Abstract
Invariant NKT (iNKT) cells are involved in host defence against microbial infections. While it is known that iNKT cells recognize glycolipids presented by CD1d, how and where they encounter antigen in vivo remains unclear. We used multi-photon microscopy to visualize the dynamics and activation of iNKT cells in lymph nodes. Following antigen administration, iNKT cells become confined in a CD1d-dependent manner in close proximity to subcapsular sinus CD169+ macrophages. These macrophages retain, internalize and present lipid antigen, and are required for iNKT cell activation, cytokine production and expansion. Thus, CD169+ macrophages can act as bona fide antigen presenting cells controlling early iNKT cell activation and favouring fast initiation of immune responses.
INTRODUCTION
Invariant NKT (iNKT) cells are a specialized subset of classical αβ T lymphocytes implicated in a wide range of immune responses including anti-viral, anti-bacterial, inflammatory and autoimmune reactions1. iNKT cells are characterised by the expression of a restricted αβ T cell receptor (TCR) repertoire comprised of the canonical Vα14-Jα18 chain in association with the Vβ2, Vβ7 or Vβ8 chains in mice (equivalent to Vα24-Jα18 and Vβ11 in humans)2. Through these TCRs iNKT cells specifically recognize lipid antigens presented by CD1d, a MHC class I-like molecule expressed by professional antigen presenting cells (APCs), including dendritic cells (DCs), macrophages and B cells3. Several lipid antigens capable of directly activating iNKT cells have been identified including glycolipids from LPS-negative bacteria such as Sphingomonas, Erlichia and Borrelia4-6. Furthermore, it has been suggested that in response to TLR stimulation of professional APCs, a combination of enhanced IL-12 secretion and up-regulation of endogenous lipid(s) may also activate iNKT cells in a CD1d dependent6-9 or independent manner10. Activated iNKT cells very rapidly secrete large quantities of cytokines, such as IFN-γ and IL-4, and induce downstream activation of a variety of other cell types, including DCs, NK cells, B cells and classical T cells. Thus iNKT cells can play a pivotal position in coordinating both innate and adaptive immune responses1,2.
iNKT cells occupy a similar tissue distribution to classical αβ T cells2 though the relative proportion of iNKT cells varies according to the particular location. For example, iNKT cells comprise around 30%, 2-3% and up to 0.5% of the total αβ T cell population in the liver, spleen and lymph nodes (LNs) respectively. Under physiological conditions of clonal abundance, iNKT cells exceed the number of antigen-specific αβ T cells by around 500 to 5000-fold in the LN (~1 in 105-106 T cells11). Thus, though the relative number of iNKT cells in secondary lymphoid tissues may appear small on first examination, it is likely that they make an important contribution in the regulation of immune responses in these sites. However, very few studies up to date have attempted to investigate the role of iNKT cells in the LNs12,13.
In spite of the key role of iNKT cells in host defence against microbial infections14, the mechanism by which these cells encounter antigen and become activated in vivo has not been fully investigated. As CD1d is expressed on a wide variety of cell types15, many cells could be potentially involved in mediating antigen presentation and subsequent activation of iNKT cells in vivo. Several studies have suggested that DCs and Kupffer cells play an important role in presenting glycolipids to iNKT cells in spleen and liver16,17. In fact, administration of DCs pulsed in vitro with bacteria such as Sphingomonas and Borrelia or different glycolipids can stimulate activation of iNKT cells in vivo4-6,16. Two studies have analyzed the behaviour of a population of CXCR6+ cells (mainly comprised of NKT cells) in the liver by confocal microscopy18, 19. These studies demonstrate that CXCR6+ cells become arrested within hepatic sinusoids following administration of antigenic lipids. However, the events underlying iNKT cell activation, including the cells involved in antigen internalization and presentation, have not been fully investigated in living lymphoid organs.
Much of the current understanding of the mechanisms of antigen presentation and lymphocyte activation in secondary lymphoid organs has been uncovered through innovative intravital imaging in the LN20. Following administration, a first wave of antigen is rapidly delivered to draining LNs through afferent lymphatic vessels and moved around the subcapsular sinus (SCS). Large antigens such as viruses, pathogens and immune complexes, are restricted from gaining access to the LN interior as they are retained by cells lining the SCS and presented to antigen-specific B and T cells directly in the LN periphery21-25. Several hours after the initial administration, a second wave of antigen arrives to the LN in association with migratory peripheral DCs allowing presentation to cognate T lymphocytes deep in the LN cortex26. It is likely that during an immune response these two waves of presentation collaborate to maximise the probability of lymphocytes encountering their cognate antigen; however the contribution (if any) that either could make to iNKT cell activation in the LN is not fully understood.
