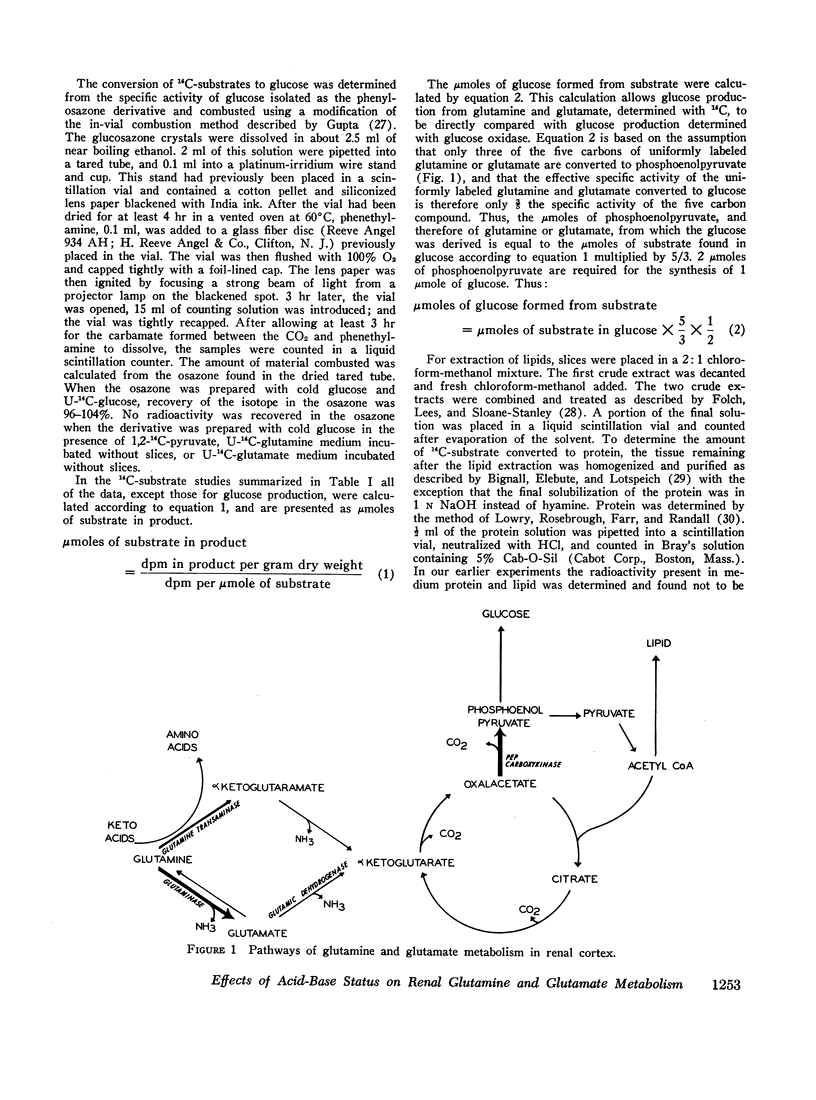
Abstract
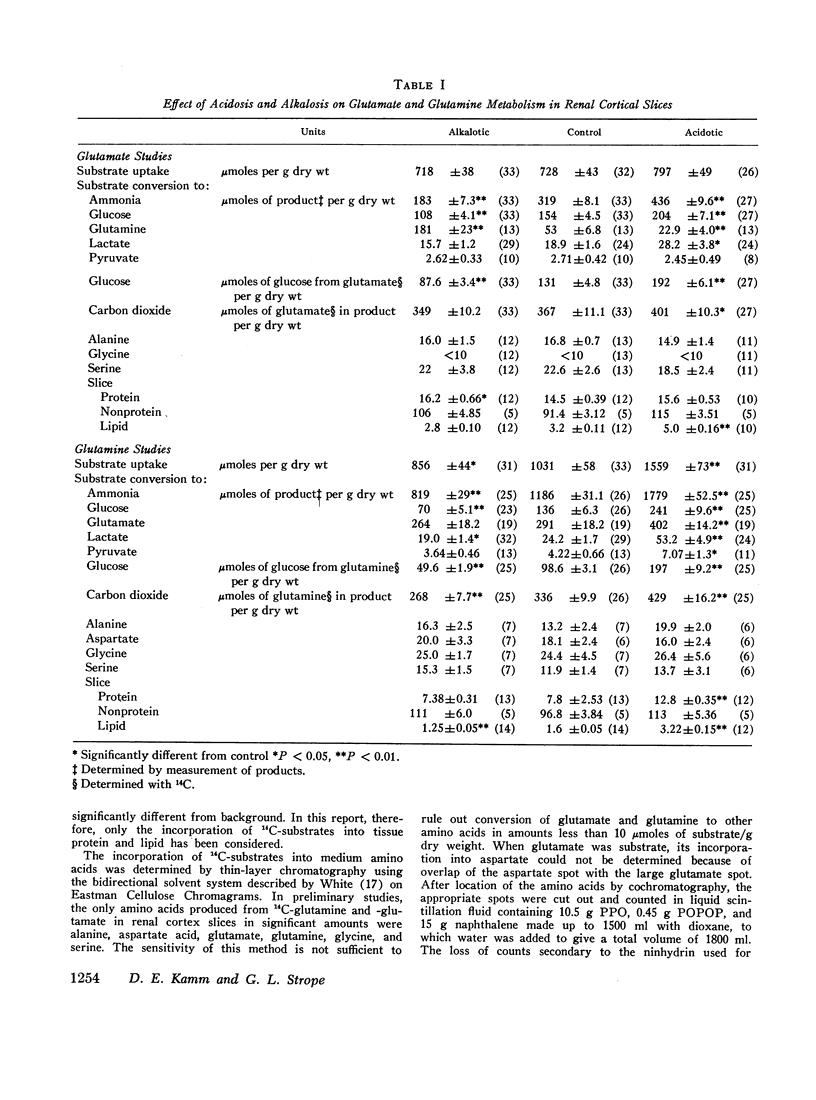
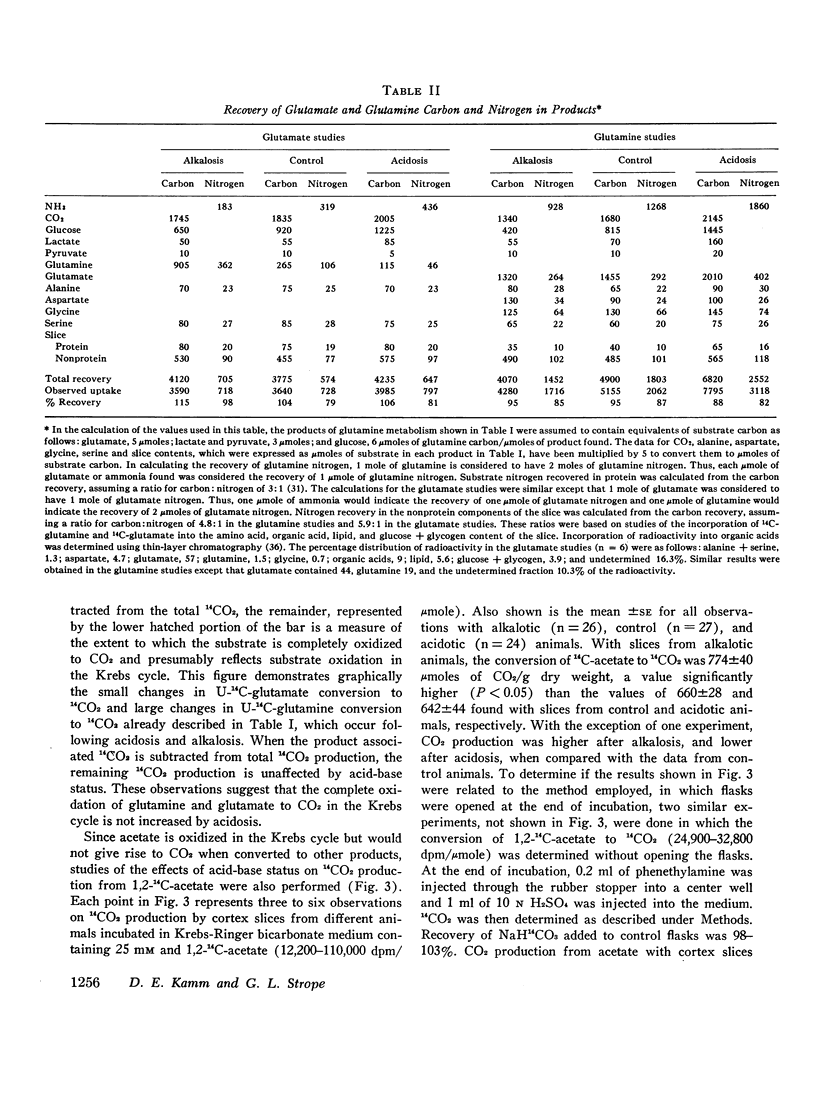
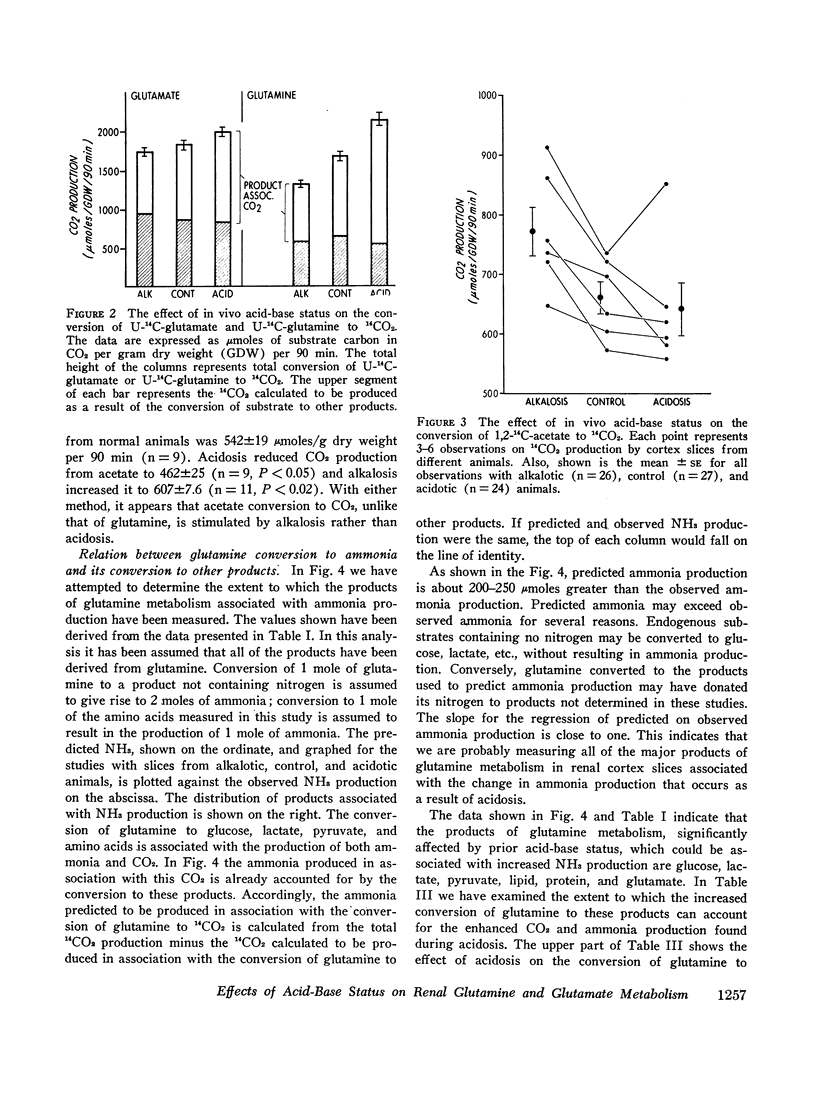
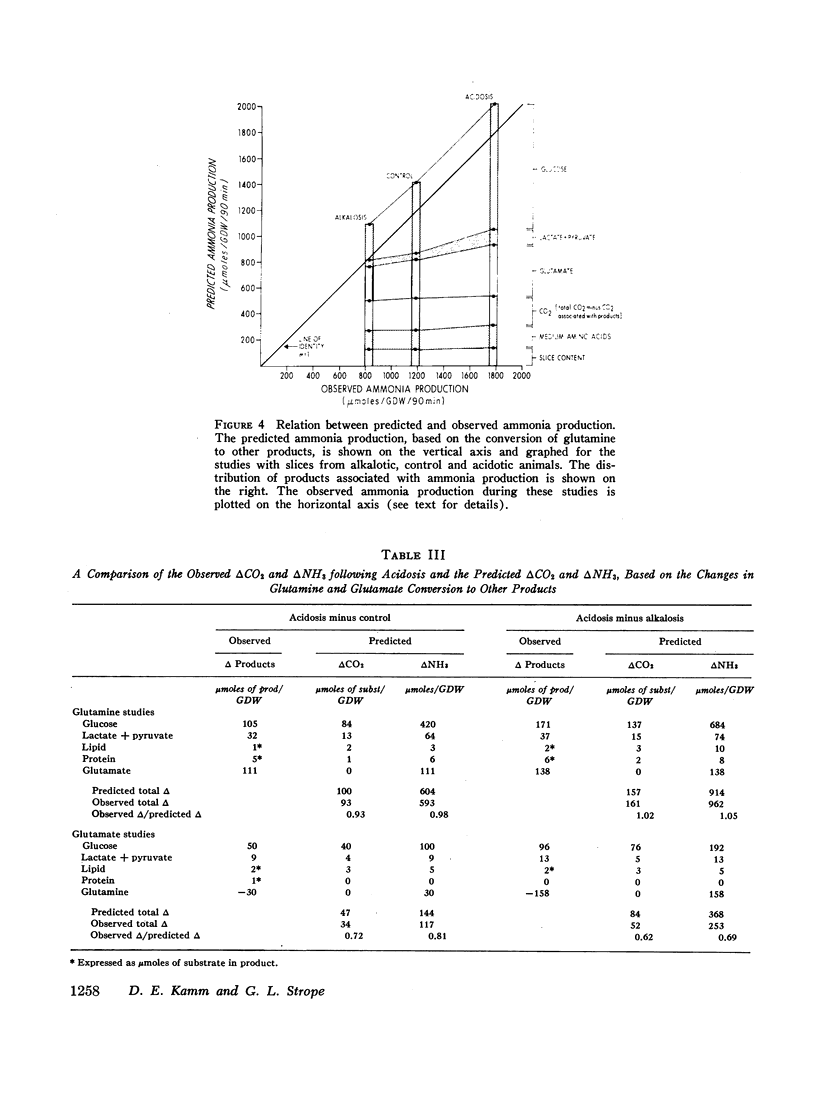
Studies of the metabolism of glutamine and glutamate by renal cortex slices from acidotic, alkalotic, and control rats were performed. 88-95% of the glutamine and 104-115% of the glutamate taken up from the medium could be accounted for by the products found. Acidosis increased glutamine uptake and conversion to ammonia, CO2, glucose, lactate, pyruvate, lipid, and protein. The increase in glutamine conversion to ammonia after acidosis could be completely accounted for by the associated increase in its conversion to glucose, glutamate, lactate, and pyruvate. When glutamate metabolism was examined, acidosis did not affect substrate uptake but did increase its conversion to ammonia, glucose, lactate, CO2, and lipid. The increase in 14CO2 from U-14C-glutamine and U-14C-glutamate found with cortex slices from acidotic animals could be explained by the CO2 production calculated to be associated with the enhanced conversion of these substrates to other products during acidosis. 14CO2 production from 1.2-14C-acetate was found to be significantly increased in alkalosis rather than acidosis. These studies suggest that in the rat, the rate at which glutamine is completely oxidized in the Krebs cycle is