Abstract
Cucurbitaceous plants (cucurbits) have long been preferred models for studying phloem physiology. However, these species are unusual in that they possess two different phloem systems, one within the main vascular bundles [fascicular phloem (FP)] and another peripheral to the vascular bundles and scattered through stem and petiole cortex tissues [extrafascicular phloem (EFP)]. We have revisited the assumption that the sap released after shoot incision originates from the FP, and also investigated the long-standing question of why the sugar content of this sap is ~30-fold less than predicted for requirements of photosynthate delivery. Video microscopy and phloem labeling experiments unexpectedly reveal that FP very quickly becomes blocked upon cutting, whereas the extrafascicular phloem bleeds for extended periods. Thus, all cucurbit phloem sap studies to date have reported metabolite, protein, and RNA composition and transport in the relatively minor extrafascicular sieve tubes. Using tissue dissection and direct sampling of sieve tube contents, we show that FP in fact does contain up to 1 M sugars, in contrast to low-millimolar levels in the EFP. Moreover, major phloem proteins in sieve tubes of FP differ from those that predominate in the extrafascicular sap, and include several previously uncharacterized proteins with little or no homology to databases. The overall compositional differences of the two phloem systems strongly indicate functional isolation. On this basis, we propose that the fascicular phloem is largely responsible for sugar transport, whereas the extrafascicular phloem may function in signaling, defense, and transport of other metabolites.
Keywords: cucurbitaceae, symplastic loading, extrafascicular, exudate, P-protein
Phloem transport systems have been extensively researched over the past century, because they perform vital functions in plants, including distribution of photoassimilates, nutrients, and signaling molecules to spatially separated organs (1). A predominant concept in phloem physiology is that of a unified interconnected transport conduit (2). Both the complexity of phloem tissue organization and complications arising from rapid wound responses hamper studies on intact plants and limit access to the transported sap of most plant species (3). However, cucurbitaceous species (cucurbits) are preferred models for phloem physiology because of both the ease of sampling phloem sap and the facile visualization of their large phloem sieve elements. The earliest work dates back to Fisher (4), with the original observations of cucurbit phloem exudation by Crafts (5, 6) and Eschrich (7). Unusual among higher plants, cucurbits have two spatially separated phloem systems: fascicular phloem (FP) (7), also known as bundle phloem (8, 9), contained within vascular bundles, and extrafascicular phloem (EFP) (5). The fascicular phloem is located both internal and external to the xylem, an arrangement known as bicollateral. The EFP is distributed as scattered elements throughout the cortex, peripheral to the vascular bundles (5, 8), and both outside (ectocyclic sieve tubes) and inside (endocyclic) the ring of sclerenchyma (see ref. 10 for an excellent drawing).
Cucurbit phloem transport has several noteworthy characteristics. Although many plants load sucrose apoplastically into phloem either against a concentration gradient (11) or with no loading step observed (12), cucurbits have been proposed instead to use a polymer trapping strategy (13). In this model, sucrose diffuses symplastically into intermediary cells (a form of companion cell), where it is converted into raffinose-family oligosaccharide sugars (RFOs). The RFOs remain in the sieve element/companion cell complex (SE/CCC) due to a combination of their increased molecular mass and the different plasmodesmal size exclusion limits between mesophyll–intermediary cell and intermediary cell–sieve element junctions (13).
However, several aspects of cucurbit phloem transport and the polymer trapping model remain unresolved. The first problem is that the total RFO sugar content in cucurbit phloem exudates is around 30-fold lower than predicted from photosynthetic output and mass flow calculations (14) and from equivalent data on phloem of other species. In contrast, amino acid content of cucurbit phloem exudate is similar to other species (15). It has been widely assumed, sometimes implicitly, that cucurbit exudates derive largely or exclusively from the much more substantial FP system, with only minor contributions from the EFP (16). Many explanations have been advanced. One suggestion is that the low RFO concentration in exudates is due to dilution by surrounding cells or by xylem sap (17), but this can be discounted based on the magnitude of dilution required, and from the fact that amino acid levels are similar to other species (18). These controversies have recently been reviewed (19).
The second problem concerns the extensively studied phloem exudate proteins (20). The two dominant proteins, known as PP1 and PP2, are thought to be major constituents of so-called P-protein bodies visible in sieve elements (21, 22). Microscopy shows that upon wounding, proteins appear to seal sieve tubes and should immediately block phloem transport (22). Why rapid blockage does not occur has long been a mystery, as exudation continues for several minutes. However, immunocytochemical evidence indicates that PP1 and PP2 may be absent from or only weakly present in FP, whereas they are abundant in EFP (8, 23). Images from tissue printing were equivocal (24).
In a previous report, the summed sugar concentration in cucurbit phloem exudates was found to be <30 mM (18), consistent with many published studies but far less than the typical 1 M sugar content required to satisfy predictions of the mass flow mechanism. Cucurbit phloem should have highly concentrated sugars (19), considering the sugar transport required to sustain the rapid growth of fruits (25). Therefore, we questioned the consensus interpretations of the origins of phloem exudates and have revisited the systems using a combination of video microscopy and transport experiments, together with metabolite and proteome analysis. We show that cucurbit phloem exudates originate from extrafascicular phloem and not from fascicular phloem. The data reveal massively divergent metabolite and protein contents between exudates and fascicular phloem tissue, suggesting that cucurbits have evolved two spatially and functionally distinct phloem systems.