Here we investigate the early events that control the activation of iNKT cells in response to specific antigens. We have used multi-photon microscopy to visualize the dynamic behaviour of iNKT cells in intact LNs. We observed that iNKT cells are mainly located in the paracortical region, similar to classical CD4+ T cells, while they search the LN for specific antigen. Within hours of administration lipid antigen retained at the SCS region stimulates the arrest of iNKT cells at this site. Furthermore we identified that CD169+ macrophages in the SCS mediate long-lasting interactions with iNKT cells in a CD1d-dependent manner, which results in very fast iNKT cell activation and cytokine secretion. Thus, we have identified a further role for SCS macrophages in presenting lipid antigen to mediate rapid activation of iNKT cells in the LN, likely to be important during the early stages of immune responses.
RESULTS
Distribution and dynamics of iNKT cells in the LNs
We wanted to investigate the distribution and dynamics of iNKT cells in the LNs. In view of the relatively small number (Fig. 1a and 1b), heterogeneity12,27,28 and the lack of reagents for reliable unambiguous identification of iNKT cells using immunohistochemistry in situ29 we used adoptive transfer and multi-photon microscopy to visualize iNKT cells in the LNs. To overcome the limitations that might be associated with the isolation of heterogeneous populations, we used two complementary flow cytometry sorting strategies that allowed us to obtain highly pure iNKT cells. We define these two strategies as: iNKT-S1, iNKT cells sorted from LNs as α-galactosylceramide (α-GalCer)-loaded CD1d-tetramer (CD1d-tet)+TCRβ+B220− cells (>97% purity; Supplementary Fig. 1a and 1b); and iNKT-S2, iNKT cells sorted from spleen and liver as TCRβ+NK1.1+B220− cells (> 95% CD1d-tet+ cells; Fig. 1c and Supplementary Fig. 2a). While larger numbers of iNKT cells were obtained from spleen and liver, iNKT cells sorted from LNs more closely represented the relative proportions of NK1.1+/NK1.1− iNKT cells found in LNs of WT animals (Supplementary Fig. 1). The resulting iNKT cells sorted by these two approaches were transferred independently into C57BL/6 recipient mice. Regardless of the tissue of origin or the purification strategy, transferred iNKT cells home to LNs where they represent around 0.05 - 0.1% of total TCRβ+ T cells (Fig. 1d, Supplementary Fig. 1c and Supplementary Fig. 2b). Thus both strategies allow analysis of iNKT cell distribution in conditions resembling those of clonal abundance in resting LNs where endogenous iNKT cells represent around 0.3% of total T cells.
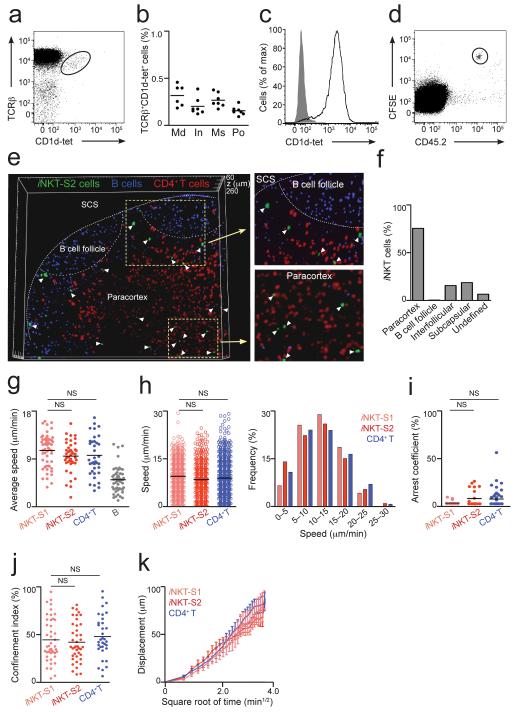
Figure 1.