not a factor regulating renal ammonia production. A comparison of the effects of acidbase status on glutamine and glutamate metabolism suggests that either glutamine transport or glutamine transaminase activity are significantly increased by acidosis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addae S. K., Lotspeich W. D. Relation between glutamine utilization and production in metabolic acidosis. Am J Physiol. 1968 Aug;215(2):269–277. doi: 10.1152/ajplegacy.1968.215.2.269. [DOI] [PubMed] [Google Scholar]
- Alleyne G. A. Renal metabolic response to acid-base changes. II. The early effects of metabolic acidosis on renal metabolism in the rat. J Clin Invest. 1970 May;49(5):943–951. doi: 10.1172/JCI106314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleyne G. A., Scullard G. H. Renal metabolic response to acid base changes. I. Enzymatic control of ammoniagenesis in the rat. J Clin Invest. 1969 Feb;48(2):364–370. doi: 10.1172/JCI105993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignall M. C., Elebute O., Lotspeich W. D. Renal protein and ammonia biochemistry in NH4Cl acidosis and after uninephrectomy. Am J Physiol. 1968 Aug;215(2):289–295. doi: 10.1152/ajplegacy.1968.215.2.289. [DOI] [PubMed] [Google Scholar]
- COHEN J. J. High respiratory quotient of dog kidney in vivo. Am J Physiol. 1960 Sep;199:560–568. doi: 10.1152/ajplegacy.1960.199.3.560. [DOI] [PubMed] [Google Scholar]
