Abstract
Bile salt metabolism was studied in fetal dogs 1 wk before term. The size and distribution of the fetal bile salt pool were measured, and individual bile salts were identified. The hepatic excretion of endogenous bile salts was studied in bile fistula fetuses, and the capacity of this excretory mechanism was investigated by the i.v. infusion of a load of sodium taurocholate-14C up to 20 times the endogenous pool size.
The total fetal bile salt pool was 30.9±2.7 μmoles, of which two-thirds was in the fetal gallbladder. Expressed on a body weight basis, this was equal to approximately one-half the estimated pool size in the adult dog (119.2±11.3 vs. 247.5±33.1 μmoles/kg body wt). Measurable quantities of bile salt were found in small bowel (6.0±1.8 μmoles), large bowel (1.1±0.3 μmoles), liver (1.2±0.5 μmoles), and plasma (0.1±0.03 μmoles). Plasma bile salt levels were significantly greater in fetal than in maternal plasma (1.01±0.24 μg/ml vs. 0.36±0.06 μg/ml; P < 0.05).
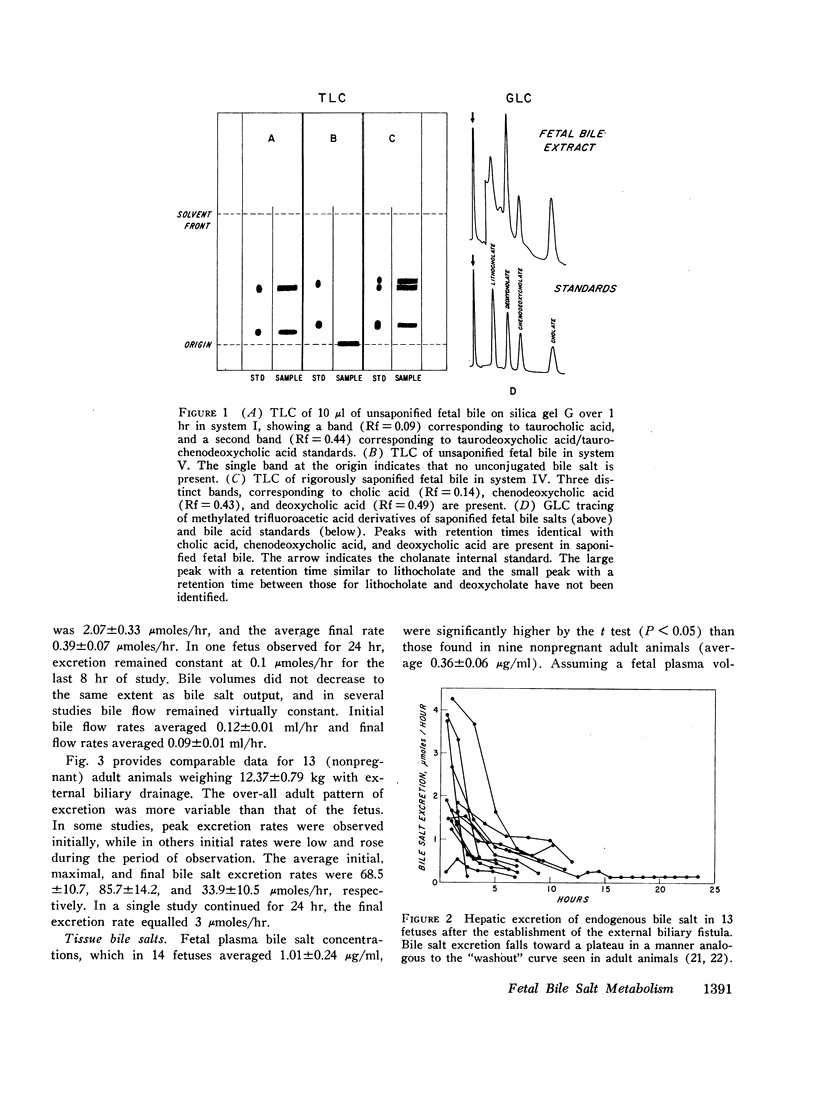
Fetal hepatic bile salt excretion showed a fall over the period of study from 2.04±0.34 to 0.30±0.07 μmoles/hr. The maximal endogenous bile salt concentration in fetal hepatic bile was 18.7±1.5 μmoles/ml. The concentration in fetal gallbladder bile was 73.9±8.6 μmoles/ml; and, in those studies in which hepatic and gallbladder bile could be compared directly, the gallbladder appeared to concentrate bile four- to fivefold.