Distribution and dynamics of iNKT cells in LNs. (a) iNKT cells in mediastinal LNs from WT animals were identified by flow cytometry as TCRβ+CD1d-tet+ cells. (b) Percentage of iNKT from TCRβ+B220− cells in various LNs. Data is from two independent experiments with 3-4 animals per group. Md, mediastinal; In, inguinal; Ms, mesenteric; Po, popliteal. (c) CD1d-tet staining of iNKT-S2 cells. (d) iNKT-S2 cells were labelled with CFSE, transferred into CD45.1 congenic mice and detected in LNs of recipient animals as CFSE+CD45.2+B220−. (e,f) iNKT-S2 (green), CD4+ T (red) and B cells (blue) were adoptively transferred into WT recipients. LNs were fixed and imaged by multi-photon microscopy. (e) Three-dimensional reconstruction from a 200 μm section of a popliteal LN showing the distribution of iNKT cells (white arrowheads). (f) Percentage of iNKT cells in various locations in the LN. (g-k) iNKT-S1 (pale red) and iNKT-S2 (red) cells were adoptively transferred alongside CD4+ T (blue) and/or B cells (grey). Intact LNs were imaged by multi-photon microscopy. Average speed (g), instantaneous speed distribution (h), arrest coefficient (i) and confinement index (j) for individual cells are shown with mean values as black lines. Data were obtained in at least two independent experiments and were compared with two-tailed unpaired Mann-Whitney test. (NS, not significant) (k) Displacement (mean ± s.e.m) of iNKT and CD4+ T cells plotted against the square root of time. Mean motility coefficient (M) can be calculated as M=x2/6t, where x is the slope of the linear part of the graph.
(g-i) Each symbol represents an individual cell.
To visualize iNKT cell distribution in the LN, iNKT-S1 and iNKT-S2 cells were CFSE labelled and independently transferred into C57BL/6 recipient mice, along with CMAC-labelled B cells and SNARF-1-labelled CD4+ T cells to define follicular and paracortical regions of the LNs respectively. Sixteen hours after transfer, mice were sacrificed and LNs removed, fixed and imaged intact using multi-photon microscopy. We acquired adjacent 5 μm sections through the LN and assembled them in three dimensions to allow visualization of iNKT cells up to a depth greater than 400 μm below the surface of the node (Fig. 1e and 1f; Supplementary Fig. 1d and 1e). Independently of the tissue of origin or the purification strategy, iNKT cells were preferentially located in the paracortex alongside CD4+ T cells. While on analysis of Z-projected images iNKT cells were occasionally found in the B cell follicles, careful examination of three-dimensional reconstructions of the LNs revealed that these iNKT cells were located around the periphery of follicles and were essentially excluded from the B cell areas. Around 10% of iNKT cells were detected in interfollicular regions or in close proximity to the SCS (Fig. 1e and 1f; Supplementary Fig. 1d and 1e).
To characterise the dynamics of iNKT cells in living LNs, we used multi-photon microscopy to image intact LNs from mice having undergone adoptive transfer of iNKT cells purified using both regimes described above. We observed that iNKT cells traverse the LN with an average speed of 11.0 ± 3.1 and 10.0 ± 3.2 μm/min, for iNKT-S1 and iNKT-S2 respectively (Fig. 1g and Supplementary Movies 1 and 2). iNKT cells average speed was similar to that of co-transferred CD4+ T cells and was around two-fold greater than co-transferred B cells (measured at 10.1 ± 4.0 μm/min and 5.4 ± 2.4 μm/min respectively), in agreement with previous observations20,30. A closer inspection showed that the distribution of instantaneous speeds for individual iNKT and CD4+ T cells was comparable, with maxima around 30 μm/min (Fig. 1h). To gain insight into the nature of the movements of iNKT cells in the LN, we used the arrest coefficient to represent the proportion of the time during a given track in which a cell is stopped (Fig. 1i). In addition, the confinement index denoted whether cells are restricted to a particular area during the imaging period (Fig. 1j). This analysis revealed that iNKT cells behave in a similar manner to CD4+ T cells, showing comparable arrest coefficient and confinement index values (Fig. 1i and 1j). Furthermore, the mean absolute displacement increased proportionally with the square root of time (Fig. 1k) indicating that both iNKT and CD4+ T cells move in a random walk mode20,30. Based on this representation we calculated a motility coefficient of 70.3 ± 17.1 and 65.9 ± 4.6 μm2/min for iNKT-S1 and iNKT-S2 cells respectively, and 73.8 ± 14.4 μm2/min for CD4+ T cells, indicating that these cells are able to scan similar volumes of the LN in a given time.
