Abstract
Background
Acute renal failure secondary to ischemia and reperfusion (I/R) injury poses a significant burden on both surgeons and patients. It carries a high morbidity and mortality rate and no specific treatment currently exists. Major causes of renal I/R injury include trauma, sepsis, hypoperfusion, and various surgical procedures. We have demonstrated that adrenomedullin (AM), a novel vasoactive peptide, combined with AM binding protein-1 (AMBP-1), which augments the activity of AM, is beneficial in various disease conditions. However, it remains unknown whether human AM/AMBP-1 provides any beneficial effects in renal I/R injury. The objective of our study therefore was to determine whether administration of human AM/AMBP-1 can prevent and/or minimize damage in a rat model of renal I/R injury.
Methods
Male adult rats were subjected to renal I/R injury by bilateral renal pedicle clamping with microvascular clips for 60 min followed by reperfusion. Human AM (12 µg/kg BW) and human AMBP-1 (40 µg/kg BW) or vehicle (52 µg/kg BW human albumin) were given intravenously over 30 min immediately following the clip removal (i.e., reperfusion). Rats were allowed to recover for 24 h post treatment, and blood and renal tissue samples were collected. Plasma levels of AM were measured using a radioimmunoassay specific for rat AM. Plasma AMBP-1 was measured by Western analysis. Renal water content and serum levels of systemic markers of tissue injury were measured. Serum and renal TNF-α levels were also assessed.
Results
At 24 h after renal I/R injury, plasma levels of AM were significantly increased while plasma AMBP-1 was markedly decreased. Renal water content and systemic markers of tissue injury (e.g., creatinine, BUN, AST and ALT) were significantly increased following renal I/R injury. Serum and renal TNF-α levels were also increased post injury. Administration of human AM/AMBP-1 decreased renal water content, and plasma levels of creatinine, BUN, AST and ALT. Serum and renal TNF-α levels were also significantly decreased after AM/AMBP-1 treatment.
Conclusion
Treatment with human AM/AMBP-1 in renal I/R injury significantly attenuated organ injury and the inflammatory response. Thus, human AM combined with human AMBP-1 may be developed as a novel treatment for patients with acute renal I/R injury.
Keywords: Renal ischemia and reperfusion injury, adrenomedullin, adrenomedullin bindin protein, inflammation
INTRODUCTION
Acute renal injury induced by ischemia and reperfusion (I/R) is a major cause of morbidity and mortality in hospitalized patients. Acute renal injury is classified according to the RIFLE (acronym indicating Risk of renal dysfunction; Injury to the kidney; Failure of kidney function; Loss of kidney function and End-stage kidney disease) criteria, which subdivides the severity into 5 stages (1, 2). As the severity of injury increases, the window of opportunity for intervention becomes smaller. Causes for acute renal failure (ARF) secondary to I/R injury include trauma, sepsis, global hypoperfusion, and various surgical procedures notably open aortic bypass surgery. Acute renal failure can be sub-divided into two distinct categories: community acquired and hospital acquired. Even though the annual incidence of community-acquired ARF is approximately 100 cases per 1 million people, it is diagnosed in at least 1% of hospital admissions at presentation (3).
Hospital-acquired ARF, using the RIFLE classification, is more prevalent and has been found in 4–9% of hospital admissions (3–7). The incidence of hospital-acquired ARF has risen dramatically in the intensive care patients, where a rate of 7–17% has been observed (8, 9) and close to 50% of these cases are caused by renal I/R injury. Single center institutions report a rising trend in all-cause renal injury and case fatality rate has approached 50% among patients requiring dialysis (10–14). There are only a few strategies implemented to prevent or limit renal injury, which include fluid resuscitation, pharmacological interventions, or simply avoidance of the insulting factor. Diuretics and vasodilators are commonly used to treat ARF. However, in large randomized studies, these agents have failed to prove effective in various disease conditions. As such, there is an urgent need for developing effective strategies to combat renal I/R injury.
The pathophysiology of renal I/R injury is complex (15–19). Renal ischemia occurs when the blood flow into the renal tissue is interrupted. With an absence of blood into the tissue, a hypoxic state ensues and causes the local accumulation of anaerobic metabolites and free radicals. When blood flow is restored into the tissue, the majority of the damage occurs, which are mediated by oxygen free radicals, inflammatory mediators, and local cytokine activation. Histopathologically, there is extensive tubular damage, tubular cell necrosis, glomerular injury and tubular obstruction (16, 17). The eventual production of pro-inflammatory cytokine TNF-α has a direct cytotoxic effect on renal tissue, leading to cell necrosis and a continuation of the inflammatory cycle (17).
Adrenomedullin (AM), a 52-amino acid peptide with potent vasoactive properties, was originally isolated from a human pheochromocytoma in 1993 (20). It is widely distributed in the endocrine and neuroendocrine system (21), suggesting that AM plays an important role in the control of systemic and local circulation, as well as cardiovascular and fluid regulation, regulation of growth and differentiation, and secretions of other hormones (22). A specific binding protein to AM, adrenomedullin binding protein-1 (AMBP-1) was identified in human plasma and the purified protein was reported to be identical to human complement factor H (23).
Our recent studies show that AMBP-1 augments the biological activity of AM and produces significant beneficial effects under various pathophysiological conditions (24–27). We have shown that plasma AM levels are significantly increased in experimental models of organ injuries (24, 28) and that the vascular responsiveness to AM is decreased in these conditions (25, 29). Furthermore, the decrease in AMBP-1 levels are responsible for the decreased vascular responsiveness to AM and that the combined treatment of AM and AMBP-1 produces significant beneficial effects under these conditions (25). These initial studies were conducted with rat AM and human AMBP-1. Recently, we have also reported that combined treatment of human AM and human AMBP-1 reduced organ injury and inflammatory responses, and improved survival in rat models of hemorrhagic shock and gut ischemia/reperfusion injury (30, 31). In the current study, we examined whether administration of human AM combined with human AMBP-1 can minimize or prevent the damage induced by acute renal I/R injury in rats.
MATERIALS AND METHODS
Experimental animals
Male Sprague-Dawley rats (250–300g), purchased from Charles River Laboratories (Wilmington, MA), were used for this study. The rats were housed in a temperature controlled room and on a 12-h light/dark cycle. The rats were fed a standard Purina rat chow diet and allowed water ad libitum. Animal experimentation was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources). This project was approved by the Institutional Animal Care and Use Committee (IACUC) of the Feinstein Institute for Medical Research.
