Abstract
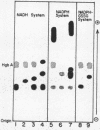
The electrophoretic mobility of erythrocyte NADH methemoglobin reductase in five hereditary methemoglobinemia patients from three Puerto Rican kindreds was 118% of normal at pH 8.6. The methemoglobin ferrocyanide reductase activity of the enzyme in erythrocyte hemolysates was 3.2-6.4% of normal. Electrophoresis of hemolysates prepared from the blood of patients from two different families at six pH values between 4.6 and 9.3 did not differentiate between the variant enzymes. Examination of the deficient enzymes extracted from the erythrocytes of one patient from each kindred revealed altered affinity for NADH and dichloroindophenol dye and decreased thermal stability. The quantitative similarity of the abnormal findings, together with the Puerto Rican origin of the kindreds, suggested that the cyanotic patients possessed the same abnormal enzyme and were thus homozygous for the same rare mutant gene. Consanguinity of the kindreds could not be established.
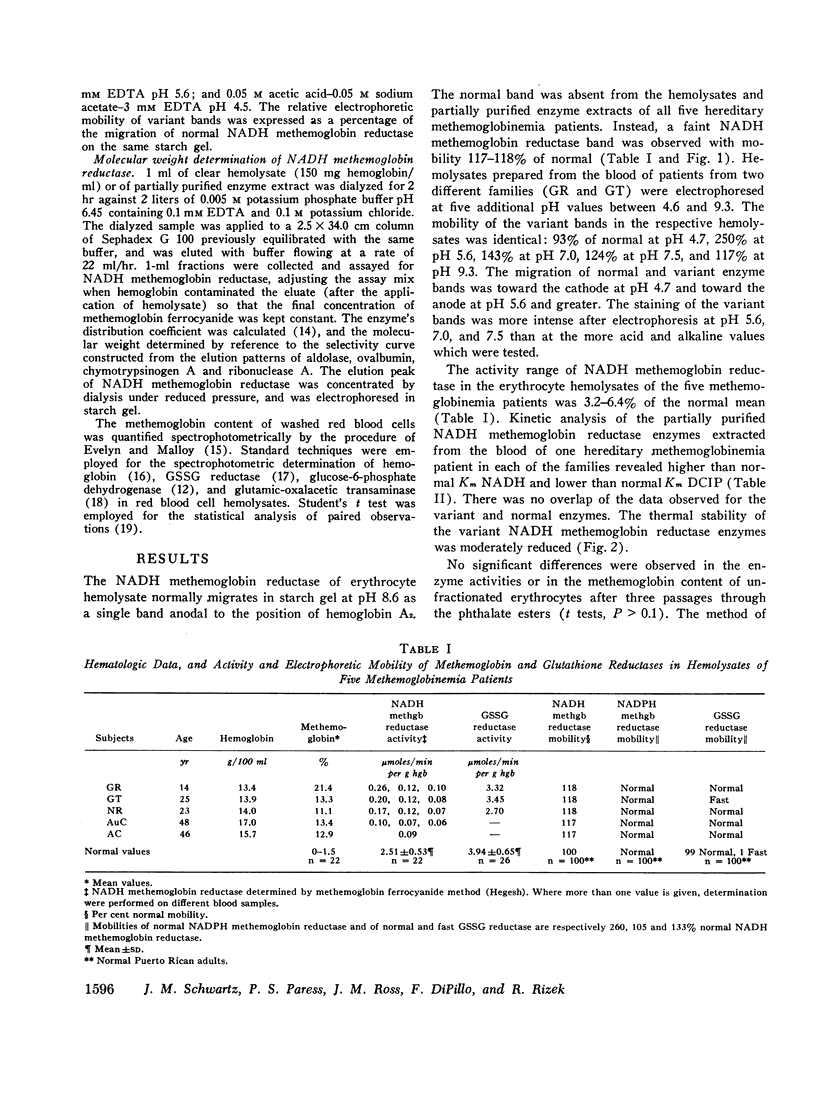
