Abstract
Previous studies of digitalis glycoside metabolism and excretion have indicated that these compounds undergo a significant enterohepatic cycle in some species. It has been suggested that the existence of such a cycle in man contributes to the prolonged action of certain cardiac glycosides. Previous studies have demonstrated that cholestyramine binds digitoxin and digoxin in vitro and accelerates the metabolic disposition of digitoxin in rats and guinea pigs, presumably by interrupting the enterohepatic circulation.
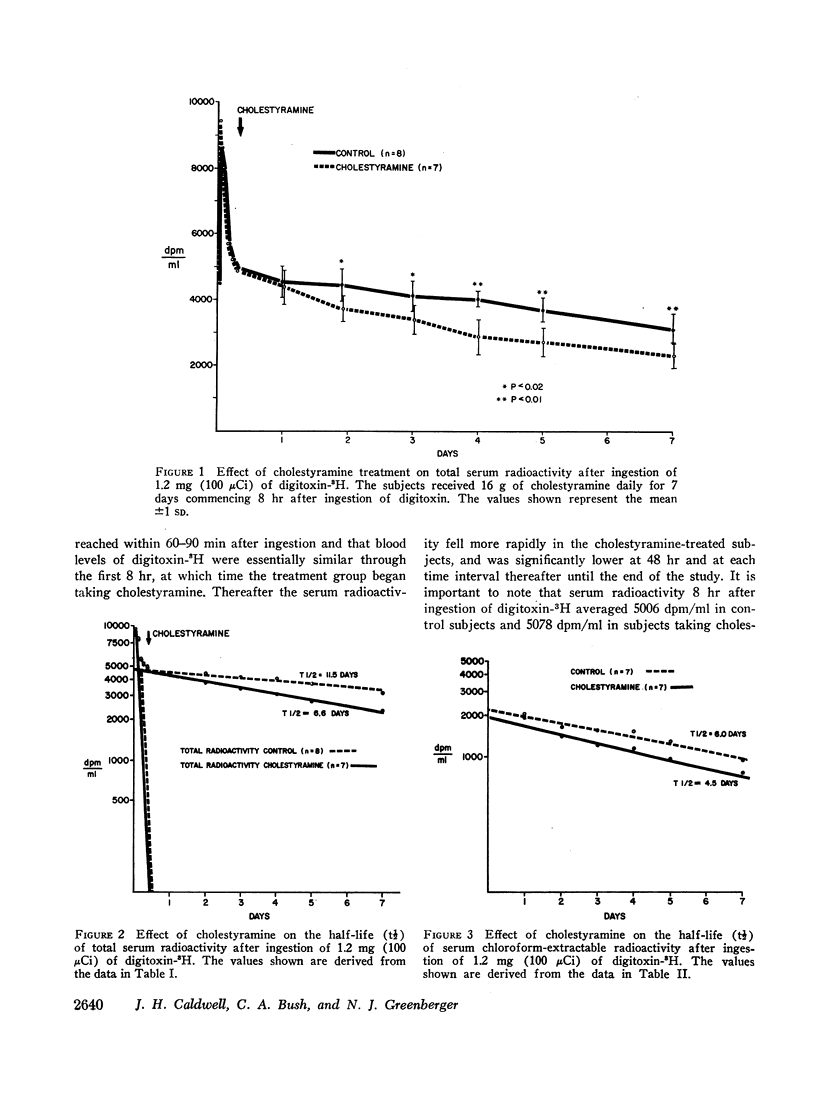
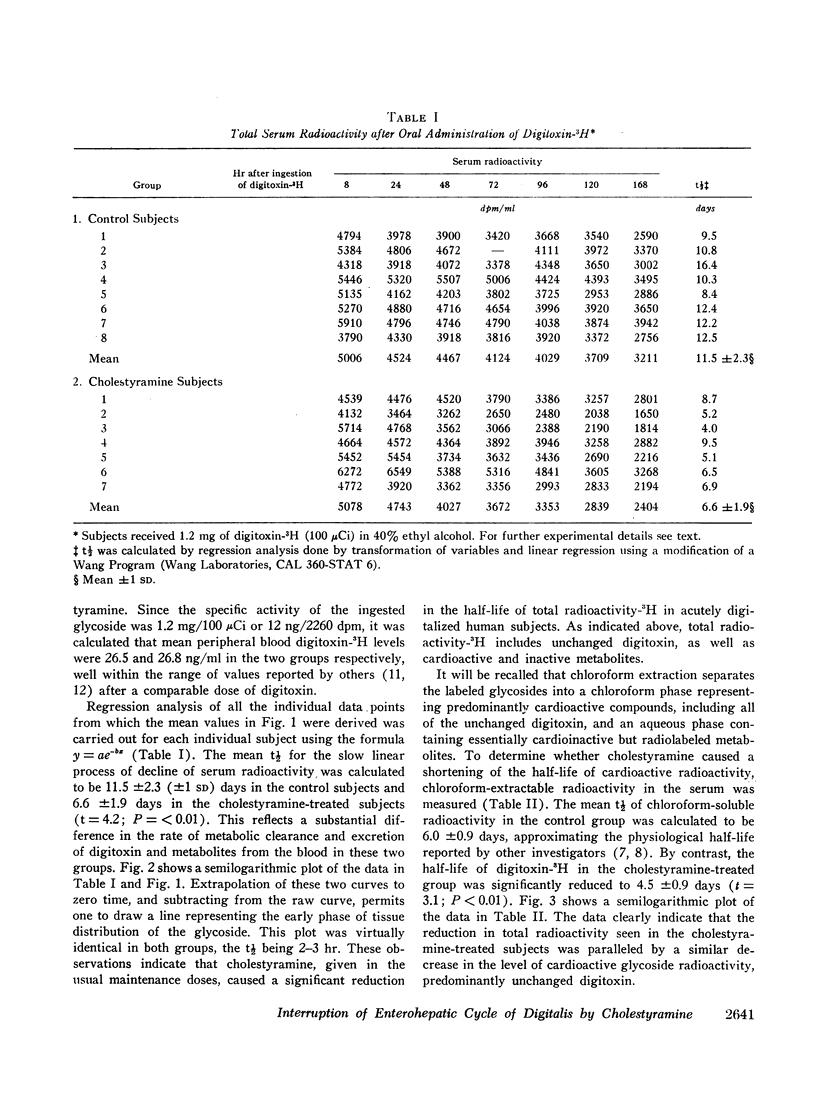
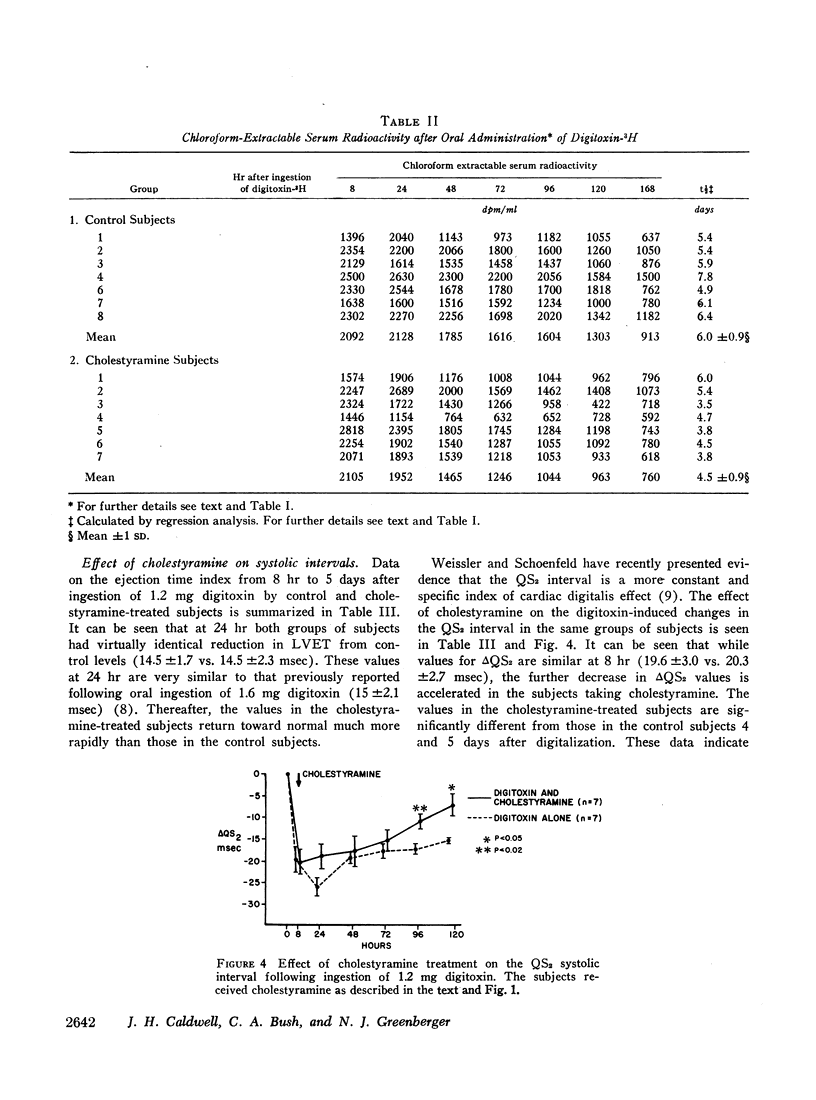
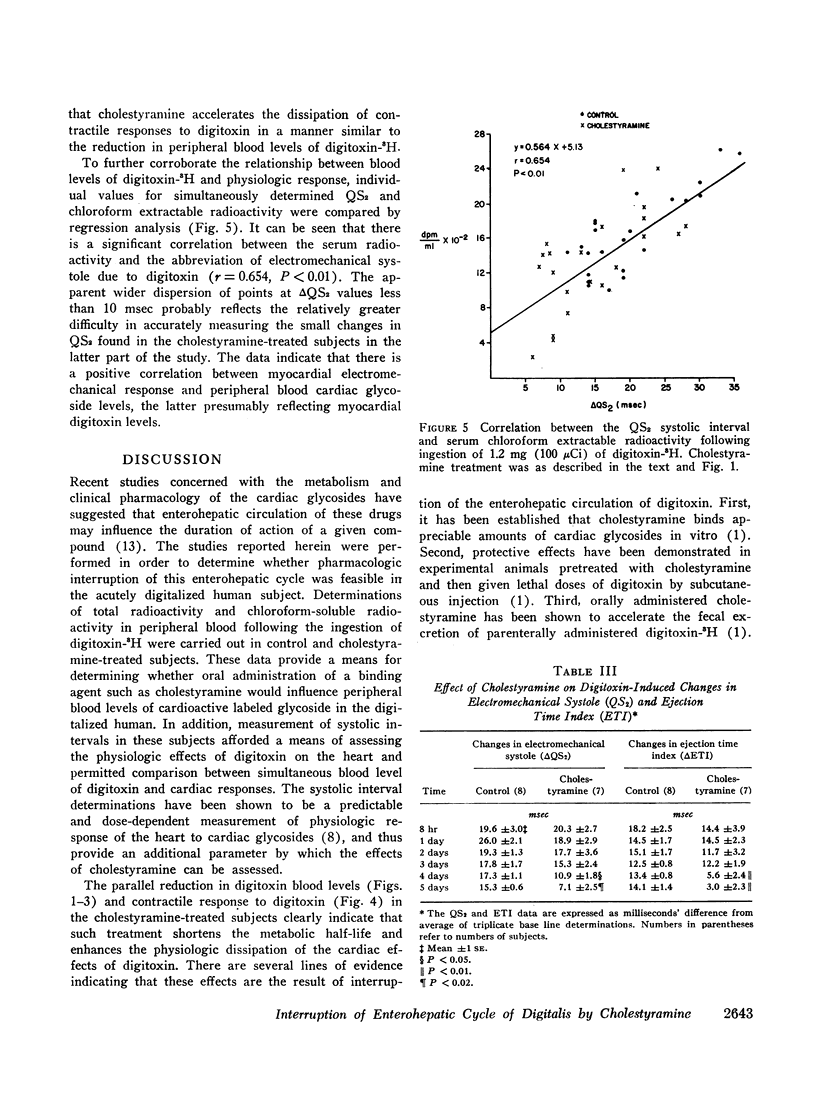
In order to assess the role of the enterohepatic circulation in the metabolism of digitalis glycosides in humans, maintenance doses of cholestyramine were administered to 7 of 15 normal human subjects beginning 8 hr after digitalization with 1.2 mg of digitoxin-3H. All subjects had frequent measurements of serum radioactivity, left ventricular ejection time (LVET), and electromechanical