Animal model of renal I/R injury
Prior to surgery, rats were fasted overnight but water was given ad libitum. Rats were anesthetized with isoflurane inhalation maintained under anesthesia. Renal ischemia/reperfusion was performed as previously described (32, 33). Briefly, a midline laparotomy incision was made to expose the abdomen. The intestines were covered in warm, moist gauze and first retracted to the right to expose the left renal pedicle. A microvascular clamp was placed around the left renal pedicle, and visual inspection of the kidney was done to confirm blanching and cessation of blood flow. The intestines were mobilized to the left to expose the right renal pedicle, and a microvascular clamp was placed in the same manner. The small intestines were then returned into the abdominal cavity. The total clamp time was 60 min, after which the clamps were removed. The occlusion time of 60 min was chosen to closely parallel scenarios one encounters in the clinical setting, i.e. aortic cross-clamping during emergency surgery, where the clamping time will not be longer than 60 min. Restoration of blood flow into the kidneys was confirmed visually. The incision was closed in layers, and the animals were returned to their cages with food and water, and allowed to recover. At 24 h, the animals were euthanized and blood and tissue samples were harvested for analyses. Prior studies have shown that serum creatinine and BUN levels peaked at 24 h following renal I/R injury (18, 34). Due to the fact that these parameters indicate renal dysfunction, we chose to use 24 h time period in our studies.
Experimental groups
The following experimental groups were studied. Group 1, renal I/R rats treated with human AM and human AMBP-1 (n=8), underwent renal pedicle clamping for 60 min and immediately following removal of the microvascular clamps, received human AM (12 µg/kg BW, Phoenix Pharmaceuticals, Belmont, CA) plus human AMBP-1 (40 µg/kg BW) in 1 ml normal saline. Human AMBP-1 (purity >99%) was purified from normal human serum by us (35) according to a published method (36) with some modifications. Since AM is a potent vasodilator, infusion of AM/AMBP-1 was done over 30 min to prevent any increase in vasodilation which can lead to hypotension. The dosage of AM/AMBP-1 used was similar to that was utilized previously in a rat model of sepsis (26). Group 2, renal I/R rats treated with vehicle (n=8), underwent renal pedicle clamping for 60 min followed by removal, and received intravenous injection of human albumin (52 µg/kg BW) for a period of 30 min in 1 ml normal saline. Group 3, sham operated animals (n=8), underwent a midline laparatomy incision and kidneys were isolated, but neither clamping nor infusion was performed.
Determination of plasma levels of AM
Plasma AM levels were assayed using a radioimmunoassay (RIA) kit specific for AM according to the protocols provided by the manufacturer (Peninsula Labs, Belmont, CA). Briefly, 1.5 ml blood was collected into a polypropylene tube containing 1mg/ml EDTA and 500 KIU/ml aprotinin at 24 h after reperfusion, and plasma was separated immediately. The plasma was then used for AM extraction by C18 Sep-Column. RIA was performed as described previously (37) and AM levels were calculated.
Determination of plasma levels of AMBP-1
Two microliters of plasma was fractionated on a 4–12% Bis-Tris gel and then transferred to a 0.2-µm nitrocellulose membrane. Nitrocellulose blots were blocked by incubation in TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween-20) containing 5% milk for 1 h. Blots were then incubated with rabbit anti-human complement factor H polyclonal antibodies (1:5000, Quidel Corp, San Diego, CA) overnight at 4°C. The blots were then washed 3 times with TBST for 15 min, incubated with horseradish peroxidase-linked anti-rabbit immunoglobulin G for 1 hour at room temperature, and then washed 4 times in TBST for 10 min each. A chemiluminescent peroxidase substrate (ECL, Amersham Biosciences, Piscataway, NJ) was applied according to the manufacturer’s instructions, and the membranes were exposed briefly to radiography film. The levels of AMBP-1 in band densities were determined using a Bio-Rad Laboratories Imaging System (Hercules, CA).
Determination of renal water content
The difference in water content in the kidneys was determined by the difference in the weight of the kidneys after 72 h of desiccation in 70°C from the initial weight, divided by the initial weight and the results are expressed as percentage.
Determination of serum levels of organ injury markers
Blood samples were centrifuged for 15 min at 2000 g to collect serum, and stored at −8°C for determination of serum levels of creatinine, and blood urea nitrogen (BUN) (15), aspartate aminotransferase (AST), alanine aminotransferase (ALT). The levels were measured using commercially available assay kits according to manufacturer’s specifications (Pointe Scientific, Canton, MI).
Determination of serum and renal tissue levels of TNF-α
The concentration of TNF-α in the serum and renal tissue samples was measured using a commercially obtained enzyme-linked immunosorbent assay (ELISA) kit specifically for rat TNF-α (BD Biosciences, San Jose, CA). Renal tissue samples were thoroughly homogenized in lysis buffer (10 mM Tris-HCl, pH 7.5, 100 mM NaCl, 50 mM EDTA, 50 mM EGTA,1% Triton-X-100 with protease inhibitors), and the supernatant was used for tissue analysis.
Statistical analysis
All data are expressed as means ± SEM and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls (SNK) method for multiple group analyses or Student’s t-test for two-group analyses. Differences in value were considered significant when P<0.05.
