction followed by slower, but more effective, coverage in the liver by the PEGylated vector.
One study described the therapeutic effect of a first-generation adenoviral vector to treat a neonatal lethal mouse model for methylmalonic acidemia (MMA) (Chandler and Venditti, 2008). MMA is caused by a deficiency of methylmalonyl-CoA-mutase. This enzyme is used to convert methylmalonyl-CoA into succinyl-CoA, and is found one step epistatic to propionyl-CoA carboxylase (Fig. 1). Because the reported phenotypes of the PA and MMA mice are so similar, and the described study showed an effective treatment of the MMA model to weaning, this intrahepatic injection technique was tested in the PA mouse model. Unfortunately, intrahepatic injection was no better than other routes in the PA mouse model.
Exactly why we observe this difference in efficacy in the two models is unclear. There could be minor differences in how the mice were generated, or it could reflect other metabolic issues. It is of note that the PA mice were originally on a mixed genetic background. To homogenize this background to reduce intermouse variations, these PA mice were back-crossed for six generations onto a C57BL/6 background. During this process the phenotypes of the mice increased, suggesting that the C57BL/6 background might be exacerbating the PA phenotype. Work is underway to explore the effects of this genetic background on PA phenotype with and without drug and gene therapy.
Given the short-term correction we have observed with first-generation and helper-dependent adenoviral vectors, we tested adeno-associated virus type 8 (AAV8) expressing hPCCA. In a pilot test in four PCCA−/− mice, injection of 5×109 VP of this alternative vector mediated slight increases in survival, but all animals still died within 42 hr (Fig. 4E). These data suggest that the short-term correction may not be due to vector deficiencies, but may be due to the underlying disease or an already decaying phenotype in this model.
Although these approaches may afford some level of genetic correction, they cannot repair the genetic defect over long periods of time in these young animals because their cells are rapidly dividing and will lose these episomal vectors. Integrating vector systems such as lentiviruses may be useful if insertional oncogenesis problems are circumvented. Repeat adenoviral vector administration may extend correction, but this may also provoke immune responses against adenovirus that may temper future administration. PEGylation may have utility for these repeat injections given its ability to at least partially evade neutralizing antibodies against adenovirus (O'Riordan et al., 1999; Croyle et al., 2001b). Alternately the use of serotype switching may enable repeat dosing.
Acknowledgments
The authors thank Mary Barry, Jenica Schwegel, and Shanon May for their excellent technical assistance. This work was supported by a grant to M.A.B. from the Propionic Acidemia Foundation and the Organic Acidemia Association.
Author Disclosure Statement
S.H., J.S., C.C., D.P., P.N., and M.B. have no competing financial interests.
References
- Akiyama M. Thorne S. Kirn D. Roelvink P.W. Einfeld D.A. King C.R. Wickham T.J. Ablating CAR and integrin binding in adenovirus vectors reduces nontarget organ transduction and permits sustained bloodstream persistence following intraperitoneal administration. Mol. Ther. 2004;9:218–230. doi: 10.1016/j.ymthe.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Al Essa M. Rahbeeni Z. Jumaah S. Joshi S. Al Jishi E. Rashed M.S. Al Amoudi M. Ozand P.T. Infectious complications of propionic acidemia in Saudi Arabia. Clin. Genet. 1998;54:90–94. doi: 10.1111/j.1399-0004.1998.tb03702.x. [DOI] [PubMed] [Google Scholar]
- Barshes N.R. Vanatta J.M. Patel A.J. Carter B.A. O'Mahony C.A. Karpen S.J. Goss J.A. Evaluation and management of patients with propionic acidemia undergoing liver transplantation: A comprehensive review. Pediatr. Transplant. 2006;10:773–781. doi: 10.1111/j.1399-3046.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- Campeau E. Desviat L.R. Leclerc D. Wu X. Perez B. Ugarte M. Gravel R.A. Structure of the PCCA gene and distribution of mutations causing propionic acidemia. Mol. Genet. Metab. 2001;74:238–247. doi: 10.1006/mgme.2001.3210. [DOI] [PubMed] [Google Scholar]