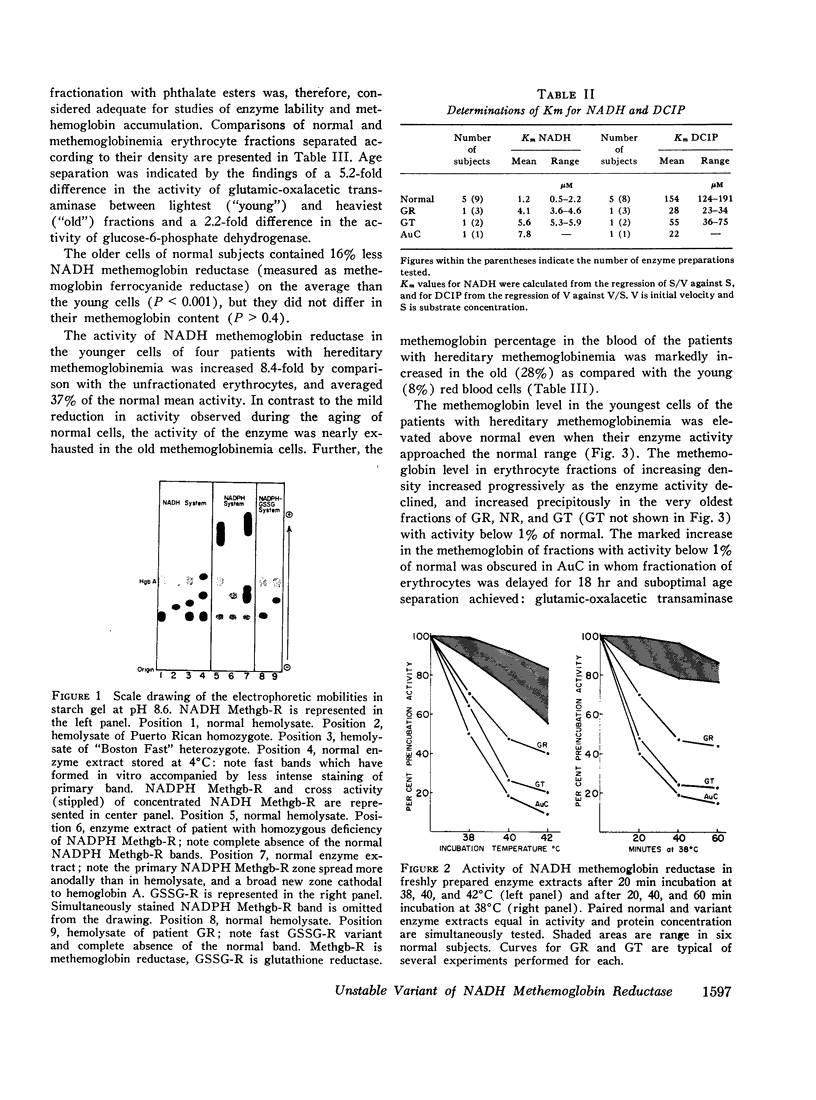
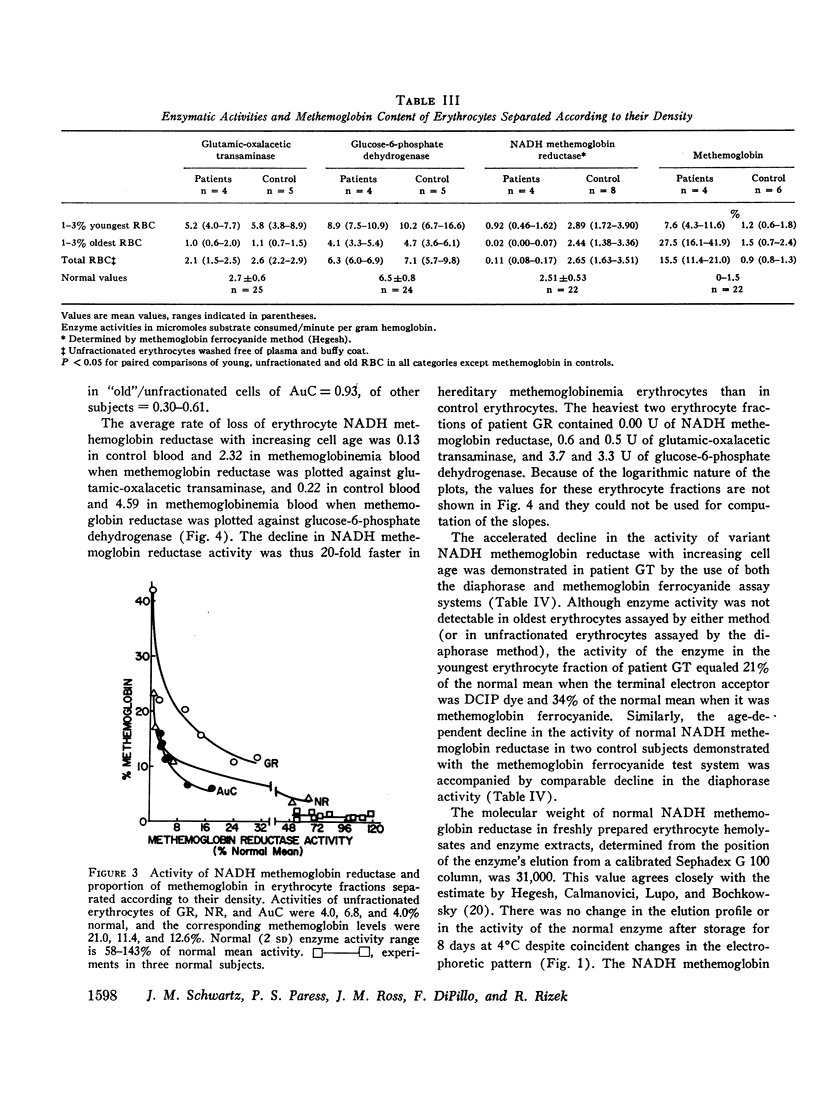
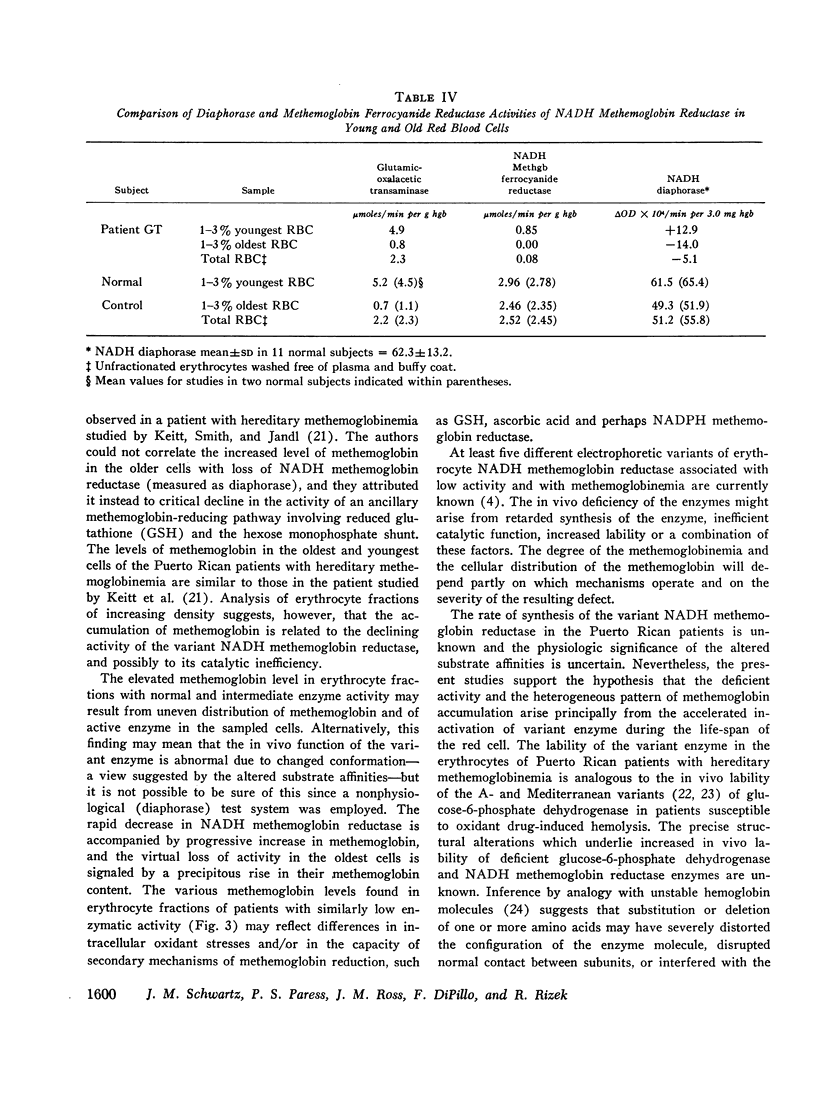
The rates of decline of the normal and variant NADH methemoglobin reductase enzymes in vivo were measured in erythrocyte fractions of increasing cell age. The rate of decline of the variant enzyme was increased 20-fold by comparison with the normal enzyme. The methemoglobin percentage in erythrocyte fractions of increasing cell age correlated inversely with the activity of the variant. The variant enzyme averaged 37% of normal mean activity in young cells and 1% in old cells. The normal enzyme, on the other hand, lost only one-sixth of its activity as the cells aged, and the methemoglobin content in old normal cells did not rise. These observations support the hypothesis that the deficient activity and the heterogeneous pattern of methemoglobin accumulation in vivo arise principally from the accelerated inactivation of variant NADH methemoglobin reductase during the life-span of the red blood cell.
Full text
PDF

