Abstract
This study was designed to define quantitatively the function of the rat glomerular mesangium in the uptake and processing of intravenously administered protein macromolecules (radiolabeled aggregated human IgG, AHIgG-125I), to relate this function to that of the general reticuloendothelial system, and to examine the effects of increased glomerular permeability to protein on the mesangial cell system.
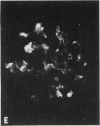
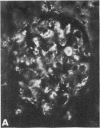
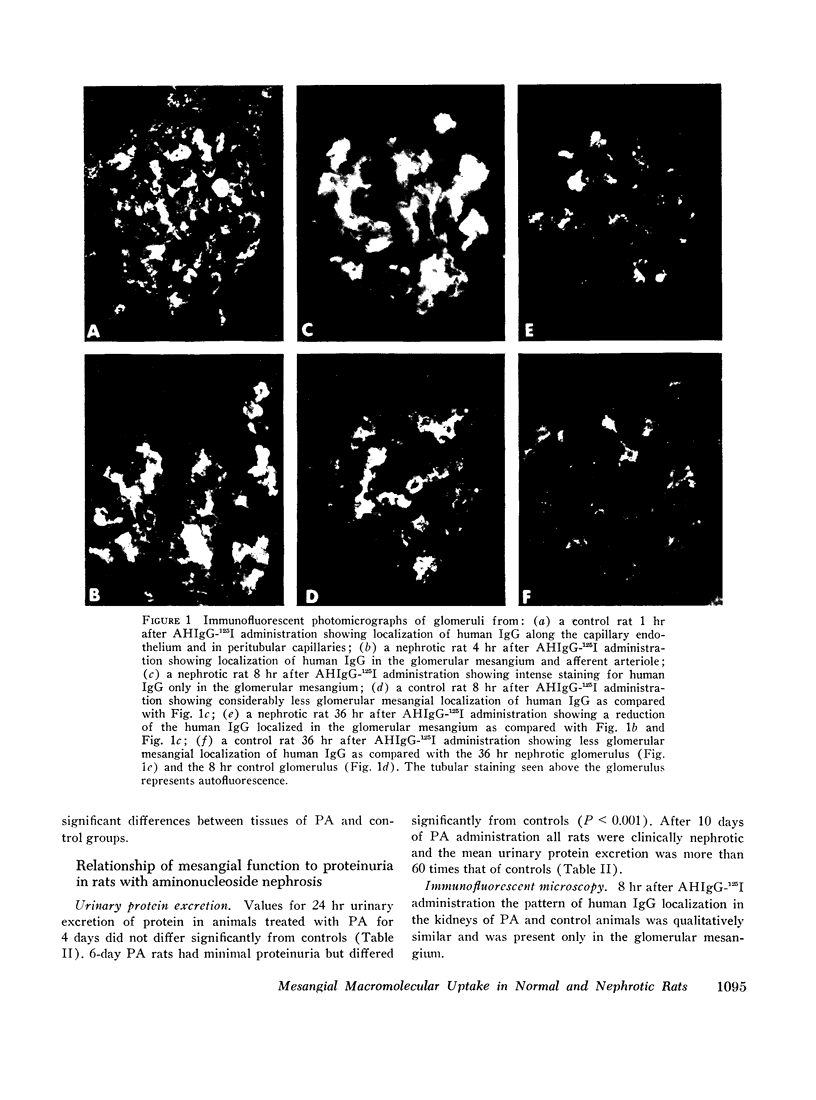
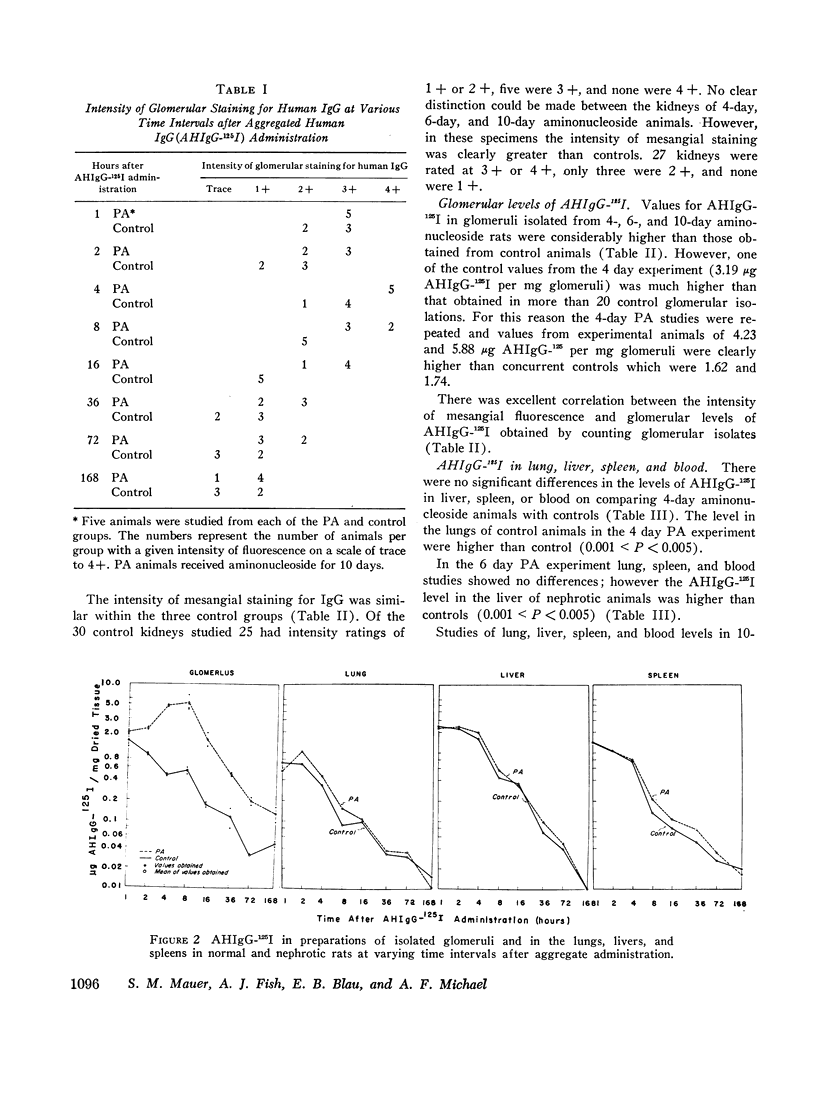
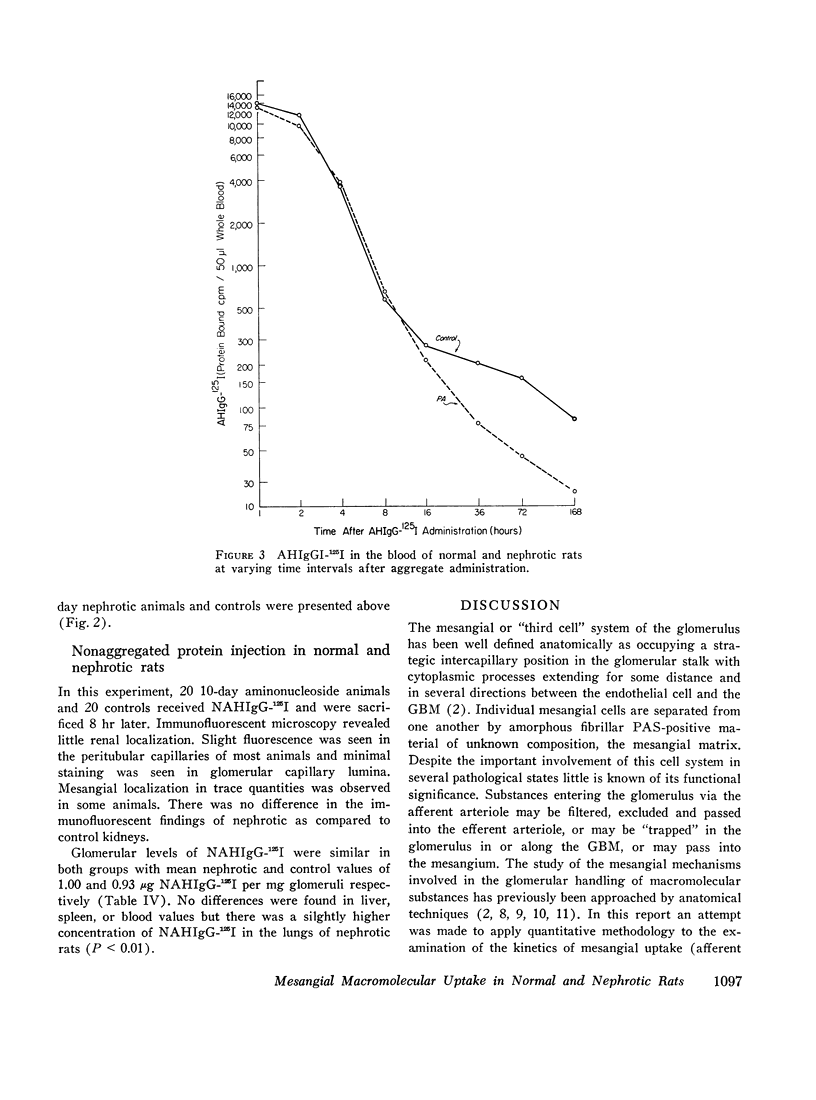
Mesangial localization of human IgG as demonstrated by immunofluorescent microscopy showed good correlation with concentrations of AHIgG-125I in preparations of isolated glomeruli. In normal rats the concentrations of AHIgG-125I in glomeruli were similar to those of lung, liver, and spleen and demonstrated a rapid decrease with increasing time intervals after aggregate administration.
