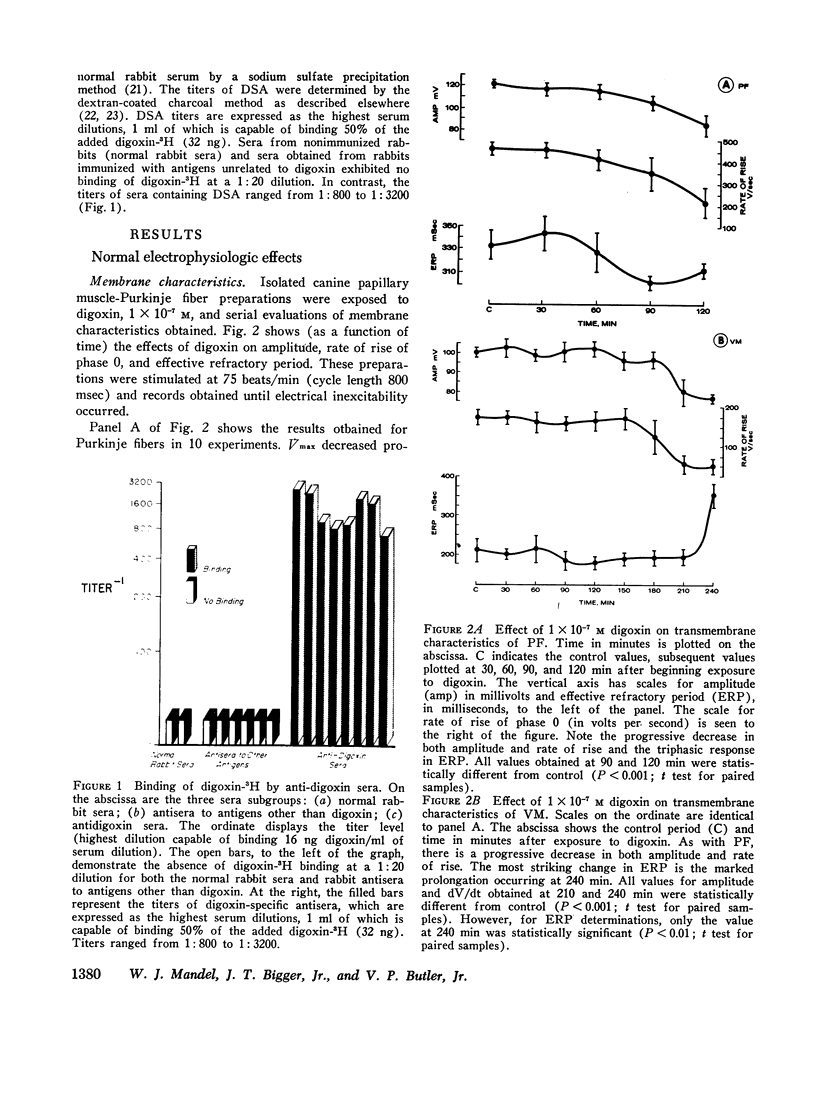
Abstract
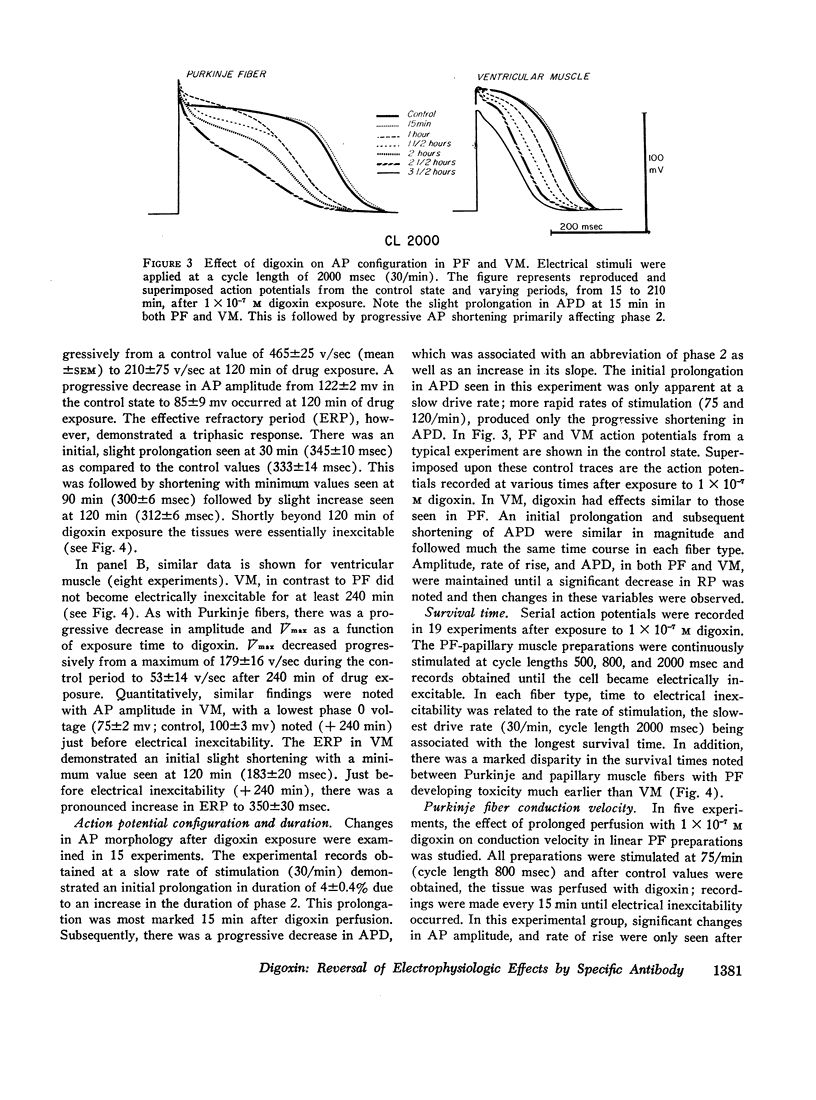
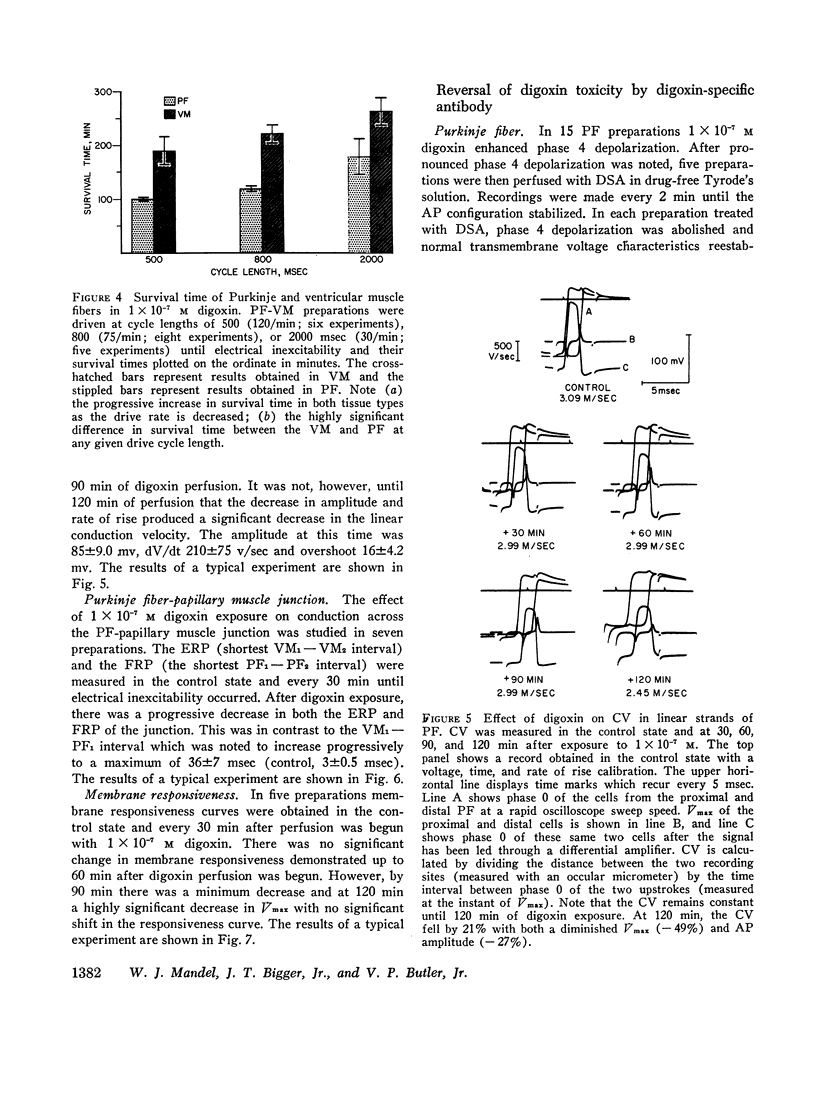
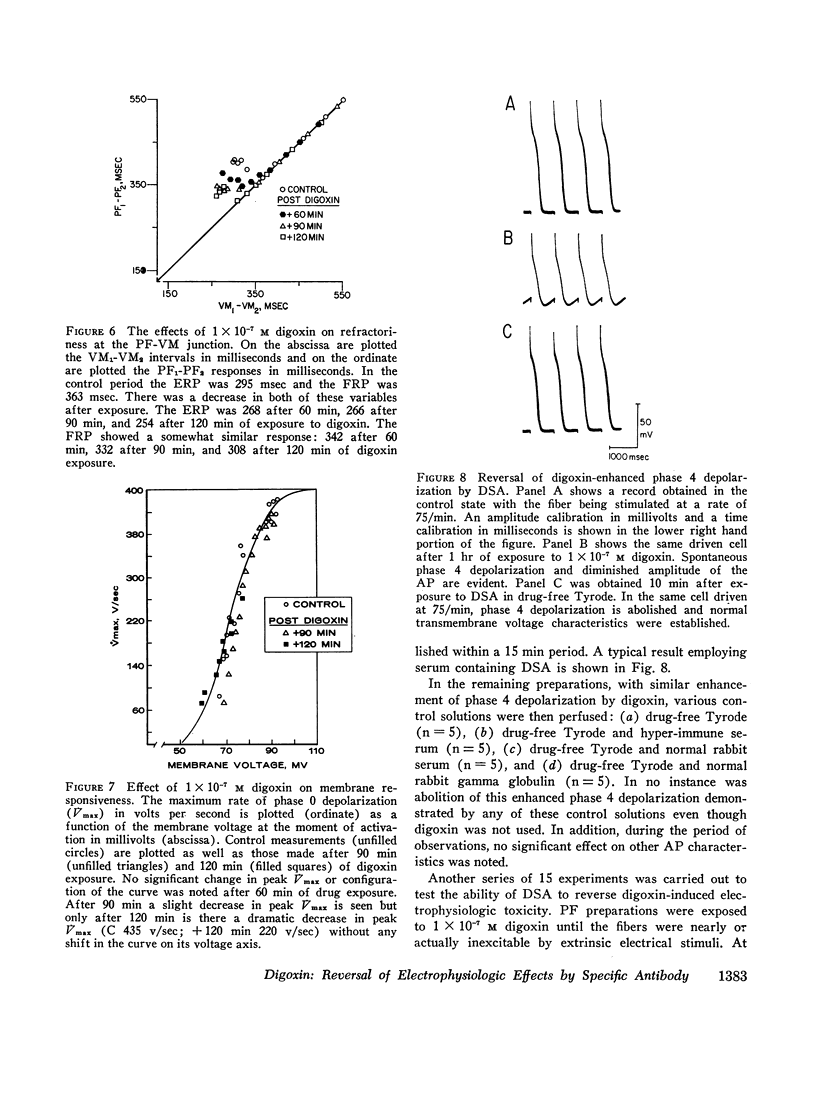
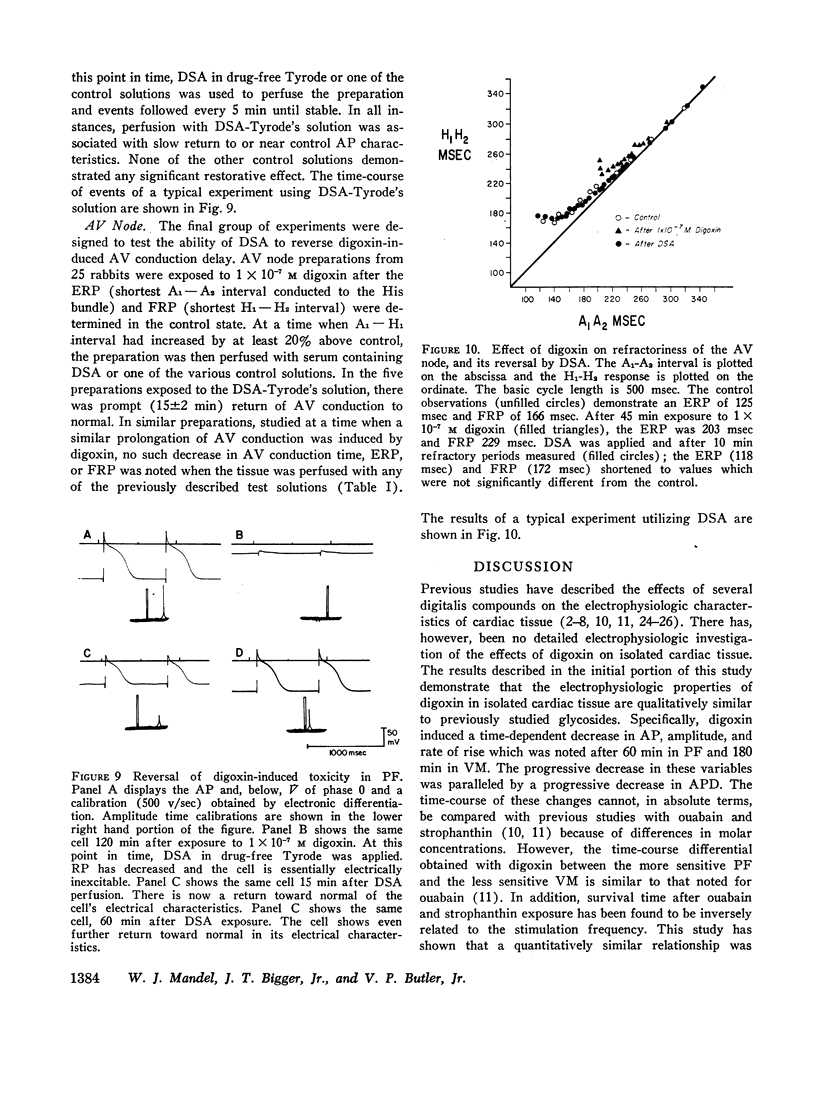
The effects of digoxin on electrophysiologic properties were evaluated in isolated perfused cardiac tissue. In canine Purkinje fiber (PF)-ventricular muscle (VM) preparations, control measurements, using microelectrode technique, were made of: resting potential (RP), action potential (AP) amplitude, rate of rise, overshoot, duration (APD), membrane responsiveness, conduction velocity (CV), and refractory period. The preparation was then exposed to 1 × 10-7 M digoxin and repeat measurements were carried out every 15 min. At slow (30/min) rates of stimulation APD initially prolonged then markedly shortened. With more rapid stimulation (75 and 120/min) no initial APD prolongation was observed. When stimulated at 75/min, RP and AP rate of rise, amplitude, and CV remained near control values for 60-75 min then rapidly decreased until electrical inexcitability (110±15 min). At that time fibers were perfused with serum containing digoxin-specific antibody (DSA) or one of a group of test solutions. In the preparations exposed to DSA, membrane characteristics improved by 15 min, and by 60 min approximated control values. No beneficial effect was seen with the various test solutions. DSA also reversed digoxin-induced enhanced phase 4 depolarization in PF.