Results
Reinvestigation of Phloem Exudation in Cucurbits.
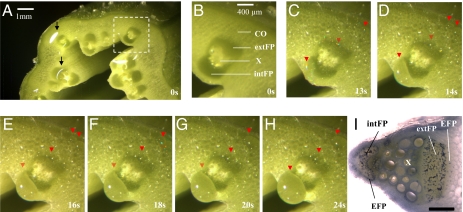
Shoots of intact pumpkin plants (Cucurbita maxima) were placed under a dissection microscope. The exudation process from petioles and stems was observed immediately after transverse cutting. Even without staining, fascicular phloem regions within the normal cucurbit bicollateral, vascular bundles were readily recognized by their darker green color and location on either side of xylem tissues (Fig. 1 A and B) (26). Fig. 1I indicates the general structural features of the bicollateral phloem in an individual vascular bundle, visualized by amido black staining of abundant phloem proteins. Identification of FP was further confirmed with decolorized aniline blue (DAB) staining of callose, which marked the sieve elements (Fig. S1).
Fig. 1.
Direct observation of phloem exudation from stem of Cucurbita maxima (pumpkin). (A) Initial spreading of rapidly exuding sap over the entire cut stem surface. Bicollateral (internal and external) fascicular phloems in each vascular bundle were readily identified by their dark green color and the distinctive intervening xylem region. Arrowheads: glistening exudates under illuminating light. (B) Close-up of boxed region in A. (C–H) Approximately 30 s later, after removal of sap with filter paper, exudation has slowed. Images shown are selected frames from video record of subsequent slower exudation. Many small droplets are visible, and a larger one adjacent to but not from the internal FP (clearest in C). The full video is available as Movie S1. Times are from the last removal of exudate with filter paper. Droplets of exudate (downward pointing red arrowheads) can clearly be seen from extrafascicular phloem sites surrounding fascicular phloem region, and from extrafascicular phloem regions in cortex. In no case was exudation seen to originate from fascicular phloem. Image brightness and contrast have been modified for enhanced clarity. (I) Main structural feature of individual vascular bundle visualized by aniline blue staining, indicating location of different phloem components: fascicular phloem (FP) both internal and external to xylem, extrafascicular phloem (EFP) adjacent to, but separate from, both internal and external FP. (Scale bar: 1mm.) intFP, internal fascicular phloem; extFP, external fascicular phloem; CO, cortex; X, xylem.
Upon cutting, the instantaneous onset of phloem exudation was too rapid to allow the exudate origins to be clearly discerned. Sap immediately spread over much of the cut surface with individual droplets usually not visible, and it was not apparent whether drops were emanating from FP or from other locations (Fig. 1A and Movie S1). However, by repeatedly removing the exudates with filter paper, it was observed that the flow decreased substantially over a period of ~1–5 min. It then became clear that the exudate droplets came largely or exclusively from EFP and not from FP (Fig. 1 C–H). The low resolution of the stereomicroscope impaired direct recognition of unstained extrafascicular sieve tubes. However, the pattern of exudate droplets emanating from the stem surface exactly matched the known distribution of cucurbit EFP strands—i.e., immediately surrounding vascular bundles, scattered through the cortex, and both outside (ectocyclic) and inside (endocyclic) the ring of sclerenchyma (10). These observations were confirmed from the video record of this exudation (Movie S1) and by detailed scrutiny of time-series still images. Even though exudation slowed with time after cutting, the sites from which exudate emerged did not change. Careful mapping of the sites from which the sap emerged showed that they were all coincident with EFP and none with FP.
We next compared exudation in three other cucurbits (Cucumis sativus, Cucurbita pepo, and Citrullus lanatus) widely used in phloem studies. Their behavior was identical: no exudates were seen from FP regions, and patterns of exudation sites were in complete agreement with known EFP distribution.
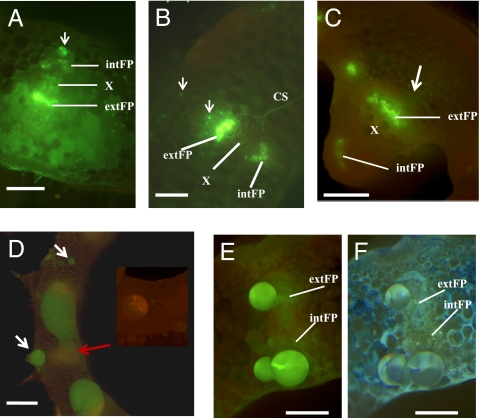
Because symplastic trapping of an externally applied phloem tracer {5(6)-carboxyfluorescein [CF5(6)] or its diacetate form} to phloem systems is well documented (27, 28), we further assessed the origin of exudates in pumpkin by applying this tracer to two sites: the leaf surface and the petiole surface, in separate experiments (Table S1). One to two hours after application of CF5(6) to leaves, petioles were cut and observed. Exudates, FP tissues, and EFP were all heavily labeled with yellow-green fluorescence (Fig. 2A). After exhaustive exudation, labeled FP and EFP were still visible (Fig. 2B). This demonstrates that both phloem systems can load and transport CF5 (6), and that CF5(6) remains largely contained within the phloem. However, this experiment cannot distinguish whether the exudate was from FP, EFP, or both. When stems more distant from CF5(6)-treated leaves were observed, only the FP was labeled, but not EFP (Fig. 2C): in contrast to Fig. 2B, bright specks indicating EFP labeling were not visible. Small amounts of fluorescence, released from FP tissue immediately after cutting, were seen spreading over the cut stem surface onto otherwise unlabeled areas (Fig. 2C), but the exudate thereafter was unlabeled. This indicates slower transport in, or loading of tracers into, EFP compared with FP, and further suggests that most or all of the exudate does not originate from FP.