- Churchill P. C., Malvin R. L. Relation of renal gluconeogenesis to ammonia production in the dog. Am J Physiol. 1970 Jan;218(1):241–245. doi: 10.1152/ajplegacy.1970.218.1.241. [DOI] [PubMed] [Google Scholar]
- Damian A. C., Pitts R. F. Rates of glutaminase I and glutamine synthetase reactions in rat kidney in vivo. Am J Physiol. 1970 May;218(5):1249–1255. doi: 10.1152/ajplegacy.1970.218.5.1249. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Goldstein L. Relation of glutamate to ammonia production in the rat kidney. Am J Physiol. 1966 Mar;210(3):661–666. doi: 10.1152/ajplegacy.1966.210.3.661. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorno W. E., Rector F. C., Jr, Seldin D. W. Relation of renal gluconeogenesis to ammonia production in the dog and rat. Am J Physiol. 1967 Oct;213(4):969–974. doi: 10.1152/ajplegacy.1967.213.4.969. [DOI] [PubMed] [Google Scholar]
- Graham L. T., Jr, Werman R., Aprison M. H. Microdetermination of glutamate in single cat spinal roots. Life Sci. 1965 May;4(10):1085–1090. doi: 10.1016/0024-3205(65)90228-6. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Kamm D. E., Asher R. R. Relation between glucose and ammonia production in renal cortical slices. Am J Physiol. 1970 Apr;218(4):1161–1165. doi: 10.1152/ajplegacy.1970.218.4.1161. [DOI] [PubMed] [Google Scholar]