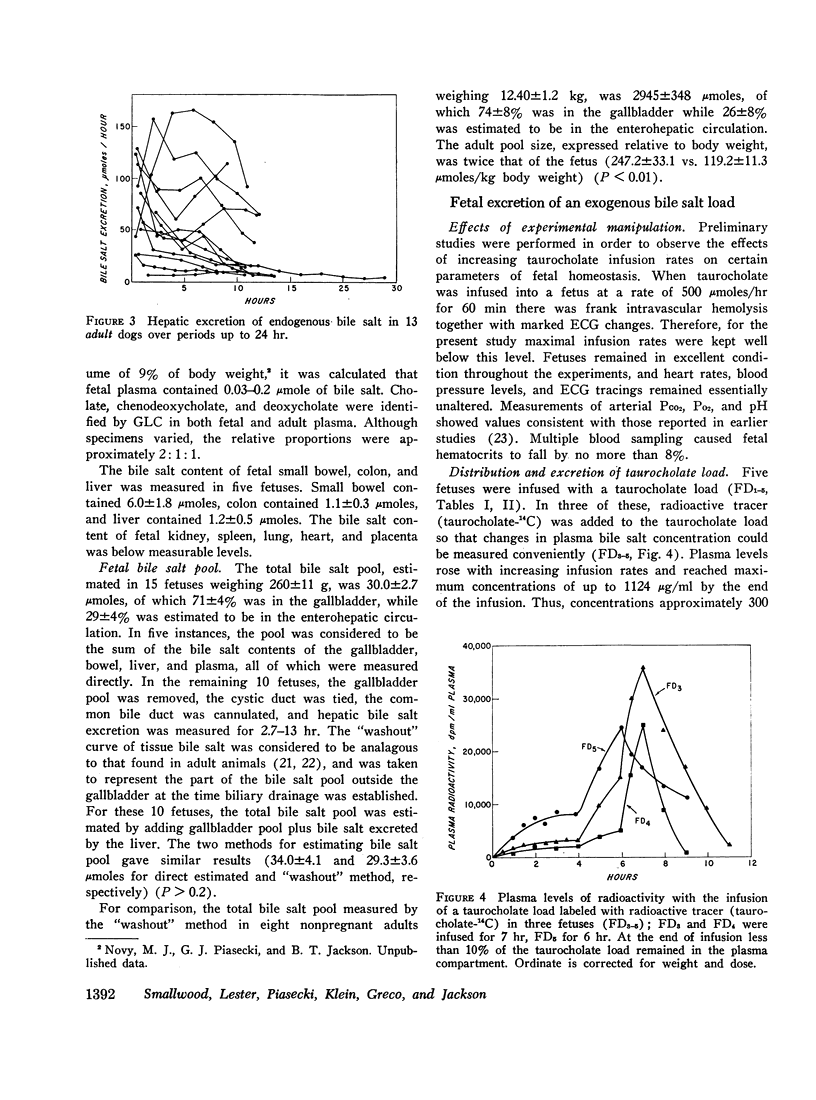
Taurocholate, taurochenodeoxycholate, and taurodeoxycholate were present in fetal bile, but no free bile salts were identified. The presence of deoxycholate was confirmed by thin-layer chromatography and gas liquid chromatography, and the absence of microorganisms in fetal gut suggests that it was probably transferred from the maternal circulation.