RESULTS
Alterations in serum AM and AMBP-1 levels after renal I/R injury
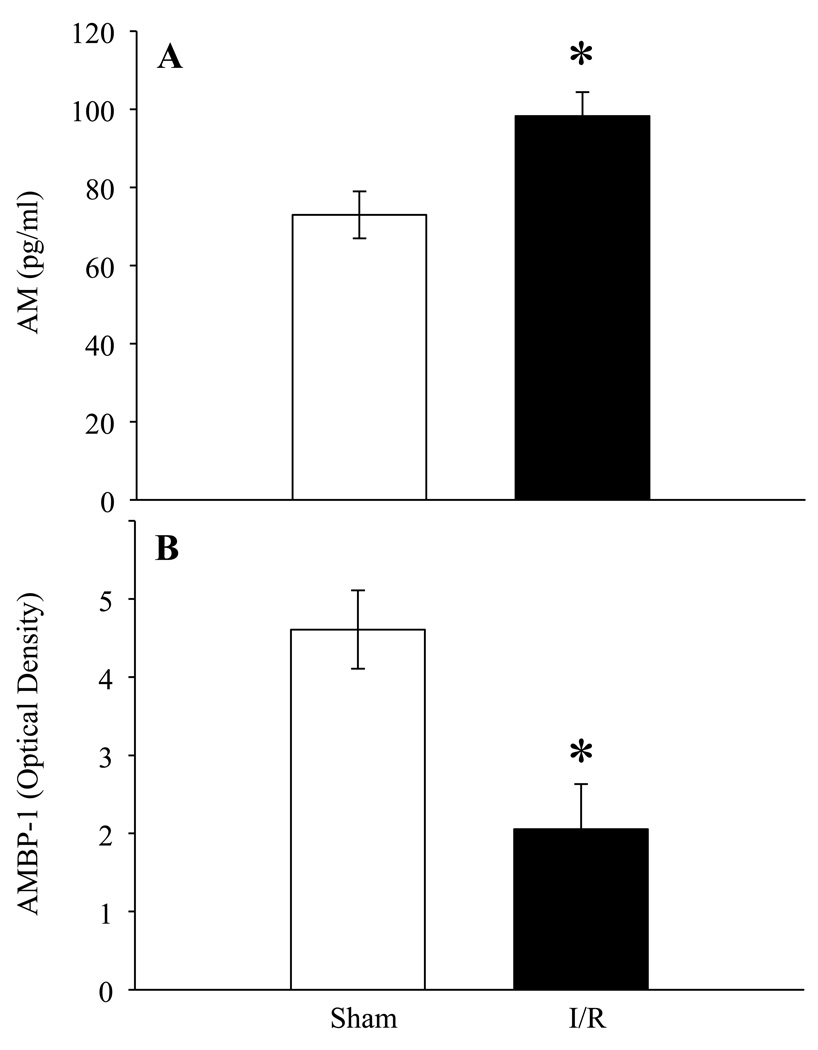
To determine if AM and AMBP-1 levels were altered in renal I/R injury, serum samples from sham and renal I/R rats were examined for AM and AMBP-1 levels. At 24 h after renal I/R injury, AM levels were significantly increased as compared to sham operated rats (Fig. 1A) whereas, as shown in Fig. 1B, rats subjected to renal I/R injury had a decrease in serum AMBP-1 by 54% as compared to sham operated animals (P <0.05).
Figure 1. Alterations in serum levels of AM and AMBP-1 after renal I/R injury.
A. Plasma samples from sham and renal I/R rats at 24 h post injury were assessed for AM using specific RIA kit. Results are shown as pg/ml estimated from known standards (n=6). B. Plasma samples were subjected to Western blotting using human anti-AMBP-1 antibody. Results are shown as arbitrary densitometric units (n=4). Data are presented as means ± SE and compared by Student’s t- test: *P < 0.05 versus Sham group.
Human AM/AMBP-1 reduces renal water content after renal I/R injury
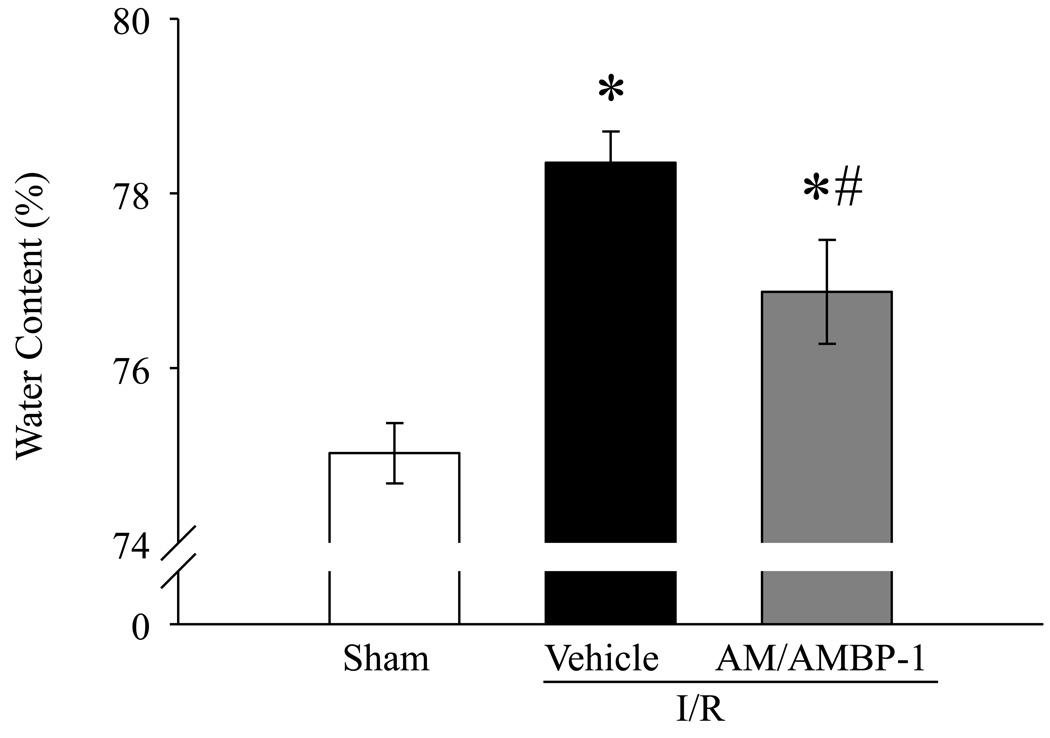
Tissue water content is a well recognized parameter to assess organ injury. As indicated in Fig. 2, rats subjected to renal I/R injury had a significant increase in renal water content from 75.0 ± 0.3% in sham-operated animals to 78.4 ± 0.4% in vehicle treated animals. Human AM/AMBP-1 treatment decreased the renal water content to 76.9 ± 0.6% in renal I/R injured rats (P <0.05). Although the difference in renal water content in the treatment group is statistically significant from that of the vehicle group, such a difference is probably nonsignificant in a clinical standpoint.
Figure 2. Alterations in renal water content after renal I/R injury.
Kidneys from sham and renal I/R rats (vehicle or human AM/AMBP-1 treatment) were collected at 24 h post I/R injury. Data are presented as means ± SE (n=7–8) and compared by one-way analysis of variance (ANOVA) and Student–Newman–Keuls method: *P < 0.05 versus Sham group, #P < 0.05 versus Vehicle group.