Importantly, iNKT cells purified through our two different strategies display comparable distributions and dynamics in the LN, independent of their tissues of origin or purification method. Therefore we conclude that iNKT cells are predominantly located in the paracortical region and patrol resting LNs with a random walk exhibiting similar dynamic characteristic to classical CD4+ T cells.
Early activation of LN iNKT cells
Next we examined whether iNKT cells are activated by lipid antigens drained into LNs. To mimic conditions occurring during infection with bacteria encoding iNKT cell agonists4-6,14, we injected silica particles of 200 nm in diameter31 coated with α-GalCer (200 ng α-GalCer per 1μl particles, ~109 particles/μl). Particles coated with non stimulatory lipids were used as controls in all experiments31. WT C57BL/6 mice were injected with particulate α-GalCer and, by 3 days after injection, LNs were found to be enlarged, exhibiting an increase in cell number around 3 times compared with LNs from animals injected with control particles (Fig. 2a and 2b). This enlargement was dependent on the presence of iNKT cells as it was not detected in Jα18 KO animals (lacking NKT cells) injected with α-GalCer particles (Fig. 2b). In spite of the increased cellularity, the relative proportion of different cell types (including B, T, NK and DCs) was unaffected, except for iNKT cells that were highly enriched in the LNs of animals injected with particulate α-GalCer with respect to control animals (Fig. 2c-f). Accordingly, the frequency of iNKT cells in draining LNs, but not in non-draining nodes, was increased up to 10-fold compared with total TCRβ+ cells (Fig. 2d-f). As such, intraperitoneal (i.p.) injection of particulate antigen increased the frequency of iNKT cells in the mediastinal LN (Fig. 2e), consistent with the notion that mediastinal LNs drain the peritoneal cavity32,33, while subcutaneous (s.c.) footpad injection enlarged the population of iNKT cells in the draining popliteal LN (Fig. 2f). We detected no change in the frequency of iNKT cells in draining LNs following injection of control particles coated with non-stimulatory lipids (Fig. 2d). Moreover, we observed CFSE dilution of adoptively transferred iNKT cells 2 days after injection of particulate lipid antigen, indicating that the increased frequency of iNKT cells in draining LNs was due, at least in part, to antigen-induced proliferation (Fig. 2g).
Figure 2.
Early activation of LN iNKT cells. (a-f) Animals were injected with α-GalCer or control particles and 3 days later LNs were analyzed. (a) Images of popliteal LNs from WT animals injected with control (top) or α-GalCer (bottom) particles. (b) Fold increase in total cell number (b) or in the depicted populations (c) in LNs of WT (b,c) and Jα18 (NKT KO, b) mice after injection with α-GalCer particles relative to animals injected with control particles; *p<0.05 (d) iNKT cells in mediastinal LNs after i.p. injection of control (left) or α-GalCer (right) particles (e,f) Percentage of iNKT cells in various LNs after i.p. (e) or s.c. foot-pad (f) injection of α-GalCer particles. +,draining; −,non-draining LNs. Each dot represents one LN, black lines represent mean. Data were compared with two-tailed t-test. (p**<0.005; ***p=0.0007). (g) CFSE-labelled adoptively transferred iNKT cells were detected as CFSE+CD1d-tet+TCRβ+B220−DAPI− two days after injection of α-GalCer (black) or control (grey) particles. (h-k) iNKT cell activation was measured as expression of CD25 (h-j) and CD69 (k) after i.p. injection of α-GalCer (black) or control particles (grey). MFI were normalized to mice injected with control particles. (l) NK1.1 expression in LN iNKT cells (left). Expression of CD25 (middle) and CD69 (right) in NK1.1+ (black) and NK1.1− (red) iNKT cells in mediastinal LNs 12 h after injection with α-GalCer (empty profiles) or control particles (filled profiles). (m) Intracellular IFN-γ staining for mediastinal LN-iNKT cells 12 h after injection of α-GalCer (black) or control (grey) particles.
When we analyzed the activation of endogenous iNKT cells within the first hours after antigen administration we detected that, as soon as 6 h after injection, iNKT cells in draining LNs up-regulated the expression of CD25 (Fig. 2h-2j) and CD69 (Fig. 2k), indicating that antigen recognition takes place in the nodes in the very early hours after administration. Moreover, both NK1.1+ and NK1.1− iNKT cell populations in the LNs showed identical CD25 and CD69 up-regulation after injection of particulate lipid antigen (Fig. 2l). In agreement with the ability of iNKT cells to rapidly secrete large amounts of cytokines after activation27, we observed production of IFN-γ by LN iNKT cells at 12 h after antigen injection, which was detected by ex vivo intracellular staining of LN single cell suspensions without addition of brefeldin A (Fig. 2m).