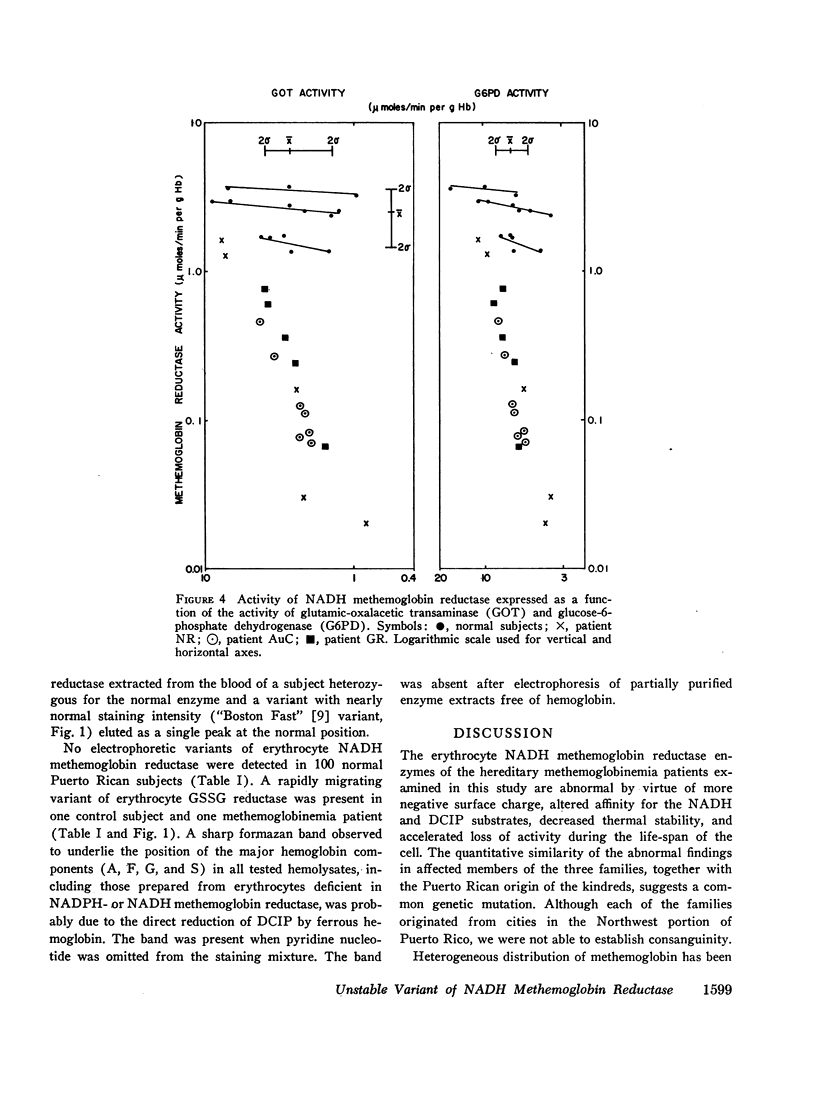

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. E., Zarkowsky H. S. Heterogeneity of the enzymatic defect in congenital methemoglobinemia. N Engl J Med. 1969 Oct 23;281(17):919–922. doi: 10.1056/NEJM196910232811702. [DOI] [PubMed] [Google Scholar]
- DANON D., MARIKOVSKY V. DETERMINATION OF DENSITY DISTRIBUTION OF RED CELL POPULATION. J Lab Clin Med. 1964 Oct;64:668–674. [PubMed] [Google Scholar]
- GERALD P. S., EFRON M. L. Chemical studies of several varieties of Hb M. Proc Natl Acad Sci U S A. 1961 Nov 15;47:1758–1767. doi: 10.1073/pnas.47.11.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. T., Bracci R., Rudolph N., Schroeder E., Kochen J. A. Hydrogen peroxide toxicity and detoxification in the erythrocytes of newborn infants. Blood. 1967 Apr;29(4):481–493. [PubMed] [Google Scholar]
- Hegesh E., Calmanovici N., Avron M. New method for determining ferrihemoglobin reductase (NADH-methemoglobin reductase) in erythrocytes. J Lab Clin Med. 1968 Aug;72(2):339–344. [PubMed] [Google Scholar]