Fig. 2.
Examination of sites of pumpkin phloem exudation by phloem-mobile fluorescent tracer. Epifluorescence stereomicroscope images of cut surfaces following application of CF5(6) phloem-mobile tracer to distant tissues. Details of the design are in Table S1. (A and B) Petiole cut surface after applying CF5(6) to intact leaf surface. (C) Stem cut surface, after applying CF5(6) to intact leaf surface. (D–F) Petiole cut surface after applying CF5(6) to distal intact petiole surface. Each image shows only part of the cut surface, including one or a few vascular bundles. (A) Spreading of fluorescent exudates over petiole surface immediately after cutting. Fascicular phloem (FP) regions, especially external FP, were intensely labeled. The arrow points to a discrete phloem exudate droplet from extrafascicular phloem region outside internal FP. (B) Exudation from cut petiole eventually stops after repeated removal of exudates. Both external and internal FP and extrafascicular phloem (small bright dots, arrowed) remain labeled, including commissural sieve tubes (CS), which belong to extrafascicular phloem. (C) Stem surface immediately after cutting shows labeled fascicular phloem. An arrow indicates faint spreading of label from external fascicular phloem, interpreted as being derived from broken sieve tubes. (D) Labeled phloem exudates spread on petiole cut surface. White arrows point to smaller exudate droplets from cortical extrafascicular phloem elements not adjacent to vascular bundles. A red arrow indicates the vascular bundle region. Neither external nor internal fascicular phloem was labeled. (Inset) Background autofluorescence of control petiole without CF5 (6). (E) After repeated removal of exudates from cut petiole surface, droplets continued to form and were still labeled. Fascicular phloem (internal and external FP) appear as more yellow-green indistinct regions. These regions are unlabeled or minimally labeled (compare with A–C) and do not underlie the exudate droplets. (F) Petiole surface shown in E was counterstained with DAB and then re-photographed. Light blue specks indicate sieve elements and demarcate both fascicular phloem regions. Exudate droplets are from extrafascicular phloem near to fascicular phloem, but not from fascicular phloem itself. CS, commissural sieve tubes. See Fig. 1 for expansions of other abbreviations. (Scale bars: A, B, E, and F, 400 μm; C, 2 mm; D, 1 mm.)
Tracer applied to petioles resulted in different labeling patterns but again strongly indicated that most or all of the exudate is from EFP. After an overnight uptake and transport period, exudates emerging from the cut surface of petioles sectioned some distance from the site of application were labeled with fluorescence (Fig. 2D). After removing the exudates once or twice, exudation slowed and smaller fluorescent droplets formed (Fig. 2E). However, in contrast to leaf-feeding experiments (Fig. 2 A–C), minimal or no FP tissue labeling was observed. Callose staining with DAB marked the FP sieve elements and confirmed that exudation was not from FP (Fig. 2F). The selective labeling also suggests a lack of symplastic transport from EFP to FP. Taking all of the labeling experiments together, none of the observations lends support to the idea that the exudate is from FP. In particular, when only stem FP is fluorescently labeled, the exudates are clearly not labeled (Fig. 2C).
The video and labeling experiments establish that cucurbit phloem exudates originate largely or exclusively from EFP, not from FP. In contrast to rapid, sustained exudation from EFP, FP becomes blocked almost immediately upon cutting. A small amount of FP sap from broken FP sieve elements may become mixed with phloem exudates immediately after cutting, but this does not continue. Therefore, the sampled exudate, after discarding the initial droplets, is essentially pure EFP sap.
Differences in Metabolome Composition Between Two Phloem Systems in Pumpkin.
The difference in exudation behavior between the two phloem systems indicated that the systems may be functionally distinct, something that has not previously been demonstrated in other species, even those with complex phloem anatomy. A rapid sealing mechanism hampers sampling of FP sap, so we instead separately dissected external and internal FP tissues from lyophilized stem segments to estimate FP sap composition (Fig. S2). For EFP sap composition, we used phloem exudates. In previous reports (18, 29), the metabolome of cucurbit phloem exudates was found to contain amino acids and secondary metabolites at concentrations similar to phloem of other plants. However, many of the amino acids and secondary metabolites present in phloem exudates are not detectable or are at trace levels in FP tissues (Fig. 3).