Human AM/AMBP-1 improves renal function after renal I/R injury
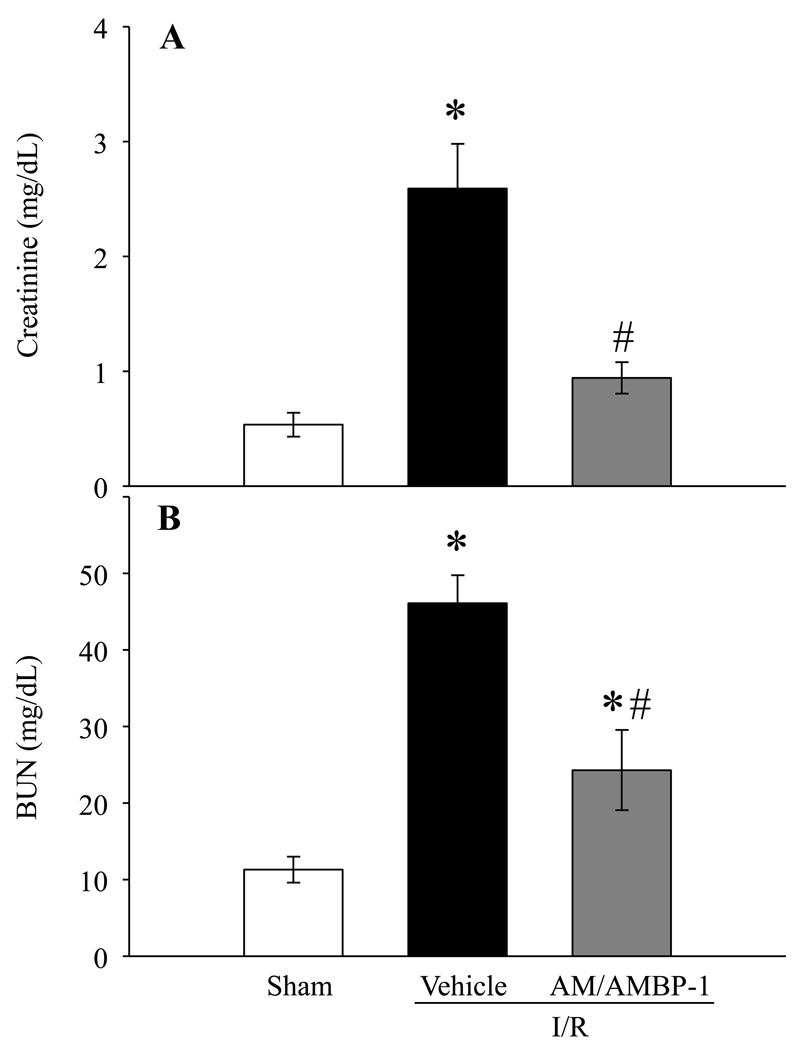
Serum levels of creatinine and BUN are considered as kidney specific markers of injury. Serum creatinine and BUN were significantly increased at 24 h after renal I/R injury in vehicle treated animals by 420% and 308%, respectively. Administration of human AM/AMBP-1 after renal I/R injury markedly improved renal function by decreasing serum creatinine and BUN levels by 64% and 47%, respectively (Figs. 3A and 3B, P < 0.05).
Figure 3. Alterations in serum levels of renal injury markers after renal I/R injury.
Serum samples from sham and renal I/R rats (vehicle or human AM/AMBP-1 treatment) at 24 h post I/R injury were assessed for creatinine (A) and BUN (B). Data are presented as means ± SE (n=5–7) and compared by one-way analysis of variance (ANOVA) and Student–Newman–Keuls method: *P < 0.05 versus Sham group, #P < 0.05 versus Vehicle group.
Human AM/AMBP-1 attenuates organ injury after renal I/R injury
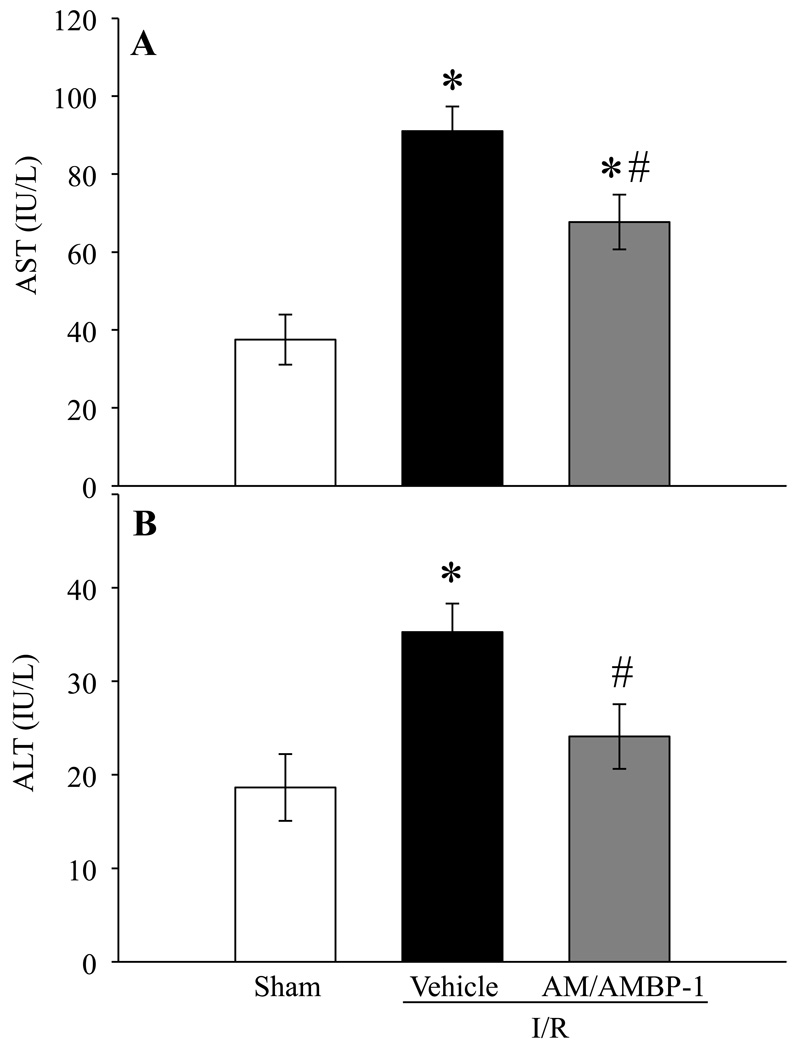
In addition to the kidney specific injury indicators, the effect of AM/AMBP-1 on systemic injury markers such as liver enzymes, serum AST and ALT, on renal I/R injury were also assessed. Serum AST and ALT levels increased by 143% and 89% after renal I/R injury, respectively (Figs. 4A–B). Administration of human AM/AMBP-1 in the injured rats significantly reduced AST and ALT levels in the serum by 26% and 32%, respectively (P < 0.05).