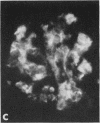
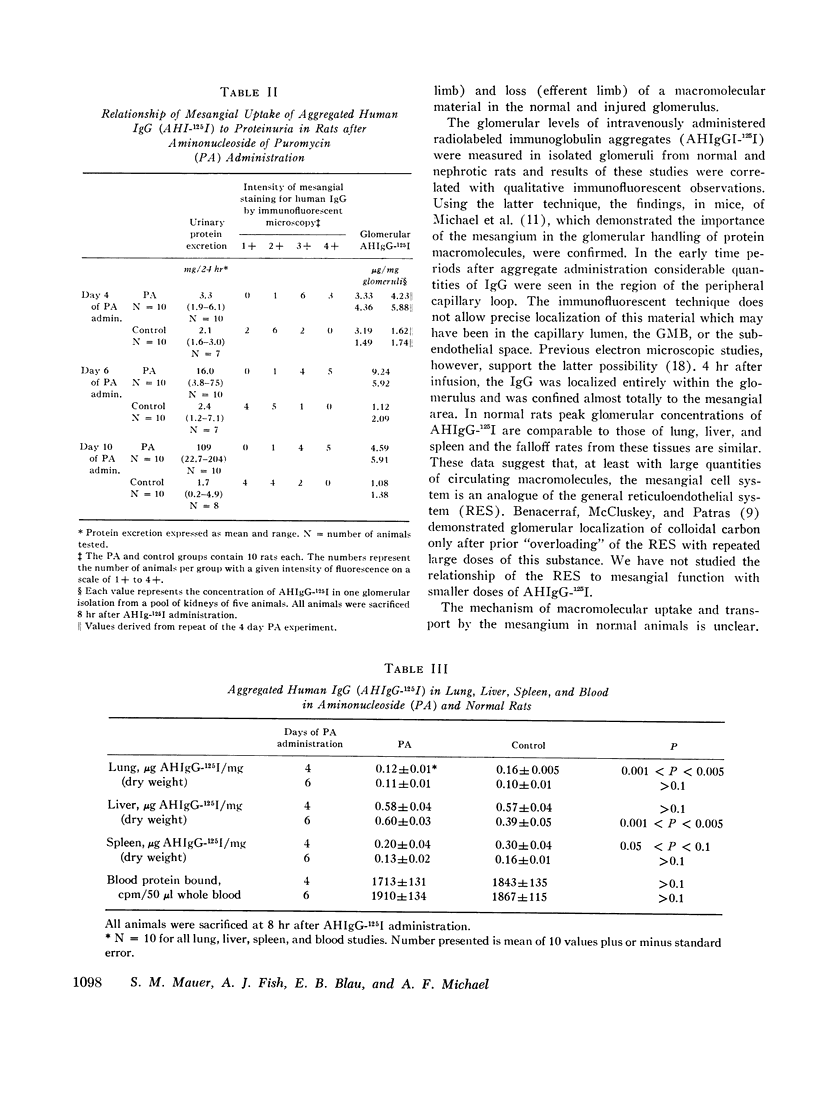
In rats given aminonucleoside of puromycin a marked increase in mesangial uptake of aggregates was found while studies of nephrotic lungs, liver, spleen, and blood showed no such differences. Glomerular levels of AHIgG-125I in aminonucleoside animals could not be correlated with the quantity of proteinuria.