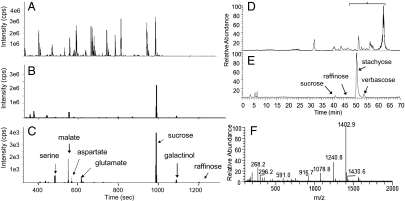
Fig. 3.
Metabolite profiles of dissected fascicular phloem tissues and phloem exudates. (A–C) GC/MS profiles. (A) Total ion chromatogram (TIC) of phloem exudates. (B) TIC of dissected external fascicular phloem tissues. (C) overlay of extracted ion chromatograms from B, to emphasize the major components detected in fascicular phloem tissues. cps, unit of absolute GC/MS signal intensity in counts per second. (D–F) LC/MS profiles. (D) TIC of phloem exudates. (E) TIC of dissected external fascicular phloem tissues. (F) Average of full-scan mass spectra for marked chromatogram segment in bracketed region in D, indicating the glycan series present in phloem exudates but not in fascicular phloem. F displays the (M+H)+ ions of all major glycans detected in phloem exudates, with the 162 m/z increment (one hexose unit) starting from either of the two aglycones (268.2 and 296.2 m/z, respectively).
The majority of small compounds detected by GC/MS (mostly <500 Da) in phloem exudates are either missing or at much lower concentrations in profiles of FP tissues (Fig. 3 A–C; for a list of compounds in phloem exudates, refer to ref. 18). The dominant metabolites detected by GC/MS in dissected FP tissues are sucrose and raffinose. Stachyose and verbascose are too large to be detected by GC/MS, but were found by LC/MS (Fig. 3 D and E). Similarly, LC/MS reveals that other compounds present in exudates, including two families of glycans and various secondary metabolites (refer to ref. 29 for elucidated structures), are absent from or minimally present in dissected FP (Fig. 3 D–F). By far the most dominant metabolites in FP are therefore RFOs.
To estimate RFO concentrations in dissected tissues, we calculated the tissue volume and determined the proportion occupied by sieve elements through microscopic measurement. In the entire dissected external and internal FP tissues, the RFO sugar concentration exceeds 500 mM. Based on total sugars measured from leaf tissue, 15 mM summed RFOs was estimated for phloem parenchyma. Approximately 45% of the fascicular phloem tissue volume was sieve elements and companion cell complexes (SE/CCC). From these numbers, FP sap was calculated to contain ~1 M total RFOs, mainly as stachyose, in contrast to 29 mM RFOs in phloem exudates. Of the five sample types analyzed (leaf disk, phloem exudates, xylem tissue, and external and internal FP tissues), only the FP contains high concentrations of RFOs (Table 1).
Table 1.
RFO sugar concentrations in different tissues measured by LC/MS
| Tissue type | Sucrose | Raffinose | Stachyose | Verbascose | Sum of RFOs | N |
| Total dissected external fascicular phloem tissue | 60.0 ± 9.8 | 14.0 ± 2.4 | 521.0 ± 126.1 | 55.9 ± 14.5 | 651.0 ± 142.4 | 12 |
| Total dissected internal fascicular phloem tissue | 43.2 ± 27.1 | N.D. | 425.9 ± 269.7 | 79.7 ± 51.4 | 548.7 ± 348.3 | 2 |
| External fascicular phloem SE/CCC (by estimation) | 88.3 ± 17.5 | 6.6 ± 4.3 | 908.0 ± 224.1 | 81.1 ± 25.7 | 1139.1 ± 253.2 | 12 |
| Internal fascicular phloem SE/CCC (by estimation) | 58.4 ± 48.3 | N.D. | 738.7 ± 479.5 | 123.3 ± 91.4 | 957.2 ± 619.2 | 2 |
| Leaf disk | 3.1 ± 0.7 | 3.8 ± 0.6 | 9.0 ± 1.5 | 0.5 ± 0.1 | 16.5 ± 2.3 | 6 |
| Phloem exudate | 0.4 ± 0.4 | 17.5 ± 10.9 | 9.5 ± 7.0 | 1.7 ± 1.7 | 29.4 ± 19.8 | 3 |
| Xylem | 0.7 ± 0.3 | 0.1 ± 0.0 | 5.2 ± 2.6 | 1.5 ± 0.9 | 7.4 ± 2.8 | 4 |
The first two rows show the RFO sugar concentration, which was calculated based on the total volume of dissected external and internal fascicular phloem tissues. The third and fourth rows show the estimated RFO sugar concentration in external and internal fascicular SE/CCCs. The last three rows show RFO sugar concentrations calculated from total sample volume. The number format is mean ± SE. The unit of concentration is mM. N, number of observations; N.D., not detected.
Differences in Proteomes Between Two Phloem Systems.
The preceding discoveries suggested that the proteomes of the two phloem systems might also differ. One important question was whether the major P-proteins present in exudates are also components of the P-protein bodies that block FP.