Figure 4. Alterations in serum levels of systemic injury indicators after renal I/R injury.
Serum samples from sham and renal I/R rats (vehicle or human AM/AMBP-1 treatment) at 24 h post I/R injury were measured for AST (A) and ALT (B). Data are presented as means ± SE (n=5–6) and compared by one-way analysis of variance (ANOVA) and Student–Newman–Keuls method: *P < 0.05 versus Sham group, #P < 0.05 versus Vehicle group.
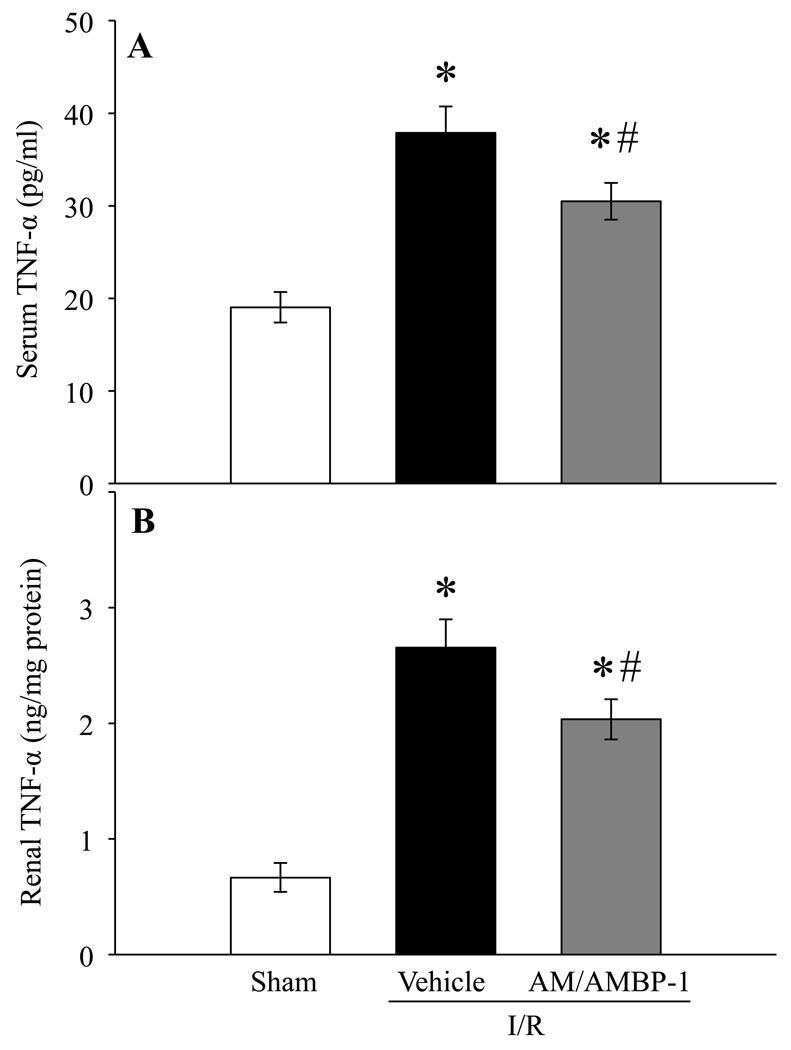
Human AM/AMBP-1 inhibits TNF-α after renal I/R injury
To further determine if treatment with AM/AMBP-1 is effective in downregulating pro-inflammatory cytokines generally increased in renal I/R injury, serum and renal content of TNF-α were measured. As indicated in Fig. 5A, serum TNF- α levels were increased by 99% in vehicle treated animals at 24 h post renal I/R injury and reduced by 20% following AM/AMBP-1 treatment (P < 0.05). Similarly, the TNF-α protein in the kidney increased by 297% and treatment with human AM/AMBP-1 reduced these levels by 23% (P < 0.05).
Figure 5. Alterations in TNF-α levels after renal I/R injury.
TNF-α from serum (A) and renal tissue (B) samples of sham and renal I/R rats (vehicle or human AM/AMBP-1 treatment) at 24 h post I/R injury were measured. Data are presented as means ± SE (n=6–8) and compared by one-way analysis of variance (ANOVA) and Student–Newman–Keuls method: *P < 0.05 versus Sham group, #P < 0.05 versus Vehicle group.
DISCUSSION
Recent advances in medical, surgical, and pharmacological interventions have improved the outcome in patients suffering from renal injury. Despite these improvements, however, ARF continues to pose a major physical and financial burden on the U.S. healthcare industry. Treatment for ARF is dependent on the cause whether it is due to pre-renal, renal, or post-renal failure. Pre-renal failure is generally caused by a low flow or hypotensive state, when fluid resuscitation and possible inotropic intervention is needed to restore flow. Another cause of pre-renal failure is from shock and/or sepsis, causing damage to the kidneys secondary to a low-flow state and damage secondary to free radical production, inflammation, and local macrophage/neutrophil migration. Currently, only supportive measures are the treatment options exist for patients with ARF. Therefore, it is obvious that a specific and effective treatment is urgently needed to prevent or at least minimize the mortality in patients with ARF.
Adrenomedullin has been shown to increase in ischemia/reperfusion injury, hemorrhagic shock, sepsis and following major surgeries and hypoxia (38–42). In vivo, the primary function of AM is to produce long-lasting effects in lowering of blood pressure, along with reduced peripheral vascular resistance in a relatively short time (20, 43). Previously we have demonstrated that AMBP-1, a specific binding protein for AM that potentiates its effects, is able to enhance AM-induced relaxation of aortic rings taken from normal animals (29). Additionally, a decreased level of AMBP-1 in humans is associated with higher susceptibility to recurrent infections (21, 44). We have also shown that the decreased level of AMBP-1 in animal studies leads to reduced vascular responsiveness in AM, thus contributing to the vascular collapse after hemorrhagic shock and severe sepsis (25, 26). In the present study, we have measured the level of AM and AMBP-1 in the serum of rats 24 h following renal I/R injury. Our results showed a significant increase in serum AM levels and a marked decrease in the amount of circulating AMBP-1 in these animals as compared to sham. This indicated the deficiency of AMBP-1 in renal I/R injury which compromises the bioactivity of AM and provided the basis for a combined intervention with human AM and human AMBP-1.