systole (QS2), the latter recorded as the interval from onset of Q wave to first major component of second heart sound. Measurement of the LVET and QS2 intervals affords a sensitive index of the cardiac response to digitalis. In addition, chloroform extraction of serum was performed to separate unchanged digitoxin and active metabolites from cardioinactive metabolites of digitoxin. Cholestyramine treatment resulted in reduction in half-life to total serum radioactivity from 11.5 to 6.6 days, and in chloroform-extractable radioactivity from 6.0 to 4.5 days, as compared to controls. In addition, cholestyramine treatment was accompanied by more rapid return to base line values of digitoxin-induced changes in the LVET and QS2 intervals. A significant positive correlation was found between QS2 values and chloroform-extractable radioactivity, the latter reflecting unchanged digitoxin-H3 (r=0.64; P=<0.01).
The results indicate that administration of cholestyramine to digitalized human subjects accelerates the metabolic disposition of digitoxin and abbreviates the physiologic response to the glycoside. This effect is presumably mediated by interruption of the enterohepatic circulation of digitoxin by cholestyramine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doherty J. E. The clinical pharmacology of digitalis glycosides: a review. Am J Med Sci. 1968 Jun;255:382–414. doi: 10.1097/00000441-196806000-00006. [DOI] [PubMed] [Google Scholar]
- Greenberger N. J., MacDermott R. P., Martin J. F., Dutta S. Intestinal absorption of six tritium-labeled digitalis glycosides in rats and guinea pigs. J Pharmacol Exp Ther. 1969 Jun;167(2):265–273. [PubMed] [Google Scholar]
- Jelliffe R. W., Buell J., Kalaba R., Sridhar R., Rockwell R., Wagner J. G. An improved method of digitoxin therapy. Ann Intern Med. 1970 Apr;72(4):453–464. doi: 10.7326/0003-4819-72-4-453. [DOI] [PubMed] [Google Scholar]
- Katzung B. G., Meyers F. H. Biotransformation of digitoxin in the dog. J Pharmacol Exp Ther. 1966 Dec;154(3):575–580. [PubMed] [Google Scholar]
- Katzung B. G., Meyers F. H. Excretion of radioactive digitoxin by the dog. J Pharmacol Exp Ther. 1965 Aug;149(2):257–262. [PubMed] [Google Scholar]
- Lukas D. A., Peterson R. E. Double isotope dilution derivative assay of digitoxin in plasma, urine, and stool of patients maintained on the drug. J Clin Invest. 1966 May;45(5):782–795. doi: 10.1172/JCI105393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKITA G. T., TALSO P. J., CURRY J. H., Jr, SMITH F. D., Jr, GEILING E. M. Metabolic fate of radioactive digitoxin in human subjects. J Pharmacol Exp Ther. 1955 Dec;115(4):371–379. [PubMed] [Google Scholar]
- Okita G. T. Species difference in duration of action of cardiac glycosides. Fed Proc. 1967 Jul-Aug;26(4):1125–1130. [PubMed] [Google Scholar]
- Smith T. W., Haber E. Current techniques for serum or plasma digitalis assay and their potential clinical application. Am J Med Sci. 1970 May;259(5):301–308. doi: 10.1097/00000441-197005000-00001. [DOI] [PubMed] [Google Scholar]
- Weissler A. M., Schoenfeld C. D. Effect of digitalis on systolic time intervals in heart failure. Am J Med Sci. 1970 Jan;259(1):4–20. doi: 10.1097/00000441-197001000-00002. [DOI] [PubMed] [Google Scholar]