- Hegesh E., Calmanovici N., Lupo M., Bochkowsky R. The diaphorase bands of human erythrocytes. J Lab Clin Med. 1971 May;77(5):859–866. [PubMed] [Google Scholar]
- Hsieh H. S., Jaffé E. R. Electrophoretic and functional variants of NADH-methemoglobin reductase in hereditary methemoglobinemia. J Clin Invest. 1971 Jan;50(1):196–202. doi: 10.1172/JCI106473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. R., Hsieh H. S. DPNH-methemoglobin reductase deficiency and hereditary methemoglobinemia. Semin Hematol. 1971 Oct;8(4):417–437. [PubMed] [Google Scholar]
- Kaplan J. C., Beutler E. Electrophoresis of red cell NADH- and NADPH-diaphorases in normal subjects and patients with congenital methemoglobinemia. Biochem Biophys Res Commun. 1967 Nov 30;29(4):605–610. doi: 10.1016/0006-291x(67)90529-3. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Beutler E. Electrophoretic study of glutathione reductase in human erythrocytes and leucocytes. Nature. 1968 Jan 20;217(5125):256–258. doi: 10.1038/217256a0. [DOI] [PubMed] [Google Scholar]
- Keitt A. S., Smith T. W., Jandl J. H. Red-cell "pseudomosaicism" in congenital methemoglobinemia. N Engl J Med. 1966 Aug 25;275(8):399–405. doi: 10.1056/NEJM196608252750801. [DOI] [PubMed] [Google Scholar]
- LODER P. B., DEGRUCHY G. C. RED-CELL ENZYMES AND CO-ENZYMES IN NON-SPHEROCYTIC CONGENITAL HAEMOLYTIC ANAEMIAS. Br J Haematol. 1965 Jan;11:21–31. doi: 10.1111/j.1365-2141.1965.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- Piomelli S., Corash L. M., Davenport D. D., Miraglia J., Amorosi E. L. In vivo lability of glucose-6-phosphate dehydrogenase in GdA- and GdMediterranean deficiency. J Clin Invest. 1968 Apr;47(4):940–948. doi: 10.1172/JCI105786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT E. M. The relation of diaphorase of human erythrocytes to inheritance of methemoglobinemia. J Clin Invest. 1960 Jul;39:1176–1179. doi: 10.1172/JCI104131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG D., OSTROW B. H. Serum transaminase as a measure of myocardial necrosis. Proc Soc Exp Biol Med. 1955 May;89(1):31–34. doi: 10.3181/00379727-89-21705. [DOI] [PubMed] [Google Scholar]