- Kamm D. E., Cahill G. F., Jr Effect of acid-base status on renal and hepatic gluconeogenesis in diabetes and fasting. Am J Physiol. 1969 May;216(5):1207–1212. doi: 10.1152/ajplegacy.1969.216.5.1207. [DOI] [PubMed] [Google Scholar]
- Kamm D. E., Fuisz R. E., Goodman A. D., Cahill G. F., Jr Acid-base alterations and renal gluconeogenesis: effect of pH, bicarbonate concentration, and PCO2. J Clin Invest. 1967 Jul;46(7):1172–1177. doi: 10.1172/JCI105610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. Metabolism of amino-acids: The synthesis of glutamine from glutamic acid and ammonia, and the enzymic hydrolysis of glutamine in animal tissues. Biochem J. 1935 Aug;29(8):1951–1969. doi: 10.1042/bj0291951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lueck J. D., Miller L. L. The effect of perfusate pH on glutamine metabolism in the isolated perfused rat liver. J Biol Chem. 1970 Oct 25;245(20):5491–5497. [PubMed] [Google Scholar]
- Lyon M. L., Pitts R. F. Species differences in renal glutamine synthesis in vivo. Am J Physiol. 1969 Jan;216(1):117–122. doi: 10.1152/ajplegacy.1969.216.1.117. [DOI] [PubMed] [Google Scholar]
- Nagata N., Rasmussen H. Renal gluconeogenesis: effects of Ca2+ and H+. Biochim Biophys Acta. 1970 Jul 21;215(1):1–16. doi: 10.1016/0304-4165(70)90382-x. [DOI] [PubMed] [Google Scholar]