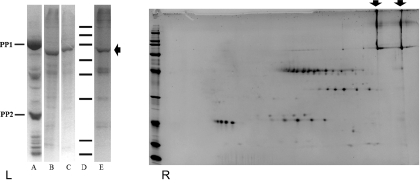
Proteins were sampled as aggregates from unfixed, live FP (Fig. S3), and from extracted, dissected FP tissues. These proteins were separated alongside exudate samples by 1D SDS/PAGE to compare the protein profiles from both phloem systems. The well defined P-proteins (PP1 and PP2) in EFP exudates appear to be absent in the FP (Fig. 4). Instead, at least five major protein bands were seen in FP samples. Additional proteins may be present, but their abundances were too low to be identified. Two-dimensional PAGE analysis similarly showed five groups of FP proteins. We designated them as groups because each share similar masses and only show a horizontal shift in the IEF dimension of the 2D gel. None of these groups is dominant in 2D PAGE of phloem exudates (Fig. S4 A and B). Likewise, the major phloem exudate proteins detected on either 1D or 2D gels are not prominent in FP samples. The smearing in 1D gels of FP proteins is suggestive of extensive posttranslational modifications, which was confirmed by 2D gel analysis (Fig. 4B). One group of FP proteins (66 kDa, as judged from 2D gel) was detected as phosphorylated by phospho-staining (Fig. S4 C and D).
Fig. 4.
Comparing proteome contents between pumpkin phloem exudates and fascicular phloem. (Left) SDS/PAGE (10%): Lanes A–E represent: phloem exudates, dissected external fascicular phloem extracts, dissected internal fascicular phloem extracts, molecular weight markers and fascicular phloem proteins sampled by needle-tips, respectively. MW markers: from top to bottom, 200, 150, 100, 75, 50, 37, 25, 20, kDa. PP1 and PP2 are the most abundant proteins in pumpkin phloem exudates. (Right) 2D PAGE of fascicular phloem proteins sampled by needle-tips. IEF, pI 3–10 nonlinear; SDS/PAGE, 8–16%; MW markers, 200, 150, 100, 75, 50, 37, 25, 20, 15, and 10 kDa. For both gels, arrows indicate one of the major fascicular phloem proteins, designated as CmFPP1, which is a single band in 1D gel (Left) and two longitudinal smeared bands in 2D gel (Right).
MASCOT searches of LC/MS peptide spectra confirmed that PP1 and PP2 are the dominant proteins in exudate samples and are not detected from FP samples. Searching of the NCBInr database returned no significant hits for any of the FP proteins analyzed from gel spots. However, the recently released cucumber genome (30) and pumpkin phloem sap proteome (31), together with translated cucurbit EST and Unigene databases (www.icugi.org), contained significant hits to de novo sequenced peptides from one of the most abundant proteins [designated as CmFPP1, for C. maxima fascicular phloem protein, ~80 kDa (Fig. 4 and Table S2)]. However, all other peptides from FP samples lack homology to any proteins previously reported in phloem exudates or, for that matter, in any tissue from any organism. Thus, the FP proteome represents a newly identified functional system within cucurbits that is distinct from the extensively studied proteome of phloem exudates. The horizontal protein spot series in 2D gels of FP proteins were confirmed to be isoforms by manual examination of MS/MS spectra. Full-length sequences have yet to be obtained for these novel proteins, and there are further unidentified minor proteins visible on the gels.
Discussion
Dual Phloem Transport Systems.
By carefully revisiting the question of the origin of cucurbit phloem exudates and by comparing the metabolite and protein contents of both cucurbit phloem systems, we have established that cucurbit phloem sap samples obtained by standard exudation methods following tissue cutting represent primarily or exclusively the contents of EFP and not the major FP system. This is an unexpected result because there is a large body of literature on metabolites, hormones, proteins, and RNA in cucurbit phloem exudates resting on the assumption that cucurbit phloem exudates are largely from FP or are a mixture from both FP and EFP.
Rapid exudation from EFP presents methodological challenges for drawing correct conclusions regarding its true origin. This may be one reason why exudation sites were previously misinterpreted and assigned to FP (5–7). Unfortunately, most subsequent research on cucurbit phloem exudates and phloem transport has been based on these reports. Space precludes a full listing, but examples include refs. 24 and 32–34, and further examples are reviewed in refs. 16, 21, and 35. In light of the findings reported here, some conclusions may need to be carefully reevaluated.
We have discovered huge divergence in metabolome and proteome contents between the two cucurbit phloem systems. This runs contrary to the notion that phloem represents a unified conduit. The new model of dual, functionally divergent transport systems provides a framework for future research using cucurbits as model species for studying phloem transport.
Phloem Metabolite Composition and Transport.
As mentioned in a recent review (19), since the very first extensive cucurbit phloem transport studies with radioactive tracers (36), it has been clear that stachyose and sucrose are the major forms of carbon exported from mature leaves. High rates of carbon mass flow in phloem are predicted from the fast growth of cucurbit vegetative and fruit tissues (25). However, no previous reports showed high RFO content in cucurbit phloem exudates, for example (15, 37). The total sugar content of around 1 M in FP is consistent with other species, and with measurements in cucurbit leaf cells (17), but is in sharp contrast to the low sugar content of the extrafascicular exudate. The paradox of low sugar in the exudate is therefore now resolved. We can further conclude that sugar transport is, as always suspected, a major function of FP. Although internal FP was difficult to sample, there was no substantial difference between the composition of external and internal FP within the bicollateral stem vascular bundles, suggesting that they may have similar function. Extrafascicular phloem exudates contained abundant amino acids and many unidentified secondary metabolites (38), in contrast to the low levels of sugars. It is possible that some of these metabolites have protective functions, perhaps providing rings of defense both as the ectocyclic elements immediately below the epidermis and as the tissue surrounding each vascular bundle.