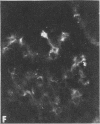
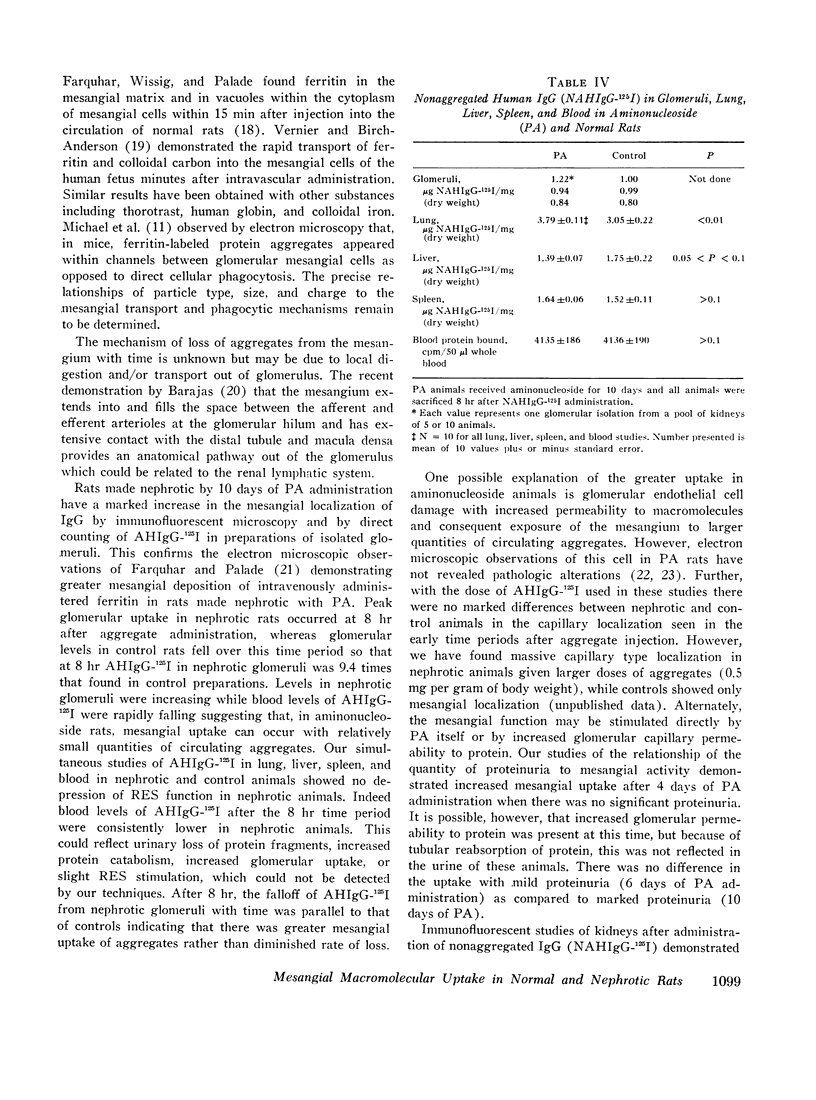
Nephrotic and control animals given unaggregated human IgG showed little glomerular localization by immunofluorescent microscopy; no difference in the concentration of this protein in nephrotic as compared to control glomerular isolates was found.
Thus, the mesangium in normal animals functions in a manner analogous to that of the general reticuloendothelial system. In nephrotic rats the mesangial uptake of macromolecules is makedly increased, a finding not observed in other tissues.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENACERRAF B., McCLUSKEY R. T., PATRAS D. Localization of colloidal substances in vascular endothelium: a mechanism of tissue damage. I. Factors causing the pathologic deposition of colloidal carbon. Am J Pathol. 1959 Jan-Feb;35(1):75–91. [PMC free article] [PubMed] [Google Scholar]
- Barajas L. The ultrastructure of the juxtaglomerular apparatus as disclosed by three-dimensional reconstructions from serial sections. The anatomical relationship between the tubular and vascular components. J Ultrastruct Res. 1970 Oct;33(1):116–147. doi: 10.1016/s0022-5320(70)90121-8. [DOI] [PubMed] [Google Scholar]
- Blau E., Michael A. F. Rat glomerular basement membrane composition and metabolism in aminonucleoside nephrosis. J Lab Clin Med. 1971 Jan;77(1):97–109. [PubMed] [Google Scholar]
- CHRISTIAN C. L. Studies of aggregated gamma-globulin. I. Sedimentation, electrophoretic and anticomplementary properties. J Immunol. 1960 Jan;84:112–116. [PubMed] [Google Scholar]
- CHRISTIAN C. L. Studies of aggregated gamma-globulin. II. Effect in vivo. J Immunol. 1960 Jan;84:117–121. [PubMed] [Google Scholar]
- Ericsson J. L., Andres G. A. Electron Microscopic Studies on the Development of the Glomerular Lesions in Aminonucleoside Nephrosis. Am J Pathol. 1961 Dec;39(6):643–663. [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J Exp Med. 1961 Nov 1;114:699–716. doi: 10.1084/jem.114.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., WISSIG S. L., PALADE G. E. Glomerular permeability. I. Ferritin transfer across the normal glomerular capillary wall. J Exp Med. 1961 Jan 1;113:47–66. doi: 10.1084/jem.113.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germuth F. G., Jr, Valdes A. J., Senterfit L. B., Pollack A. D. A unique influence of cortisone on the transit of specific macromolecules across vascular walls in immune complex disease. Johns Hopkins Med J. 1968 Mar;122(3):137–153. [PubMed] [Google Scholar]