- Campos S.K. Parrott M.B. Barry M.A. Avidin-based targeting and purification of a protein IX-modified, metabolically biotinylated adenoviral vector. Mol. Ther. 2004;9:943–955. doi: 10.1016/j.ymthe.2004.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler R.J. Venditti C.P. Adenovirus-mediated gene delivery rescues a neonatal lethal murine model of mut0 methylmalonic acidemia. Hum. Gene Ther. 2008;19:53–60. doi: 10.1089/hum.2007.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs B. Nyhan W.L. Borden M. Bard L. Cooke R.E. Idiopathic hyperglycinemia and hyperglycinuria: A new disorder of amino acid metabolism. I. Pediatrics. 1961;27:522–538. [PubMed] [Google Scholar]
- Chillon M. Lee J.H. Fasbender A. Welsh M.J. Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene Ther. 1998;5:995–1002. doi: 10.1038/sj.gt.3300665. [DOI] [PubMed] [Google Scholar]
- Croyle M.A. Cheng X. Wilson J.M. Development of formulations that enhance physical stability of viral vectors for gene therapy. Gene Ther. 2001a;8:1281–1290. doi: 10.1038/sj.gt.3301527. [DOI] [PubMed] [Google Scholar]
- Croyle M.A. Chirmule N. Zhang Y. Wilson J.M. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J. Virol. 2001b;75:4792–4801. doi: 10.1128/JVI.75.10.4792-4801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desviat L.R. Perez B. Perez-Cerda C. Rodriguez-Pombo P. Clavero S. Ugarte M. Propionic acidemia: Mutation update and functional and structural effects of the variant alleles. Mol. Genet. Metab. 2004;83:28–37. doi: 10.1016/j.ymgme.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Desviat L.R. Clavero S. Perez-Cerda C. Navarrete R. Ugarte M. Perez B. New splicing mutations in propionic acidemia. J. Hum. Genet. 2006;51:992–997. doi: 10.1007/s10038-006-0068-3. [DOI] [PubMed] [Google Scholar]
- Hofherr S.E. Mok S. Gushiken F.C. Lopez J.A. Barry M.A. Polyethylene glycol modification of adenovirus reduces platelet activation, endothelial cell activation, and thrombocytopenia. Hum. Gene Ther. 2007;18:837–848. doi: 10.1089/hum.2007.0051. [DOI] [PubMed] [Google Scholar]
- Hofherr S.E. Shashkova E.V. Weaver E.A. Khare R. Barry M.A. Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol. Ther. 2008;16:1276–1282. doi: 10.1038/mt.2008.86. [DOI] [PubMed] [Google Scholar]
- Hsia Y.E. Scully K.J. Rosenberg L.E. Inherited propionyl-Coa carboxylase deficiency in “ketotic hyperglycinemia.”. J. Clin. Invest. 1971;50:127–130. doi: 10.1172/JCI106466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard J. Lochmuller H. Acsadi G. Jani A. Massie B. Karpati G. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995;2:107–115. [PubMed] [Google Scholar]
- Kayler L.K. Merion R.M. Lee S. Sung R.S. Punch J.D. Rudich S.M. Turcotte J.G. Campbell D.A., Jr. Holmes R. Magee J.C. Long-term survival after liver transplantation in children with metabolic disorders. Pediatr. Transplant. 2002;6:295–300. doi: 10.1034/j.1399-3046.2002.02009.x. [DOI] [PubMed] [Google Scholar]
- Lamhonwah A.M. Barankiewicz T.J. Willard H.F. Mahuran D.J. Quan F. Gravel R.A. Isolation of cDNA clones coding for the α and β chains of human propionyl-CoA carboxylase: Chromosomal assignments and DNA polymorphisms associated with PCCA and PCCB genes. Proc. Natl. Acad. Sci. U.S.A. 1986;83:4864–4868. doi: 10.1073/pnas.83.13.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshutz G.S. Sarkar R. Flebbe-Rehwaldt L. Kazazian H. Gaensler K.M. Short-term correction of factor VIII deficiency in a murine model of hemophilia A after delivery of adenovirus murine factor VIII in utero. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13324–13329. doi: 10.1073/pnas.96.23.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer I.A. Keppler-Hafkemeyer A. Beers R.A. Pegram C.N. Bigner D.D. Pastan I. Recombinant immunotoxins specific for a mutant epidermal growth factor receptor: Targeting with a single chain antibody variable domain isolated by phage display. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14815–14820. doi: 10.1073/pnas.93.25.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrane M.M. Yun J.S. Moorman A.F. Lamers W.H. Hendrick G.K. Arafah B.M. Park E.A. Wagner T.E. Hanson R.W. Metabolic effects of developmental, tissue-, and cell-specific expression of a chimeric phosphoenolpyruvate carboxykinase (GTP)/bovine growth hormone gene in transgenic mice. J. Biol. Chem. 1990;265:22371–22379. [PubMed] [Google Scholar]