Thus, iNKT cells are rapidly activated in the LNs in response to antigen administration, indicating that they can play a role in the early initiation of immune responses in draining nodes.
iNKT cell dynamics in response to specific antigen
Previous investigations have revealed that the dynamics of B and T cells in LNs are altered following administration of cognate antigen20. Accordingly, we used multi-photon microscopy to examine the dynamics of iNKT cells adoptively transferred into C57BL/6 WT mice in the LN at various times after administration of particulate lipid antigen.
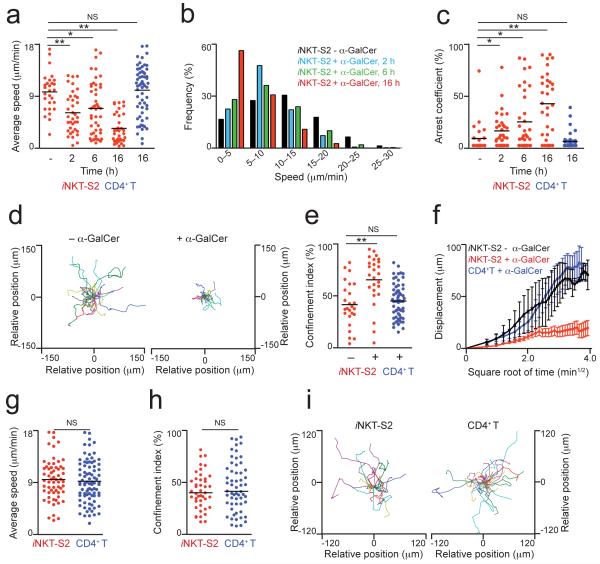
We observed that iNKT cells exhibit a decrease in average speed as soon as 2 h after injection of particulate lipid antigen (6.5 ± 3.4 μm/min for iNKT-S2 cells) which became more apparent by 16 h after injection (3.8 ± 2.4 μm/min and 2.9 ± 1.8 μm/min for iNKT-S2 and iNKT-S1 cells respectively; Fig. 3a, Supplementary Fig. 3a and Supplementary Movies 3 and 4). This decrease in speed was accompanied by an increase in long-lasting arrests, that is reflected both in the altered distribution of instantaneous speeds (Fig. 3b and Supplementary Fig. 3b) and in the increased arrest coefficient (Fig. 3c and Supplementary Fig. 3c). In addition we observed that iNKT cells become more confined following antigen administration, as evidenced by the constriction of the migratory trajectories (Fig. 3d and Supplementary Fig. 3e) and increase in confinement index (Fig. 3e and Supplementary Fig. 3d). Furthermore, the motility coefficient of iNKT cells is reduced around 10-fold to 5.0 ± 2.8 μm2/min and 3.8 ± 3.3 μm2/min for iNKT-S2 and iNKT-S1 cells respectively in response to particulate lipid antigen (Fig. 3f and Supplementary Fig. 3f). The speed and confinement of CD4+ T cells were not affected following injection of particles coated with lipid antigen (Fig. 3a-3f and Supplementary Fig. 3). iNKT cells purified using our two different strategies showed identical dynamic behaviour in response to the administration of specific antigen.
Figure 3.
iNKT cells arrest in the LNs in response to specific antigen. (a-i) iNKT-S2 cells (red), alongside CD4+ T cells (blue), were labelled and injected i.v. into recipient mice and allowed to home for 14-16 h. (a-f) WT recipient mice were injected i.p. with particles coated with α-GalCer (+ α-GalCer) or control lipids (− α-GalCer) and at various time points draining mediastinal LNs were removed and imaged by multi-photon microscopy. The average speed (a), instantaneous speed distribution (b) and arrest coefficient (c) of iNKT cells are shown at different time-points. (d,e) Representative migratory tracks (d) and confinement index (e) for iNKT cells at 16 h after injection of particulate lipids are shown. (f) Displacement (mean ± s.e.m.) of iNKT cells from mice injected with particles coated with α-GalCer (red) or control lipids (black) is plotted against the square root of time. Dynamic parameters for CD4+ T cells (blue, a,c,e,f) at 16 h after injection of particulate α-GalCer are shown. (g-i) CD1d-KO recipient mice were i.p. injected with particles coated with α-GalCer and mediastinal LNs were imaged at 16 h after antigen administration. The average speed (g), confinement index (h) and representative migratory tracks (i) of iNKT and CD4+ T cells are shown. Mean values are shown as black lines. Data were obtained from at least two independent experiments per condition and were compared with a two-tailed unpaired Mann-Whitney test. *p<0.01; **p<0.0005 NS, not significant.