- Iidaka K., McCoy J., Kimmelsteil P. The glomerular mesangium. A quantitative analysis. Lab Invest. 1968 Dec;19(6):573–579. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Salmon S., Fudenberg H. Biologic activities of aggregated gamma-globulin. 8. Aggregated immunoglobulins of different classes. J Immunol. 1967 Jul;99(1):82–91. [PubMed] [Google Scholar]
- LANNIGAN R. The production of chronic renal disease in rats by a single intravenous injection of aminonucleoside of puromycin and the effect of low dosage continuous hydrocortisone. Br J Exp Pathol. 1963 Jun;44:326–333. [PMC free article] [PubMed] [Google Scholar]
- LATTA H., MAUNSBACH A. B. Relations of the centrolobular region of the glomerulus to the juxtaglomerular apparatus. J Ultrastruct Res. 1962 Jun;6:562–578. doi: 10.1016/s0022-5320(62)80010-0. [DOI] [PubMed] [Google Scholar]
- MENEFEE M. G., MUELLER C. B., BELL A. L., MYERS J. K. TRANSPORT OF GLOBIN BY THE RENAL GLOMERULUS. J Exp Med. 1964 Dec 1;120:1129–1138. doi: 10.1084/jem.120.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A. F., Fish A. J., Good R. A. Glomerular localization and transport of aggregated proteins in mice. Lab Invest. 1967 Jul;17(1):14–29. [PubMed] [Google Scholar]
- Michael A. F., Jr, Drummond K. N., Good R. A., Vernier R. L. Acute poststreptococcal glomerulonephritis: immune deposit disease. J Clin Invest. 1966 Feb;45(2):237–248. doi: 10.1172/JCI105336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUDA R., KAPLAN M. H., CUPPAGE F. E., HEYMANN W. DEPOSITION OF AUTOLOGOUS GAMMA GLOBULIN IN KIDNEYS OF RATS WITH NEPHROTIC RENAL DISEASE OF VARIOUS ETIOLOGIES. J Lab Clin Med. 1965 Aug;66:204–215. [PubMed] [Google Scholar]
- SMITH F. G., Jr, LITMAN N., LATTA H. LUPUS GLOMERULONEPHRITIS. THE EFFECT OF LARGE DOSES OF CORTICOSTEROIDS ON RENAL FUNCTION AND RENAL LESIONS IN TWO CHILDREN. Am J Dis Child. 1965 Sep;110:302–308. [PubMed] [Google Scholar]
- UNANUE E., DIXON F. J. EXPERIMENTAL GLOMERULONEPHRITIS. IV. PARTICIPATION OF COMPLEMENT IN NEPHROTOXIC NEPHRITIS. J Exp Med. 1964 Jan 1;119:965–982. doi: 10.1084/jem.119.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urizar R. E., Michael A., Sisson S., Vernier R. L. Anaphylactoid purpura. II. Immunofluorescent and electron microscopic studies of the glomerular lesions. Lab Invest. 1968 Oct;19(4):437–450. [PubMed] [Google Scholar]
- VERNIER R. L., BIRCH-ANDERSEN A. Studies of the human fetal kidney. II. Permeability characteristics of the developing glomerulus. J Ultrastruct Res. 1963 Feb;8:66–88. doi: 10.1016/s0022-5320(63)80021-0. [DOI] [PubMed] [Google Scholar]
- VERNIER R. L., PAPERMASTER B. W., GOOD R. A. Aminonucleoside nephrosis. I. Electron microscopic study of the renal lesion in rats. J Exp Med. 1959 Jan 1;109(1):115–126. doi: 10.1084/jem.109.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]