Based on this observation, we treated renal I/R injured rats with human AM and human AMBP-1. Our results indicated that AM/AMBP-1 treatment significantly reduced renal edema, organ injury and inflammatory responses indicating that AM/AMBP-1 can be beneficial in renal I/R injury. Previously we have shown that rat AM in combination with human AMBP-1 attenuates organ injury and inflammatory responses produced by various conditions such as severe sepsis and hemorrhagic shock (25, 26). However, the current study is the first to demonstrate beneficial effect of human AM in combination with human AMBP-1 in renal I/R injured rats. Furthermore, our study show that a low dose of human AM, which do not produce significant cardiovascular side effects such as hypotension and do not have any beneficial effect (24, 45–47), produced significant decrease in organ injury and inflammatory responses when used in combination with human AMBP-1. In agreement with these findings, low-dose rat AM combined with human AMBP-1 produces beneficial effects in various disease conditions (24–27, 46). In contrast, treatment with rat AM or human AMBP-1 alone in these models of organ injury failed to produce a significant protection (26, 46). In our future studies, we will determine the optimal dosage of human AM/AMBP-1 in producing beneficial effect in the renal I/R injury model.
Besides its vasodilative properties, we (28, 47) and others (48–52) have shown that AM possesses anti-inflammatory properties. Studies indicate that AM suppresses secretion of TNF-α from murine RAW264.7 cells stimulated with endotoxin (53). Our recent studies indicate that while AM alone suppressed TNF-α release from Kupffer cells by 52%, combined treatment of AM/AMBP-1 decreased these levels by 90% (47). Others have shown that AM regulates chemokines such as MCP-1 expression (54) and that it is able to inhibit neutrophil activation by suppressing formyl-Met-Leu-Phe (fMLP) induced up-regulation of the adhesion molecule CD11b in human neutrophils (55).
How AM/AMBP-1 exerts its beneficial effect in renal I/R injury remains to be determined. The binding between AM and AMBP-1 has important physiological consequences. The presence of a binding protein can alter the biological function of a potent factor and determines its inhibitory or stimulatory capabilities. In the case of AM, AMBP-1 may not change the affinity of AM to its receptors rather it may bind to cell surface adhesion molecules and bring AM near to its receptors and raise the efficacy of AM (23, 56). As a result, AMBP-1 may effectively increase AM’s potency without modifying its receptor or its binding capacities. Another possibility is that since AMBP-1 is known to prevent degradation of AM (57), AM/AMBP-1 binding can make AM more functionally effective.
Although the primary function of AM is to lower blood pressure and reduce vascular resistance, these effects may not translate to renoprotective effects seen in our combined human AM and human AMBP-1 treated animals. In addition to its vasoactive properties, a number of studies including ours indicated that AM has anti-inflammatory properties (28, 47–52). The current study also showed that human AM/AMBP-1 treatment in renal I/R injury reduced inflammatory responses and organ injury generally observed in renal I/R injury. Therefore, the observed protection of the renal parenchyma post I/R injury following human AM/AMBP-1 treatment could be due to AM’s role as an anti-inflammatory agent. In this regard, we have recently shown that the protective role of AM/AMBP-1 in sepsis could be mediated by cAMP-dependent pathway and by the induction of peroxisome proliferator-activated receptor-γ (PPAR-γ) through Pyk-2-tyrosine kinase-ERK pathway (59). It is plausible that such pathways are involved in AM/AMBP-1’s anti-inflammatory properties in renal I/R injury. Future studies are warranted for such conclusions.
Due to the complexity and severity of renal I/R injury, there is an obvious need for the development of novel treatments to prevent and/or minimize the injury. Since the pathophysiology of renal I/R injury constitutes oxidative stress, inflammation and apoptosis, therapy should be directed against all aspects of the pathology. In this regard, generation of oxygen radicals play an essential role in the pathogenesis of renal I/R injury. Studies indicate that leflunomide, a novel immunomodulatory drug for the treatment of rheumatoid arthritis, provides renoprotective effects by its radical scavenging and antioxidant activities (58). Future studies will determine whether AM/AMBP-1 is able to protect against oxygen radical production and apoptosis in renal I/R injury.
To date, AM/AMBP-1 has not been used in human trials. All our prior studies on the beneficial effects of AM/AMBP-1 have been done solely in animal models of injury (i.e., preclinical trials). Nevertheless, our previous studies in other models of organ injuries clearly showed that AM/AMBP-1 produce beneficial effect in various pathophysiological conditions (25, 26). In the present study, we show that the administration of human AM combined with human AMBP-1 attenuated renal edema, organ injury, and inflammatory responses associated with renal I/R injury and suggest AM/AMBP-1 as a novel treatment that holds promise for the treatment of renal I/R injury.
ACKNOWLEDGMENTS
This study was partially supported by the National Institutes of Health grants, R01 GM057468 and R01 HL076179 (PW).