In a,c,e,g,h, each symbol represents one cell
To ascertain if the observed arrest of iNKT cells is dependent on CD1d-mediated antigen presentation, we used CD1d-deficient mice as recipients for adoptive transfer of iNKT-S2 and CD4+ T cells. 16 h after administration of particulate lipid antigen, iNKT cells behaved in a similar manner to CD4+ T cells and did not exhibit a decrease in average speed (Fig. 3g and Supplementary Movie 5). In addition, the migratory trajectories and confinement index of iNKT cells transferred into CD1d-deficient mice were not altered following administration of antigen (Fig. 3h and 3i). Thus, in the absence of CD1d-mediated presentation, the behaviour of iNKT cells in response to specific antigen administration is indistinguishable from CD4+ T cells.
Taken together, these findings demonstrate that LN iNKT cells become confined as a result of CD1d-mediated antigen presentation within hours of administration of particulate lipid antigen.
iNKT cells arrest on CD169+ SCS macrophages
Given that iNKT cells in draining LNs are activated in response to specific lipid antigen in a CD1d-dependent manner, we investigated both the site and the APCs involved in antigen presentation to iNKT cells. To achieve this we used fluorescent-labelled particulate lipids and tracked their distribution in the LNs through a combination of flow cytometry and confocal microscopy alongside multi-photon microscopy. Within minutes of injection, we detected particulate lipid antigen primarily within the SCS and medullary regions of the draining LNs (Supplementary Fig. 4a-4e). When we visualized the behaviour of iNKT-S2 cells in the LN after antigen arrival by multi-photon microscopy we observed that, as soon as 2 h after injection, adoptively transferred iNKT cells move towards the SCS and are confined in the vicinity of antigen-rich areas (Fig. 4a, Supplementary Fig. 5a and Supplementary Movie 6). Further, 16 h after antigen administration, these iNKT cells were predominantly observed in the LN periphery and, in agreement with our earlier data, exhibited long-lasting arrests (Fig. 4b, Supplementary Fig. 5b and Supplementary Movie 7). iNKT-S1 cells were also found in close proximity to the SCS region 16 h after injection of particulate lipid antigen (Supplementary Fig. 5c and Supplementary Movie 8).
Figure 4.
iNKT cells arrest on SCS CD169+ macrophages (a,b) iNKT-S2 (red) and B cells (blue), were transferred into WT recipients prior to i.p. injection of α-GalCer particles (green). Mediastinal LNs were imaged by multi-photon microscopy. (a) Time-lapse images from a movie acquired 15-65 μm below the LN surface showing an iNKT cell arrested in the SCS (white circle) 2 h after injection of particulate α-GalCer. Scale bar, 20 μm (b) Three-dimensional reconstruction of the SCS 16 h after injection of particulate α-GalCer, including tracks of B and iNKT cells (20 min movie). (c) Confocal microscopy images of mediastinal LNs 2 h after i.p. injection of α-GalCer particles (green), stained with CD169 (blue, left and middle) or CD11c (blue, right). Scale bar, 300 μm (left), 5 μm (middle), 15 μm (right) (d) Flow cytometry analyses of lipid uptake by CD169+ macrophages (left) or CD11chi DCs (right) in mediastinal LNs 4 h after injection of α-GalCer particles (black). Grey, no injected (e) Three-dimensional reconstruction of a LN (100 μm section) after injection of α-GalCer (top) or control (bottom) particles, showing iNKT cells (blue), particulate lipids (red) and CD169 cells (green). Long ticks, 50 μm. (f) Percentage of iNKT cells in direct contact with CD169+ macrophages after injection of α-GalCer or control particles. (g) Time-lapse images from two different movies (top and bottom) showing iNKT cells (arrowheads) in contact with CD169+ macrophages (green) 6 h after α-GalCer (red) injection. Time stamp is in mm:ss. Scale bar, 20 μm (top), 15 μm (bottom).