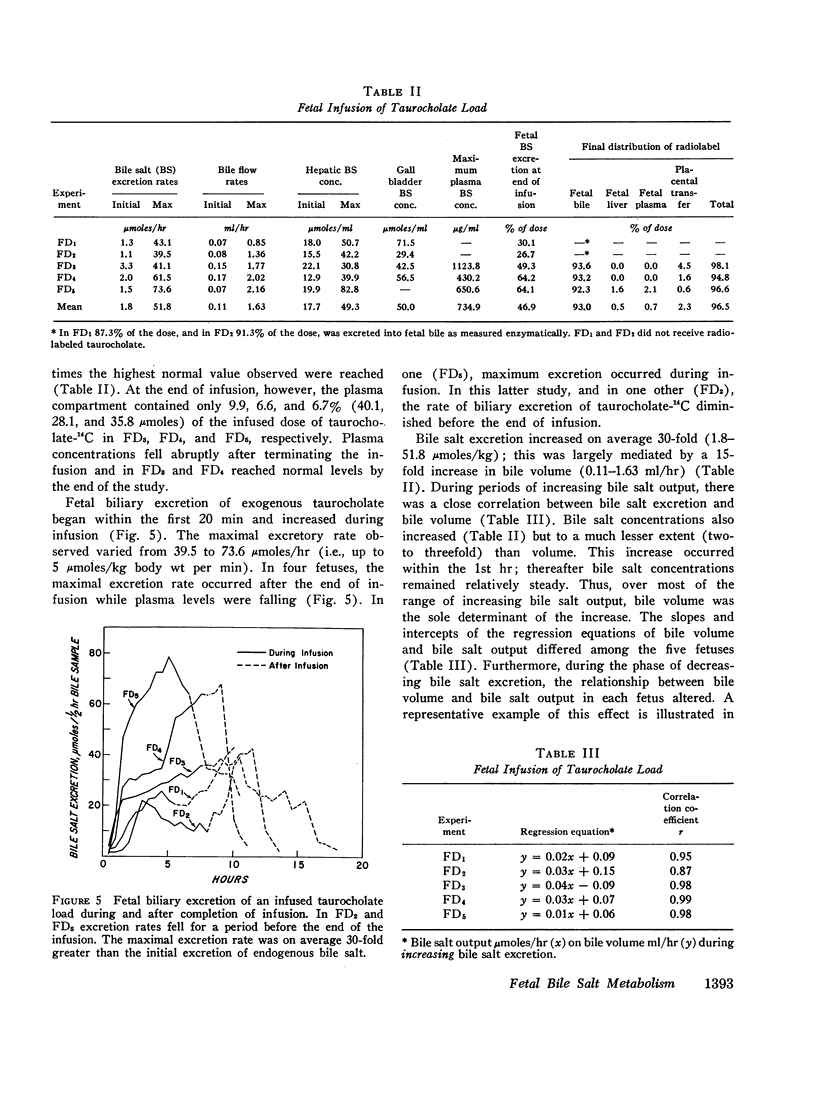
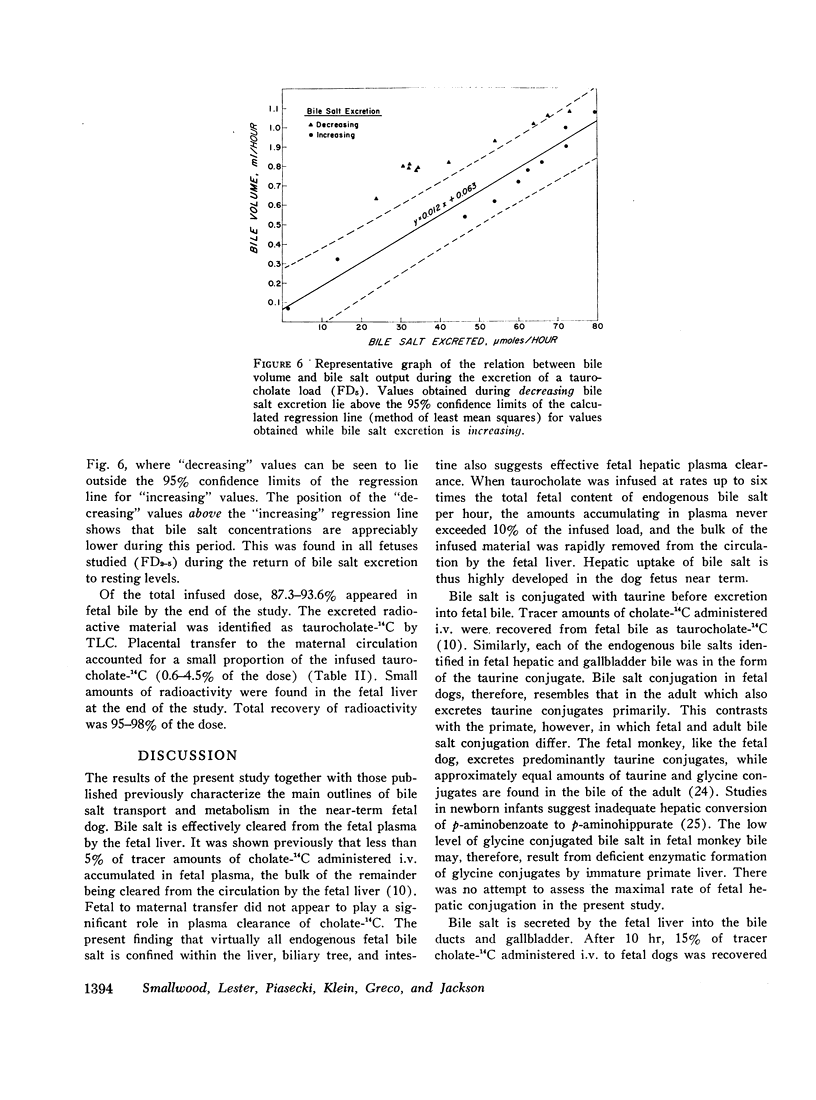
After infusion of a taurocholate load, fetal hepatic bile salt excretion increased 30-fold, so that 85-95% of the dose was excreted by the fetal liver during the period of observation. Placental transfer accounted for less than 5% of the dose. Fetal bile volume increased 15-fold on average, while bile salt concentrations increased two- to threefold.
It is concluded that bile salt is taken up, conjugated, and excreted by the fetal liver with remarkable efficiency. The excreted material is either stored and concentrated in the fetal gallbladder or released into the intestine and reabsorbed to be reexcreted in bile.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONGIOVANNI A. M. BILE ACID CONTENT OF GALLBLADDER OF INFANTS, CHILDREN AND ADULTS. J Clin Endocrinol Metab. 1965 May;25:678–685. doi: 10.1210/jcem-25-5-678. [DOI] [PubMed] [Google Scholar]
- Bernstein R. B., Novy M. J., Piasecki G. J., Lester R., Jackson B. T. Bilirubin metabolism in the fetus. J Clin Invest. 1969 Sep;48(9):1678–1688. doi: 10.1172/JCI106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M. Mechanisms for the intestinal absorption of bile acids. J Lipid Res. 1968 May;9(3):297–309. [PubMed] [Google Scholar]
- Dowling R. H., Mack E., Small D. M. Effects of controlled interruption of the enterohepatic circulation of bile salts by biliary diversion and by ileal resection on bile salt secretion, synthesis, and pool size in the rhesus monkey. J Clin Invest. 1970 Feb;49(2):232–242. doi: 10.1172/JCI106232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCRANTZ J. C., SJOVALL J. On the bile acids in duodenal contents of infants and children. Bile acids and steroids 72. Clin Chim Acta. 1959 Nov;4:793–799. doi: 10.1016/0009-8981(59)90030-0. [DOI] [PubMed] [Google Scholar]