REFERENCES
- 1.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 4.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 5.Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 6.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 7.Palevsky PM. Epidemiology of acute renal failure: the tip of the iceberg. Clin J Am Soc Nephrol. 2006;1:6. doi: 10.2215/CJN.01521005. [DOI] [PubMed] [Google Scholar]
- 8.Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- 9.Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 10.Chertow GM, Lazarus JM, Christiansen CL, Cook EF, Hammermeister KE, Grover F, Daley J. Preoperative renal risk stratification. Circulation. 1997;95:878. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 11.Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP. Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int. 2002;62:986. doi: 10.1046/j.1523-1755.2002.00509.x. [DOI] [PubMed] [Google Scholar]
- 12.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. Jama. 1996;275:1489. [PubMed] [Google Scholar]
- 13.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 14.Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le Gall JR, Druml W. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Bhalodia Y, Kanzariya N, Patel R, Patel N, Vaghasiya J, Jivani N, Raval H. Renoprotective activity of benincasa cerifera fruit extract on ischemia/reperfusion-induced renal damage in rat. Iran J Kidney Dis. 2009;3:80. [PubMed] [Google Scholar]
- 16.Finn WF. Nephron heterogeneity in polyuric acute renal failure. J Lab Clin Med. 1981;98:21. [PubMed] [Google Scholar]
- 17.Chatterjee PK, Cuzzocrea S, Thiemermann C. Inhibitors of poly (ADP-ribose) synthetase protect rat proximal tubular cells against oxidant stress. Kidney Int. 1999;56:973. doi: 10.1046/j.1523-1755.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- 18.Yeboah MM, Xue X, Duan B, Ochani M, Tracey KJ, Susin M, Metz CN. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 2008;74:62. doi: 10.1038/ki.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeboah MM, Xue X, Javdan M, Susin M, Metz CN. Nicotinic acetylcholine receptor expression and regulation in the rat kidney after ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2008;295:F654. doi: 10.1152/ajprenal.90255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 21.Pearson LJ, Rait C, Nicholls MG, Yandle TG, Evans JJ. Regulation of adrenomedullin release from human endothelial cells by sex steroids and angiotensin-II. J Endocrinol. 2006;191:171. doi: 10.1677/joe.1.06815. [DOI] [PubMed] [Google Scholar]
- 22.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 23.Pio R, Martinez A, Unsworth EJ, Kowalak JA, Bengoechea JA, Zipfel PF, Elsasser TH, Cuttitta F. Complement factor H is a serum-binding protein for adrenomedullin, and the resulting complex modulates the bioactivities of both partners. J Biol Chem. 2001;276:12292. doi: 10.1074/jbc.M007822200. [DOI] [PubMed] [Google Scholar]
- 24.Carrizo GJ, Wu R, Cui X, Dwivedi AJ, Simms HH, Wang P. Adrenomedullin and adrenomedullin-binding protein-1 downregulate inflammatory cytokines and attenuate tissue injury after gut ischemia-reperfusion. Surgery. 2007;141:245. doi: 10.1016/j.surg.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Wu R, Cui X, Dong W, Zhou M, Simms HH, Wang P. Mechanisms responsible for vascular hyporesponsiveness to adrenomedullin after hemorrhage: the central role of adrenomedullin binding protein-1. Ann Surg. 2005;242:115. doi: 10.1097/01.sla.0000167849.10599.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Zhou M, Chaudry IH, Wang P. Novel approach to prevent the transition from the hyperdynamic phase to the hypodynamic phase of sepsis: role of adrenomedullin and adrenomedullin binding protein-1. Ann Surg. 2002;236:625. doi: 10.1097/00000658-200211000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou M, Simms HH, Wang P. Adrenomedullin and adrenomedullin binding protein-1 attenuate vascular endothelial cell apoptosis in sepsis. Ann Surg. 2004;240:321. doi: 10.1097/01.sla.0000133253.45591.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang S, Zhou M, Fowler DE, Wang P. Mechanisms of the beneficial effect of adrenomedullin and adrenomedullin-binding protein-1 in sepsis: down-regulation of proinflammatory cytokines. Crit Care Med. 2002;30:2729. doi: 10.1097/00003246-200212000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, Ba ZF, Chaudry IH, Wang P. Adrenomedullin binding protein-1 modulates vascular responsiveness to adrenomedullin in late sepsis. Am J Physiol Regul Integr Comp Physiol. 2002;283:R553. doi: 10.1152/ajpregu.00544.2001. [DOI] [PubMed] [Google Scholar]
- 30.Wu R, Dong W, Qiang X, Ji Y, Cui T, Yang J, Zhou M, Blau S, Marini CP, Ravikumar TS, Wang P. Human vasoactive hormone adrenomedullin and its binding protein rescue experimental animals from shock. Peptides. 2008;29:1223. doi: 10.1016/j.peptides.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Wu R, Zhou M, Blau SA, Wang P. Human adrenomedullin combined with human adrenomedullin binding protein-1 is protective in gut ischemia and reperfusion injury in the rat. Regul Pept. 2009;152:82. doi: 10.1016/j.regpep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onem Y, Ipcioglu OM, Haholu A, Sen H, Aydinoz S, Suleymanoglu S, Bilgi O, Akyol I. Posttreatment with aminoguanidine attenuates renal ischemia/reperfusion injury in rats. Ren Fail. 2009;31:50. doi: 10.1080/08860220802546313. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez V, Trujillo J, Valdes R, Uribe N, Cruz C, Gamba G, Bobadilla NA. Adrenalectomy prevents renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2009;297:F932. doi: 10.1152/ajprenal.00252.2009. [DOI] [PubMed] [Google Scholar]
- 34.Choi DE, Jeong JY, Lim BJ, Chung S, Chang YK, Lee SJ, Na KR, Kim SY, Shin YT, Lee KW. Pretreatment of sildenafil attenuates ischemia-reperfusion renal injury in rats. Am J Physiol Renal Physiol. 2009;297:F362. doi: 10.1152/ajprenal.90609.2008. [DOI] [PubMed] [Google Scholar]