Data are representative of three (a-d) or six (e-g) experiments.
CD169+ macrophages can mediate retention of antigen at the SCS region of LNs21,23-25,34,35. In line with this, 2 h after administration we observed particulate lipid antigen arriving to the LNs and localizing within CD169+ macrophages and in association with LyVE-1+ lymphatic vessels (Fig. 4c and Supplementary Fig. 4f). Accordingly, particles were detected with more than 50% of CD169+-CD11b+ cells in the draining LNs by flow cytometry within 4 h of antigen administration (Fig. 4d). No antigen retention by CD11chi conventional DCs was observed at this time point (Fig. 4c and 4d). To further characterise the behaviour of LN iNKT cells after antigen arrival, we visualized iNKT-S2 cells in recipient animals injected with fluorescently labelled anti-mouse CD169 antibody34 alongside with labelled particulate α-GalCer or control lipids (Fig. 4e). We fixed the LNs of recipient animals, imaged them by multi-photon microscopy and analyzed the location of iNKT cells relative to SCS CD169+ macrophages. CD169+ cells formed a layer in the SCS of the LNs retaining particulate lipids (Fig. 4e). Within 6 h of antigen administration, we observed that iNKT cells are retained in the vicinity of the SCS and approximately 55% (40 out of 75 iNKT cells) establish direct contact (< 5μm apart) with CD169+ macrophages (Fig. 4e and 4f). In contrast, following administration of particles containing non-stimulatory lipids iNKT cells were predominantly located in deeper regions of the LN with few (18%, 10 out of 54 iNKT cells) making contact with SCS macrophages (Fig. 4e and 4f). Furthermore imaging of living LNs revealed iNKT cells stopping in the SCS region and establishing long-lasting interactions with particle-containing macrophages by 6 h after antigen injection. A large proportion of iNKT cell-macrophage contacts (81%, 59 out of 73) lasted for the duration of the 20 min imaging period and dissociation of these conjugates was rarely observed (19%, 14 out of 73) (Fig. 4g and Supplementary Movies 9 and 10).
Thus, iNKT cells make specific and long-lasting contacts with antigen-loaded CD169+ macrophages within few hours of antigen arriving at the draining LN.
iNKT cell activation by LN macrophages
To establish if macrophages are capable of mediating iNKT cell activation in the LNs, we depleted resident macrophages by treatment with clodronate liposomes (CLL). In agreement with previous reports23 we observed that CCL preferentially depleted macrophages only in draining LNs leaving non draining LNs intact (data not shown). Though CLL treatment induced an increase in total cell numbers in draining LNs, it did not alter the relative proportions of B, T, DC and iNKT cells (Fig. 5a and 5b; Supplementary Fig. 6a) or the gross morphological structure of the LN (Supplementary Fig. 6c). In addition, macrophage depletion did not affect the basal state of iNKT cells as the expression of CD25 remained unchanged after CLL treatment (Supplementary Fig. 6b).
Figure 5.
Macrophages are required for early iNKT cell activation in the LNs (a-f) WT mice were injected with CLL (+CLL) or not (−CLL) 6 days prior to removal and analysis of draining LNs. (a) Confocal microscopy images of LNs from CCL treated (rigth) and untreated (left) mice stained for B220 (blue) and CD169 (red). (b) Percentage of CD169+ macrophages (left) and iNKT cells (right) from total mononuclear cells in LNs of CLL treated or untreated mice. *p<0.0001 (c-f) iNKT cell activation was assessed by flow cytometry after injection with α-GalCer (linear profiles) or control (grey filled profile) particles. MFI were normalized to mice injected with control particles. (c) CD25 expression in LN iNKT cells was assessed 12 h after injection of α-GalCer particles into CLL treated (red) or untreated (black) mice. *p=0.0023 (d-f) Frequency of iNKT cells in draining LNs was assessed 3 days after injection of particles into CLL treated or untreated mice. Representative FACS profiles (d) and quantification of iNKT cell frequency (e, *p=0.0025, **p=0.0002) and numbers (f, **p=0.0015; *p=0.0408) are shown. Each data point represents one LN from individual mice. Black lines represent mean values. Data were pooled from two independent experiments and compared with a two-tailed t-test. NS, not significant.
Error bars (f), s.e.m.