- Mian A. Mane V. Ng P. Finegold M. O'Brien W.E. Rodgers J.R. Beaudet A.L. Lee B. Long-term correction of ornithine transcarbamylase deficiency by tissue restricted over-expression using a helper-dependent adenovirus. Mol. Ther. 2004;10:492–499. doi: 10.1016/j.ymthe.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Miyazaki T. Ohura T. Kobayashi M. Shigematsu Y. Yamaguchi S. Suzuki Y. Hata I. Aoki Y. Yang X. Minjares C. Haruta I. Uto H. Ito Y. Muller U. Fatal propionic acidemia in mice lacking propionyl-CoA carboxylase and its rescue by postnatal, liver-specific supplementation via a transgene. J. Biol. Chem. 2001;276:35995–35999. doi: 10.1074/jbc.M105467200. [DOI] [PubMed] [Google Scholar]
- Mok H. Palmer D.J. Ng P. Barry M.A. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol. Ther. 2005;11:66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Morral N. Parks R.J. Zhou H. Langston C. Schiedner G. Quinones J. Graham F.L. Kochanek S. Beaudet A.L. High doses of a helper-dependent adenoviral vector yield supraphysiological levels of α1-antitrypsin with neglible toxicity. Hum. Gene Ther. 1998;9:2709–2716. doi: 10.1089/hum.1998.9.18-2709. [DOI] [PubMed] [Google Scholar]
- O'Connor J.E. Costell M. Grisolia S. Carbamyl glutamate prevents the potentiation of ammonia toxicity by sodium benzoate. Eur. J. Pediatr. 1989;148:540–542. doi: 10.1007/BF00441553. [DOI] [PubMed] [Google Scholar]
- O'Riordan C.R. Lachapelle A. Delgado C. Parkes V. Wadsworth S.C. Smith A.E. Francis G.E. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum. Gene Ther. 1999;10:1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- Ogawara K. Rots M.G. Kok R.J. Moorlag H.E. Van Loenen A.M. Meijer D.K. Haisma H.J. Molema G. A novel strategy to modify adenovirus tropism and enhance transgene delivery to activated vascular endothelial cells in vitro and in vivo. Hum. Gene Ther. 2004;15:433–443. doi: 10.1089/10430340460745766. [DOI] [PubMed] [Google Scholar]
- Palmer D. Ng P. Improved system for helper-dependent adenoviral vector production. Mol. Ther. 2003;8:846–852. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Parrott M.B. Adams K.E. Mercier G.T. Mok H. Campos S.K. Barry M.A. Metabolically biotinylated adenovirus for cell-targeting, ligand screening, and vector purification. Mol. Ther. 2003;8:689–702. doi: 10.1016/s1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- Roe C.R. Millington D.S. Maltby D.A. Bohan T.P. Hoppel C.L. l-Carnitine enhances excretion of propionyl coenzyme A as propionylcarnitine in propionic acidemia. J. Clin. Invest. 1984;73:1785–1788. doi: 10.1172/JCI111387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J.S. Watabe K. Ohashi T. Eto Y. Intraventricular administration of recombinant adenovirus to neonatal twitcher mouse leads to clinicopathological improvements. Gene Ther. 2001;8:1081–1087. doi: 10.1038/sj.gt.3301495. [DOI] [PubMed] [Google Scholar]
- Smith J.S. Tian J. Lozier J.N. Byrnes A.P. Severe pulmonary pathology after intravenous administration of vectors in cirrhotic rats. Mol. Ther. 2004a;9:932–941. doi: 10.1016/j.ymthe.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Smith J.S. Tian J. Muller J. Byrnes A.P. Unexpected pulmonary uptake of adenovirus vectors in animals with chronic liver disease. Gene Ther. 2004b;11:431–438. doi: 10.1038/sj.gt.3302149. [DOI] [PubMed] [Google Scholar]
- Tang D. Johnston S.A. Carbone D.P. Butyrate-inducible and tumor-restricted gene expression by adenovirus vectors. Cancer Gene Ther. 1994;1:15–20. [PubMed] [Google Scholar]
- Ugarte M. Perez-Cerda C. Rodriguez-Pombo P. Desviat L.R. Perez B. Richard E. Muro S. Campeau E. Ohura T. Gravel R.A. Overview of mutations in the PCCA and PCCB genes causing propionic acidemia. Hum. Mutat. 1999;14:275–282. doi: 10.1002/(SICI)1098-1004(199910)14:4<275::AID-HUMU1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Ye X. Whiteman B. Jerebtsova M. Batshaw M.L. Correction of argininosuccinate synthetase (AS) deficiency in a murine model of citrullinemia with recombinant adenovirus carrying human AS cDNA. Gene Ther. 2000;7:1777–1782. doi: 10.1038/sj.gt.3301303. [DOI] [PubMed] [Google Scholar]
- Yorifuji T. Muroi J. Uematsu A. Nakahata T. Egawa H. Tanaka K. Living-related liver transplantation for neonatal-onset propionic acidemia. J. Pediatr. 2000;137:572–574. doi: 10.1067/mpd.2000.108391. [DOI] [PubMed] [Google Scholar]