Effective (ERP) and Functional (FRP) refractory periods of rabbit atrioventricular (AV) node preparations were measured in the control state. The tissue was then exposed to 1 × 10-7 M digoxin and refractory period measurements repeated. At a time when AV conduction prolonged by 20%, associated with marked prolongation of ERP and FRP, DSA or various test solutions were perfused. The prolongation in ERP, FRP, and AV conduction time rapidly returned to normal only in the DSA perfused tissue. It is concluded that DSA has the ability to reverse pronounced toxic electrophysiological effects of digoxin in in vitro cardiac tissue.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Larsen F. S., Brody T. M. Correlation of cardiac sodium- and potassium-activated adenosine triphosphatase activity with ouabain-induced inotropic stimulation. J Pharmacol Exp Ther. 1970 May;173(1):145–151. [PubMed] [Google Scholar]
- BUTLER V. P., Jr, TANENBAUM S. W., BEISER S. M. A STUDY OF THE CROSS-REACTIVITY OF ANTIPURIN-6-OYL SERUM WITH DEOXYRIBONUCLEIC ACID (DNA). J Exp Med. 1965 Jan 1;121:19–38. doi: 10.1084/jem.121.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigger J. T., Jr, Bassett A. L., Hoffman B. F. Electrophysiological effects of diphenylhydantoin on canine purkinje fibers. Circ Res. 1968 Feb;22(2):221–236. doi: 10.1161/01.res.22.2.221. [DOI] [PubMed] [Google Scholar]
- Bigger J. T., Jr, Mandel W. J. Effect of lidocaine on conduction in canine Purkinje fibers and at the ventricular muscle-Purkinje fiber junction. J Pharmacol Exp Ther. 1970 Apr;172(2):239–254. [PubMed] [Google Scholar]
- Bigger J. T., Jr, Mandel W. J. Effect of lidocaine on the electrophysiological properties of ventricular muscle and purkinje fibers. J Clin Invest. 1970 Jan;49(1):63–77. doi: 10.1172/JCI106224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler V. P., Jr, Chen J. P. Digoxin-specific antibodies. Proc Natl Acad Sci U S A. 1967 Jan;57(1):71–78. doi: 10.1073/pnas.57.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE CARVALHO A. P., DE MELLO W. C., HOFFMAN B. F. Electrophysiological evidence for specialized fiber types in rabbit atrium. Am J Physiol. 1959 Mar;196(3):483–488. doi: 10.1152/ajplegacy.1959.196.3.483. [DOI] [PubMed] [Google Scholar]
- Dudel J., Peper K., Rüdel R., Trautwein W. The dynamic chloride component of membrane current in Purkinje fibers. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;295(3):197–212. doi: 10.1007/BF01844100. [DOI] [PubMed] [Google Scholar]
- Dudel J., Peper K., Rüdel R., Trautwein W. The potassium component of membrane current in Purkinje fibers. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;296(4):308–327. doi: 10.1007/BF00362531. [DOI] [PubMed] [Google Scholar]
- FARAH A., LOOMIS T. A. The action of cardiac glycosides on experimental auricular flutter. Circulation. 1950 Nov;2(5):742–748. doi: 10.1161/01.cir.2.5.742. [DOI] [PubMed] [Google Scholar]
- FISCH C., GREENSPAN K., KNOEBEL S. B., FEIGENBAUM H. EFFECT OF DIGITALIS ON CONDUCTION OF THE HEART. Prog Cardiovasc Dis. 1964 Jan;6:343–365. doi: 10.1016/s0033-0620(64)80007-4. [DOI] [PubMed] [Google Scholar]
- GLYNN I. M. THE ACTION OF CARDIAC GLYCOSIDES ON ION MOVEMENTS. Pharmacol Rev. 1964 Dec;16:381–407. [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- KASSEBAUM D. G. Electrophysiological effects of strophanthin in the heart. J Pharmacol Exp Ther. 1963 Jun;140:329–338. [PubMed] [Google Scholar]
- LING G., GERARD R. W. The normal membrane potential of frog sartorius fibers. J Cell Physiol. 1949 Dec;34(3):383–396. doi: 10.1002/jcp.1030340304. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Ion fluxes in cardiac excitation and contraction and their relation to myocardial contractility. Physiol Rev. 1968 Oct;48(4):708–757. doi: 10.1152/physrev.1968.48.4.708. [DOI] [PubMed] [Google Scholar]
- MENDEZ C., ACEVES J., MENDEZ R. The anti-adrenergic action of digitalis on the refractory period of the A-V transmission system. J Pharmacol Exp Ther. 1961 Feb;131:199–204. [PubMed] [Google Scholar]