Phloem systems are divided into three functional zones: loading, transport, and unloading (39). Cucurbit EFP systems are outside of the photosynthate loading zone of leaf minor veins (40) and are therefore probably not involved in RFO loading. Further investigation is needed to assess whether EFP has roles in loading other molecules such as amino acids, or whether it has solely a transport function (39) that is responsible for collecting metabolites along transport systems of FP. The amino acid profile in exudates is similar to that from other species. This is consistent with a previous proposal that EFP may be specialized for delivering amino acids, based on immunolocalization of enzymes involved in nitrogen export (41).
Phloem Protein Composition.
Consistent with the divergent metabolome composition between fascicular and extrafascicular phloem, gel analysis and LC/MS indicate that the proteomes are also substantially different. None of the dominant protein groups detected from FP feature as major components of EFP exudate. Conversely, the major exudate proteins PP1 and PP2 were not found in FP. The only evidence of shared proteins is based on close homology between the CmFPP1 peptides detected in this work and a protein present in the pumpkin phloem sap proteome (31). Further multiple homologous sequences in several species suggest that this protein is common to all cucurbits and is a member of a small family (42). A more comprehensive comparative analysis of the proteomes from the two phloem systems will reveal how many proteins are common to both. If the two systems have evolved from a single ancestral phloem, then retention of some sharing is likely.
Regarding protein function, we propose that PP1 and PP2 are responsible for the slow blocking of EFP that occurs over a period of several minutes after cutting, at which point the exudates become gel-like. However, we have shown that these proteins are, at most, only very low abundance components of FP. This is consistent with earlier observations (8, 9). However, FP is blocked almost instantaneously upon wounding, and this is unlikely to be due to PP1 and PP2. Although not yet tested experimentally, some of the major proteins detected from FP may have wound-sealing functions.
In conclusion, we showed that the two structurally and spatially distinct phloem systems in cucurbits have also evolved to be functionally differentiated and physiologically separated. This contrasts with the unified phloem systems present in most plant species. Evolution of functional divergence of phloem transport is not evident in other well studied phloem model species, but may well exist outside the Cucurbitaceae. It remains to be seen whether the sum of the contents and functions in the two systems of cucurbits is equivalent to those in the single phloem of most other species. Although cucurbits cannot be considered a generic phloem model, they nevertheless represent models with unique advantages for phloem studies because of their dual transport systems. The release of the complete cucumber genome sequence (30) will greatly facilitate such explorations.
Methods
Plant Materials.
Greenhouse-grown Cucurbita maxima Duch. cv. Gelber Zentner (pumpkin) was used for all studies, and compared with Cucumis sativus L. cv. Hoffmanns Giganta (cucumber) and Cucurbita pepo L. cv. Cocozelle v. Tripolis (zucchini) and Citrullus lanatus (Thunb.) Matsum. and Nakai (watermelon) for phloem exudation. Field-grown Cucurbita maxima cv. Atlantic Giant was used during later stages of phloem protein analysis. Three-month-old, mature plants (when stem phloem is fully developed) were used for metabolite analysis and for dissecting phloem proteins from fresh stem segments.
Microscopy.
Full details are given in SI Methods. Briefly, phloem exudation was observed by bright-field and fluorescence stereo microscopy. Alkaline decolorized aniline blue was used as a callose stain, visualized under UV excitation. CF-5(6) was used as a phloem-mobile tracer throughout, in accordance with ref. 27.
Sampling.
Phloem exudates were collected according to ref. 18 for metabolite and protein analysis. Fascicular phloem tissues for metabolite analysis were microdissected from freeze-dried stem segments of pumpkin. Fascicular phloem tissues for protein analysis were dissected from frozen pumpkin stem segments. Volumes of dissected tissues and percentage of sieve elements in dissected phloem tissues were estimated by microscopic measurement. Protein aggregates from FP sieve tubes were sampled directly with syringe needle-tips from free-hand, fresh tissue sections of pumpkin stem after brief staining with 0.1% (wt/vol) amido black (pH ~ 6.8). Detailed protocols are given in SI Methods.
Metabolite and Protein Analysis.
Metabolite profiling and identification from phloem exudates and dissected tissues were done according to refs. 18, 29, and 38. Proteins collected from FP by needle-tips were processed either by direct acetone precipitation or phenol method (43). Proteins from dissected FP tissues were extracted by phenol method. Extracted proteins were analyzed by 1D or 2D SDS/PAGE. Protein extracts of needle-dissected protein aggregates and from excised gel bands or spots were also analyzed, after trypsin digestion, by nano-LC/MS/MS. MASCOT database searching was performed according to standard procedures. De novo sequencing was conducted using methods reported in ref. 33. Full details of analytical methods are given in SI Methods.