- 35.Qiang X, Wu R, Ji Y, Zhou M, Wang P. Purification and characterization of human adrenomedullin binding protein-1. Mol Med. 2008;14:443. doi: 10.2119/2008-00015.Qiang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sim RB, DiScipio RG. Purification and structural studies on the complement-system control protein beta 1H (Factor H) Biochem J. 1982;205:285. doi: 10.1042/bj2050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Wu R, Qiang X, Zhou M, Dong W, Ji Y, Marini CP, Ravikumar TS, Wang P. Human adrenomedullin and its binding protein attenuate organ injury and reduce mortality after hepatic ischemia-reperfusion. Ann Surg. 2009;249:310. doi: 10.1097/SLA.0b013e3181961d43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cejudo-Martin P, Morales-Ruiz M, Ros J, Navasa M, Fernandez-Varo G, Fuster J, Rivera F, Arroyo V, Rodes J, Jimenez W. Hypoxia is an inducer of vasodilator agents in peritoneal macrophages of cirrhotic patients. Hepatology. 2002;36:1172. doi: 10.1053/jhep.2002.36371. [DOI] [PubMed] [Google Scholar]
- 39.Ehlenz K, Koch B, Preuss P, Simon B, Koop I, Lang RE. High levels of circulating adrenomedullin in severe illness: correlation with C-reactive protein and evidence against the adrenal medulla as site of origin. Exp Clin Endocrinol Diabetes. 1997;105:156. doi: 10.1055/s-0029-1211745. [DOI] [PubMed] [Google Scholar]
- 40.Fujioka S. Increased plasma concentration of adrenomedullin during and after major surgery. Surg Today. 2001;31:575. doi: 10.1007/s005950170089. [DOI] [PubMed] [Google Scholar]
- 41.Tarui S, Tokunaga K, Fujioka S, Matsuzawa Y. Visceral fat obesity: anthropological and pathophysiological aspects. Int J Obes. 1991;15 Suppl 2:1. [PubMed] [Google Scholar]
- 42.Trollmann R, Schoof E, Beinder E, Wenzel D, Rascher W, Dotsch J. Adrenomedullin gene expression in human placental tIssue and leukocytes: a potential marker of severe tIssue hypoxia in neonates with birth asphyxia. Eur J Endocrinol. 2002;147:711. doi: 10.1530/eje.0.1470711. [DOI] [PubMed] [Google Scholar]
- 43.Ishiyama Y, Kitamura K, Ichiki Y, Nakamura S, Kida O, Kangawa K, Eto T. Hemodynamic effects of a novel hypotensive peptide, human adrenomedullin, in rats. Eur J Pharmacol. 1993;241:271. doi: 10.1016/0014-2999(93)90214-3. [DOI] [PubMed] [Google Scholar]
- 44.Naked GM, Florido MP, Ferreira de Paula P, Vinet AM, Inostroza JS, Isaac L. Deficiency of human complement factor I associated with lowered factor H. Clin Immunol. 2000;96:162. doi: 10.1006/clim.2000.4878. [DOI] [PubMed] [Google Scholar]
- 45.Cui X, Wu R, Zhou M, Dong W, Ulloa L, Yang H, Wang H, Tracey KJ, Simms HH, Wang P. Adrenomedullin and its binding protein attenuate the proinflammatory response after hemorrhage. Crit Care Med. 2005;33:391. doi: 10.1097/01.ccm.0000153416.41398.a9. [DOI] [PubMed] [Google Scholar]
- 46.Wu R, Dong W, Zhou M, Cui X, Simms HH, Wang P. A novel approach to maintaining cardiovascular stability after hemorrhagic shock: beneficial effects of adrenomedullin and its binding protein. Surgery. 2005;137:200. doi: 10.1016/j.surg.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Wu R, Zhou M, Wang P. Adrenomedullin and adrenomedullin binding protein-1 downregulate TNF-alpha in macrophage cell line and rat Kupffer cells. Regul Pept. 2003;112:19. doi: 10.1016/s0167-0115(03)00018-1. [DOI] [PubMed] [Google Scholar]
- 48.Chini EN, Chini CC, Bolliger C, Jougasaki M, Grande JP, Burnett JC, Jr, Dousa TP. Cytoprotective effects of adrenomedullin in glomerular cell injury: central role of cAMP signaling pathway. Kidney Int. 1997;52:917. doi: 10.1038/ki.1997.413. [DOI] [PubMed] [Google Scholar]
- 49.Nakayama M, Takahashi K, Murakami O, Murakami H, Sasano H, Shirato K, Shibahara S. Adrenomedullin in monocytes and macrophages: possible involvement of macrophage-derived adrenomedullin in atherogenesis. Clin Sci (Lond) 1999;97:247. [PubMed] [Google Scholar]
- 50.Nishikimi T, Saito Y, Kitamura K, Ishimitsu T, Eto T, Kangawa K, Matsuo H, Omae T, Matsuoka H. Increased plasma levels of adrenomedullin in patients with heart failure. J Am Coll Cardiol. 1995;26:1424. doi: 10.1016/0735-1097(95)00338-X. [DOI] [PubMed] [Google Scholar]
- 51.Rademaker MT, Charles CJ, Lewis LK, Yandle TG, Cooper GJ, Coy DH, Richards AM, Nicholls MG. Beneficial hemodynamic and renal effects of adrenomedullin in an ovine model of heart failure. Circulation. 1997;96:1983. doi: 10.1161/01.cir.96.6.1983. [DOI] [PubMed] [Google Scholar]
- 52.Yoshibayashi M, Kamiya T, Nishikimi T, Saito Y, Matsuo H, Kangawa K. Elevated plasma levels of adrenomedullin in congenital cyanotic heart disease. Clin Sci (Lond) 1999;96:543. [PubMed] [Google Scholar]
- 53.Kubo A, Minamino N, Isumi Y, Katafuchi T, Kangawa K, Dohi K, Matsuo H. Production of adrenomedullin in macrophage cell line and peritoneal macrophage. J Biol Chem. 1998;273:16730. doi: 10.1074/jbc.273.27.16730. [DOI] [PubMed] [Google Scholar]
- 54.Iwamoto M, Osajima A, Tamura M, Suda T, Ota T, Kanegae K, Watanabe Y, Kabashima N, Anai H, Nakashima Y. Adrenomedullin inhibits pressure-induced mesangial MCP-1 expression through activation of protein kinase A. J Nephrol. 2003;16:673. [PubMed] [Google Scholar]
- 55.Gonzalez-Rey E, Chorny A, Varela N, Robledo G, Delgado M. Urocortin and adrenomedullin prevent lethal endotoxemia by down-regulating the inflammatory response. Am J Pathol. 2006;168:1921. doi: 10.2353/ajpath.2006.051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beltowski J, Jamroz A. Adrenomedullin--what do we know 10 years since its discovery? Pol J Pharmacol. 2004;56:5. [PubMed] [Google Scholar]
- 57.Martinez A, Oh HR, Unsworth EJ, Bregonzio C, Saavedra JM, Stetler-Stevenson WG, Cuttitta F. Matrix metalloproteinase-2 cleavage of adrenomedullin produces a vasoconstrictor out of a vasodilator. Biochem J. 2004;383:413. doi: 10.1042/BJ20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karaman A, Turkmen E, Gursul C, Tas E, Fadillioglu E. Prevention of renal ischemia/reperfusion-induced injury in rats by leflunomide. Int J Urol. 2006;13:1434. doi: 10.1111/j.1442-2042.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 59.Miksa M, Wu R, Cui X, Dong W, Das P, Simms HH, Ravikumar TS, Wang P. Vasoactive hormone adrenomedullin and its binding protein: anti-inflammatory effects by up-regulating peroxisome proliferator-activated receptor-gamma. J Immunol. 2007;179:6263. doi: 10.4049/jimmunol.179.9.6263. [DOI] [PubMed] [Google Scholar]