- MENDEZ C., MENDEZ R. The action of cardiac glycosides on the excitability and conduction velocity of the mammalian atrium. J Pharmacol Exp Ther. 1957 Dec;121(4):402–413. [PubMed] [Google Scholar]
- MENDEZ R., MENDEZ C. The action of cardiac glycosides on the refractory period of heart tissues. J Pharmacol Exp Ther. 1953 Jan;107(1):24–36. [PubMed] [Google Scholar]
- MOE G. K., MENDEZ R. The action of several cardiac glycosides on conduction velocity and ventricular excitability in the dog heart. Circulation. 1951 Nov;4(5):729–734. doi: 10.1161/01.cir.4.5.729. [DOI] [PubMed] [Google Scholar]
- MUELLER P. OUABAIN EFFECTS ON CARDIAC CONTRACTION, ACTION POTENTIAL, AND CELLULAR POTASSIUM. Circ Res. 1965 Jul;17:46–56. doi: 10.1161/01.res.17.1.46. [DOI] [PubMed] [Google Scholar]
- Mason D. T., Spann J. F., Jr, Zelis R. New developments in the understanding of the actions of the digitalis glycosides. Prog Cardiovasc Dis. 1969 May;11(6):443–478. doi: 10.1016/0033-0620(69)90001-2. [DOI] [PubMed] [Google Scholar]
- Myerburg R. J. The gating mechanism in the distal atrioventricular conducting system. Circulation. 1971 Jun;43(6):955–960. doi: 10.1161/01.cir.43.6.955. [DOI] [PubMed] [Google Scholar]
- NASTUK W. L., PLESCIA O. J., OSSERMAN K. E. Changes in serum complement activity in patients with myasthenia gravis. Proc Soc Exp Biol Med. 1960 Oct;105:177–184. doi: 10.3181/00379727-105-26050. [DOI] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper K., Trautwein W. A membrane current related to the plateau of the action potential of Purkinje fibers. Pflugers Arch. 1968;303(2):108–123. doi: 10.1007/BF00592629. [DOI] [PubMed] [Google Scholar]
- Polimeni P. I., Vassalle M. On the mechanism of ouabain toxicity in Purkinje and ventricular muscle fibers at rest and during activity. Am J Cardiol. 1971 Jun;27(6):622–629. doi: 10.1016/0002-9149(71)90226-8. [DOI] [PubMed] [Google Scholar]
- Reuter H. The dependence of slow inward current in Purkinje fibres on the extracellular calcium-concentration. J Physiol. 1967 Sep;192(2):479–492. doi: 10.1113/jphysiol.1967.sp008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D. H., Butler V. P., Jr Immunological protecion against dixin toxicity. J Clin Invest. 1971 Apr;50(4):866–871. doi: 10.1172/JCI106558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D. H., Butler V. P., Jr Reversal of digoxin toxicity with specific antibodies. J Clin Invest. 1971 Aug;50(8):1738–1744. doi: 10.1172/JCI106663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Allen J. C., Harigaya S. Possible involvement of cardiac Na+, K+-adenosine triphosphatase in the mechanism of action of cardiac glycosides. J Pharmacol Exp Ther. 1969 Jul;168(1):31–41. [PubMed] [Google Scholar]
- Smith T. W., Butler V. P., Jr, Haber E. Characterization of antibodies of high affinity and specificity for the digitalis glycoside digoxin. Biochemistry. 1970 Jan 20;9(2):331–337. doi: 10.1021/bi00804a020. [DOI] [PubMed] [Google Scholar]
- VASSALLE M., KARIS J., HOFFMAN B. F. Toxic effects of ouabain on Purkinje fibers and ventricular muscle fibers. Am J Physiol. 1962 Sep;203:433–439. doi: 10.1152/ajplegacy.1962.203.3.433. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. The effect of the cardiac membrane potential on the rapid availability of the sodium-carrying system. J Physiol. 1955 Jan 28;127(1):213–224. doi: 10.1113/jphysiol.1955.sp005250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace A. G., Mignone R. J. Physiologic evidence concerning the re-entry hypothesis for ectopic beats. Am Heart J. 1966 Jul;72(1):60–70. doi: 10.1016/0002-8703(66)90628-4. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Dreifus L. S. Electrophysiologic effects of digitalis on A-V transmission. Am J Physiol. 1966 Dec;211(6):1461–1466. doi: 10.1152/ajplegacy.1966.211.6.1461. [DOI] [PubMed] [Google Scholar]