- GRUNDY S. M., AHRENS E. H., Jr, MIETTINEN T. A. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL FECAL BILE ACIDS. J Lipid Res. 1965 Jul;6:397–410. [PubMed] [Google Scholar]
- Hofmann A. F., Small D. M. Detergent properties of bile salts: correlation with physiological function. Annu Rev Med. 1967;18:333–376. doi: 10.1146/annurev.me.18.020167.002001. [DOI] [PubMed] [Google Scholar]
- JACKSON B. T., EGDAHL R. H. The performance of complex fetal operations in utero without amniotic fluid loss or other disturbances of fetal-maternal relationships. Surgery. 1960 Sep;48:564–570. [PubMed] [Google Scholar]
- JORPES J. E., MUTT V., JONSON G., THUL, SUNDMANLIN L. THE EFFECT OF SECRETIN ON BILE FLOW. Gastroenterology. 1963 Dec;45:786–788. [PubMed] [Google Scholar]
- Jackson B. T., Smallwood R. A., Piasecki G. J., Brown A. S., Rauschecker H. F., Lester R. Fetal bile salt metabolism. I. The metabolism of sodium cholate-14C in the fetal dog. J Clin Invest. 1971 Jun;50(6):1286–1294. doi: 10.1172/JCI106607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER R., SCHMID R. Intestinal absorption of bile pigments. I. The enterohepatic circulation of bilirubin in the rat. J Clin Invest. 1963 May;42:736–746. doi: 10.1172/JCI104766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICKELSEN O. Nutrition-germfree animal research. Annu Rev Biochem. 1962;31:515–548. doi: 10.1146/annurev.bi.31.070162.002503. [DOI] [PubMed] [Google Scholar]