- OWEN E. E., ROBINSON R. R. Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride. J Clin Invest. 1963 Feb;42:263–276. doi: 10.1172/JCI104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliara A. S., Goodman A. D. Relation of renal cortical gluconeogenesis, glutamate content, and production of ammonia. J Clin Invest. 1970 Nov;49(11):1967–1974. doi: 10.1172/JCI106416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkington L. A., O'Donovan D. J. Metabolism of glutamine in cortex slices from dog kidney during acid-base alterations. Am J Physiol. 1971 Jun;220(6):1634–1639. doi: 10.1152/ajplegacy.1971.220.6.1634. [DOI] [PubMed] [Google Scholar]
- Preuss H. G. Ammonia production from glutamine and glutamate in isolated dog renal tubules. Am J Physiol. 1971 Jan;220(1):54–58. doi: 10.1152/ajplegacy.1971.220.1.54. [DOI] [PubMed] [Google Scholar]
- Preuss H. G. Pyridine nucleotides in renal ammonia metabolism. J Lab Clin Med. 1968 Sep;72(3):370–382. [PubMed] [Google Scholar]
- Preuss H. G. Renal glutamate metabolism in acute metabolic acidosis. Nephron. 1969;6(3):235–246. doi: 10.1159/000179731. [DOI] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, ORLOFF J. The effect of the administration of sodium bicarbonate and ammonium chloride on the excretion and production of ammonia; the absence of alterations in the activity of renal ammonia-producing enzymes in the dog. J Clin Invest. 1959 Feb;38(2):366–372. doi: 10.1172/JCI103810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, SELDIN D. W., COPENHAVER J. H. The mechanism of ammonia excretion during ammonium chloride acidosis. J Clin Invest. 