Supplementary Material
Acknowledgments
We thank Dr. Julia Kehr for initial sequencing of tryptic peptides, Greg Dulski (Illinois Giant Pumpkin Growers Association, Hoffman Estates, IL) for providing Cucurbita maxima cv. Atlantic Giant plant materials, Bert Berla and Dr. Sophie Alvarez for assistance with 2D SDS-PAGE, and Prof. Robert Turgeon for constructive comments on an earlier version of the manuscript. This work was supported by the Max Planck Society (2000-2003) (B.Z., V.T., and O.F.) and the Donald Danforth Plant Science Center (B.Z. and L.M.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 13201.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0910558107/-/DCSupplemental.
References
- 1.Fisher DB. Long-distance transport. In: Clarke AE, editor. Biochemistry & Molecular Biology of Plants. Hoboken, NJ: Wiley; 2002. pp. 730–784. [Google Scholar]
- 2.Ruiz-Medrano R, Xoconostle-Cázares B, Lucas WJ. The phloem as a conduit for inter-organ communication. Curr Opin Plant Biol. 2001;4:202–209. doi: 10.1016/s1369-5266(00)00162-x. [DOI] [PubMed] [Google Scholar]
- 3.van Bel AJE. The phloem, a miracle of ingenuity. Plant Cell Environ. 2003;26:125–149. [Google Scholar]
- 4.Fisher A. Das Sieboehren system von Cucurbita. The phloem system of Cucurbita. Ber Dtsch Bot Ges. 1883;1:276–279. [Google Scholar]
- 5.Crafts AS. Phloem anatomy, exudation and transport of organic nutrients in cucurbits. Plant Physiol. 1932;7:i4–i225. doi: 10.1104/pp.7.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crafts AS. Further studies on exudation in cucurbits. Plant Physiol. 1936;11:63–79. doi: 10.1104/pp.11.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eschrich W, Evert RF, Heyser W. Proteins of the sieve tube exudate of Cucurbita maxima. Planta. 1971;100:208–221. doi: 10.1007/BF00387037. [DOI] [PubMed] [Google Scholar]
- 8.Smith LM, Sabnis DD, Johnson RPC. Immunocytochemical localization of phloem lectin from Cucurbita maxima using peroxidase and colloidal-gold labels. Planta. 1987;170:461–470. doi: 10.1007/BF00402980. [DOI] [PubMed] [Google Scholar]
- 9.Clark AM, et al. Molecular characterization of a phloem-specific gene encoding the filament protein, phloem protein 1 (PP1), from Cucurbita maxima. Plant J. 1997;12:49–61. doi: 10.1046/j.1365-313x.1997.12010049.x. [DOI] [PubMed] [Google Scholar]
- 10.Kempers R, Prior DAM, van Bel AJE, Oparka KJ. Plasmodesmata between sieve element and companion cell of extrafascicular stem phloem of Cucurbita maxima permit passage of 3-Kda fluorescent-probes. Plant J. 1993;4:567–575. [Google Scholar]
- 11.Lalonde S, Tegeder M, Throne-Holst M, Frommer WB, Patrick JW. Phloem loading and unloading of sugars and amino acids. Plant Cell Environ. 2003;26:37–56. [Google Scholar]
- 12.Turgeon R, Medville R. The absence of phloem loading in willow leaves. Proc Natl Acad Sci USA. 1998;95:12055–12060. doi: 10.1073/pnas.95.20.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turgeon R. Phloem loading and plasmodesmata. Trends Plant Sci. 1996;1:418–423. [Google Scholar]
- 14.Schaffer AA, Pharr DM, Madore MA. Cucurbits. In: Schaffer AA, editor. Photoassimilate Distribution in Plants and Crops: Source-sink Relationships. New York: Dekker; 1996. pp. 729–753. [Google Scholar]
- 15.Richardson PT, Baker DA, Ho LC. The chemical composition of cucurbit vascular exudates. J Exp Bot. 1982;33:1239–1247. [Google Scholar]
- 16.Thompson GA. P-Protein trafficking through plasmodesmata. In: van Bel AJE, van Kesteren WJP, editors. Plasmodesmata: Structure, Function, Role in Cell Communication. Berlin: Springer; 1999. pp. 295–314. [Google Scholar]
- 17.Haritatos E, Keller F, Turgeon R. Raffinose oligosaccharide concentrations measured in individual cell and tissue types in Cucumis melo L leaves: Implications for phloem loading. Planta. 1996;198:614–622. doi: 10.1007/BF00262649. [DOI] [PubMed] [Google Scholar]
- 18.Fiehn O. Metabolic networks of Cucurbita maxima phloem. Phytochemistry. 2003;62:875–886. doi: 10.1016/s0031-9422(02)00715-x. [DOI] [PubMed] [Google Scholar]
- 19.Turgeon R, Wolf S. Phloem transport: cellular pathways and molecular trafficking. Annu Rev Plant Biol. 2009;60:207–221. doi: 10.1146/annurev.arplant.043008.092045. [DOI] [PubMed] [Google Scholar]
- 20.Oparka KJ, Cruz SS. The great escape: Phloem transport and unloading of macromolecules. Annu Rev Plant Physiol. 2000;51:323–347. doi: 10.1146/annurev.arplant.51.1.323. [DOI] [PubMed] [Google Scholar]