- O'Máille E. R., Richards T. G., Short A. H. Factors determining the maximal rate of organic anion secretion by the liver and further evidence on the hepatic site of action of the hormone secretin. J Physiol. 1966 Oct;186(2):424–438. doi: 10.1113/jphysiol.1966.sp008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVERIO V. T., DENHAM C., DAVIDSON J. D. Oxygen flask combustion in determination of C-14 and H3 in biological materials. Anal Biochem. 1962 Aug;4:188–189. doi: 10.1016/0003-2697(62)90035-0. [DOI] [PubMed] [Google Scholar]
- POLEY J. R., DOWER J. C., OWEN C. A., Jr, STICKLER G. B. BILE ACIDS IN INFANTS AND CHILDREN. J Lab Clin Med. 1964 May;63:838–846. [PubMed] [Google Scholar]
- Peric-Golia L., Socic H. Biliary bile acids and cholesterol in developing sheep. Am J Physiol. 1968 Nov;215(5):1284–1287. doi: 10.1152/ajplegacy.1968.215.5.1284. [DOI] [PubMed] [Google Scholar]
- SANDBERG D. H., SJOEVALL J., SJOEVALL K., TURNER D. A. MEASUREMENT OF HUMAN SERUM BILE ACIDS BY GAS-LIQUID CHROMATOGRAPHY. J Lipid Res. 1965 Apr;6:182–192. [PubMed] [Google Scholar]
- TALALAY P. Enzymic analysis of steroid hormones. Methods Biochem Anal. 1960;8:119–143. doi: 10.1002/9780470110249.ch3. [DOI] [PubMed] [Google Scholar]
- VEST M. F., ROSSIER R. DETOXIFICATION IN THE NEWBORN: THE ABILITY OF THE NEWBORN INFANT TO FORM CONJUGATES WITH GLUCURONIC ACID, GLYCINE, ACETATE AND GLUTATHIONE. Ann N Y Acad Sci. 1963 Dec 30;111:183–198. doi: 10.1111/j.1749-6632.1963.tb36958.x. [DOI] [PubMed] [Google Scholar]
- Wheeler H. O., Ramos O. L. DETERMINANTS OF THE FLOW AND COMPOSITION OF BILE IN THE UNANESTHETIZED DOG DURING CONSTANT INFUSIONS OF SODIUM TAUROCHOLATE. J Clin Invest. 1960 Jan;39(1):161–170. doi: 10.1172/JCI104015. [DOI] [PMC free article] [PubMed] [Google Scholar]