1955 Jan;34(1):20–26. doi: 10.1172/JCI103058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. P., Sherrard D. J. Regulation of glutamine metabolism in vitro by bicarbonate ion and pH. J Clin Invest. 1969 Jun;48(6):1088–1096. doi: 10.1172/JCI106065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone W. J., Balagura S., Pitts R. F. Diffusion equilibrium for ammonia in the kidney of the acidotic dog. J Clin Invest. 1967 Oct;46(10):1603–1608. doi: 10.1172/JCI105652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone W. J., Pitts R. F. Pathways of ammonia metabolism in the intact functioning kidney of the dog. J Clin Invest. 1967 Jul;46(7):1141–1150. doi: 10.1172/JCI105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil-Malherbe H., Krebs H. A. Metabolism of amino-acids: The conversion of proline into glutamic acid in kidney. Biochem J. 1935 Sep;29(9):2077–2081. doi: 10.1042/bj0292077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whereat A. F., Snydman D. R., Barness L. A. Thin-layer chromatography of citric acid cycle intermediates, pyruvate and lactate. J Chromatogr. 1968 Aug 27;36(3):390–393. doi: 10.1016/s0021-9673(01)92968-1. [DOI] [PubMed] [Google Scholar]
- White H. H. Separation of amino acids in physiological fluids by two-dimensional thin-layer chromatography. Clin Chim Acta. 1968 Sep;21(3):297–302. doi: 10.1016/0009-8981(68)90059-4. [DOI] [PubMed] [Google Scholar]