- 21.Thompson GA, Schulz A. Macromolecular trafficking in the phloem. Trends Plant Sci. 1999;4:354–360. doi: 10.1016/s1360-1385(99)01463-6. [DOI] [PubMed] [Google Scholar]
- 22.Evert RF, Eschrich , Eichhorn SE. P-protein distribution in mature sieve elements of Cucurbita maxima. Planta. 1973;109:193–210. doi: 10.1007/BF00387084. [DOI] [PubMed] [Google Scholar]
- 23.la Cour Petersen M, Hejgaard J, Thompson GA, Schulz A. Cucurbit phloem serpins are graft-transmissible and appear to be resistant to turnover in the sieve element-companion cell complex. J Exp Bot. 2005;56:3111–3120. doi: 10.1093/jxb/eri308. [DOI] [PubMed] [Google Scholar]
- 24.Golecki B, Schulz A, Thompson GA. Translocation of structural P proteins in the phloem. Plant Cell. 1999;11:127–140. doi: 10.1105/tpc.11.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crafts AS, Crisp CE. Phloem Transport in Plants. San Francisco: Freeman; 1971. [Google Scholar]
- 26.Esau K. Plant Anatomy. 2nd Ed. New York: Wiley; 1965. [Google Scholar]
- 27.Grignon N, Touraine B, Durand M. 6(5)Carboxyfluorescein as a tracer of phloem sap translocation. Am J Bot. 1989;76:871–877. [Google Scholar]
- 28.Knoblauch M, van Bel AJE. Sieve tubes in action. Plant Cell. 1998;10:35–50. [Google Scholar]
- 29.Tolstikov VV, Fiehn O. Analysis of highly polar compounds of plant origin: Combination of hydrophilic interaction chromatography and electrospray ion trap mass spectrometry. Anal Biochem. 2002;301:298–307. doi: 10.1006/abio.2001.5513. [DOI] [PubMed] [Google Scholar]
- 30.Huang S, et al. The genome of the cucumber, Cucumis sativus L. Nat Genet. 2009;41:1275–1281. doi: 10.1038/ng.475. [DOI] [PubMed] [Google Scholar]
- 31.Lin MK, Lee YJ, Lough TJ, Phinney BS, Lucas WJ. Analysis of the pumpkin phloem proteome provides insights into angiosperm sieve tube function. Mol Cell Proteomics. 2009;8:343–356. doi: 10.1074/mcp.M800420-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Yoo BC, Lee JY, Lucas WJ. Analysis of the complexity of protein kinases within the phloem sieve tube system. Characterization of Cucurbita maxima calmodulin-like domain protein kinase 1. J Biol Chem. 2002;277:15325–15332. doi: 10.1074/jbc.M200382200. [DOI] [PubMed] [Google Scholar]
- 33.Walz C, Juenger M, Schad M, Kehr J. Evidence for the presence and activity of a complete antioxidant defence system in mature sieve tubes. Plant J. 2002;31:189–197. doi: 10.1046/j.1365-313x.2002.01348.x. [DOI] [PubMed] [Google Scholar]
- 34.Xoconostle-Cázares B, et al. Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science. 1999;283:94–98. doi: 10.1126/science.283.5398.94. [DOI] [PubMed] [Google Scholar]
- 35.Lough TJ, Lucas WJ. Integrative plant biology: Role of phloem long-distance macromolecular trafficking. Annu Rev Plant Biol. 2006;57:203–232. doi: 10.1146/annurev.arplant.56.032604.144145. [DOI] [PubMed] [Google Scholar]
- 36.Webb JA, Gorham PR. Translocation of photosynthetically assimilated C14 in straight-necked squash. Plant Physiol. 1964;39:663–672. doi: 10.1104/pp.39.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell DE, Gadus MV, Madore MA. Patterns of assimilate production and translocation in muskmelon Cucumis Melo L. I. diurnal patterns. Plant Physiol. 1992;99:959–965. doi: 10.1104/pp.99.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolstikov V, Fiehn O, Tanaka N. Metabolomics: Methods and Protocols, Methods in Molecular Biology. Vol. 358. Totowa, NJ: Humana; 2007. Application of liquid chromatography-mass spectrometry analysis in metabolomics; pp. 141–155. [DOI] [PubMed] [Google Scholar]
- 39.van Bel AJE. The transport phloem: Specifics of its functioning. Prog Bot. 1993;54:134–150. [Google Scholar]
- 40.Turgeon R, Webb JA, Evert RF. Ultrastructure of minor veins in Cucurbita pepo leaves. Protoplasma. 1975;83:217–232. [Google Scholar]
- 41.Chen ZH, et al. Are isocitrate lyase and phosphoenolpyruvate carboxykinase involved in gluconeogenesis during senescence of barley leaves and cucumber cotyledons? Plant Cell Physiol. 2000;41:960–967. doi: 10.1093/pcp/pcd021. [DOI] [PubMed] [Google Scholar]
- 42.Omid A, Keilin T, Glass A, Leshkowitz D, Wolf S. Characterization of phloem-sap transcription profile in melon plants. J Exp Bot. 2007;58:3645–3656. doi: 10.1093/jxb/erm214. [DOI] [PubMed] [Google Scholar]
- 43.Hurkman WJ, Tanaka CK. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986;81:802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.