To analyze the role of macrophages in iNKT cell activation we injected macrophage-depleted and control animals with particulate lipids. In the absence of macrophages, particulate lipids were predominantly detected deep in the B cell follicles and in medullary regions (Supplementary Fig. 6d). By 12 h after injection of particulate lipid antigen we observed that CD25 up-regulation by iNKT cells was decreased around two-fold in draining LNs of CCL treated with respect to untreated animals (Fig. 5c). Furthermore, macrophage depletion prevented the expansion of iNKT cells 3 days after administration of antigen as reflected in the percentage and number of iNKT cells found in draining LNs (Fig. 5d-5f). Therefore, selective macrophage depletion in the LNs leads to inefficient iNKT cell activation and prevents the expansion of the iNKT cell population.
CD169+ macrophages mediate CD1d-dependent antigen presentation
Next, we investigated the possibility that CD169+ macrophages participate directly in the presentation of antigenic lipids to iNKT cells. In line with this, we observed that CD169+ macrophages expressed cell surface CD1d by flow cytometry (Fig. 6a). Subsequently, CD11b+ cells were enriched from LN single cell suspensions by magnetic selection and CD169+-CD11b+ macrophages were highly purified by flow cytometry sorting (Fig. 6b). After pre-incubation for 2 h with particulate lipids, CD169+ macrophages were co-cultured with mouse iNKT hybridoma DN32.D3 cells. α-GalCer was efficiently presented by CD169+ cells, as defined by IL-2 production by DN32.D3 cells (Fig. 6c). To ascertain if iNKT activation is dependent on antigen internalization, we used particles containing Gal(α1→2)α-GalCer, an α-GalCer analogue that requires intracellular processing prior to recognition by iNKT cells36. Co-culture of DN32.D3 cells with macrophages pre-incubated with Gal(α1→2)α-GalCer particles induced secretion of IL-2 (Fig. 6d), indicating that iNKT cell activation is indeed dependent on antigen internalization by macrophages. Moreover we observed that CD169+ macrophages were able to present antigen to a similar extent as CD11chi DCs purified from mice LNs (Fig. 6e). In contrast, LN B cells were not able induce IL-2 secretion in co-cultured DN32.D3 iNKT cells (Fig. 6e), in line with their low phagocytic capacity in absence of specific BCR-mediated uptake31.
Figure 6.
LN CD169+ macrophages present lipids to iNKT cells. (a) CD1d expression in CD169+-CD11b+ macrophages (black). Grey, isotype control. (b) CD11b+ cells before (left, middle) or after (right) sort stained with anti-CD169 antibody (middle, right) or isotype control (left). (c-d) Purified CD169+ cells from b were incubated with α-GalCer (+,c), Gal(α1→2)α-GalCer (+,d) or control (−) particles before co-culture with DN32.D3 cells. IL-2 was the read-out for lipid presentation by CD169+ cells. (e) LN CD169+ macrophages (Mφ filled squares), CD11chi DCs (triangles) and B cells (empty squares) were incubated with α-GalCer particles before co-culture (1-5 × 104) with 5 × 104 DN32.D3 cells. (f) CD169+CD11b+CD11cint cells were stained with anti-CD11b and F4/80 antibodies before (left) and after (right) purification of SCS macrophages. (g) SCS macrophages were incubated with α-GalCer (+) or control (−) particles before co-culture with DN32.D3 cells (h,i) Mice were injected with α-GalCer (+,h), Gal(α1→2)α-GalCer (+,i) or control (−) particles and 2 h later CD169+ cells from draining LNs were purified and co-cultured with DN32.D3 cells. (j) Mice were injected with α-GalCer particles and CD169+ macrophages (Mφ), CD11chi DCs and B cells were purified and co-cultured with DN32.D3 cells. (k-l) CD169+ cells were pulsed with α-GalCer (+) or control (−) particles before co-culture (2 or 3 days) with CFSE labelled-primary iNKT cells purified from LNs. iNKT cells cultured without macrophages (No Mφ) were included as control. (k) IFN-γ was measured as a read-out for lipid presentation. (l) iNKT cell proliferation was detected as CFSE dilution. Data represent one experiment from at least two performed independently. Statistical analyses, two-tailed t-test. *p<0.0001.
Error bars (c,d,g-k), s.e.m.
CD169+ macrophages can be distinguished as SCS or medullary macrophages on the basis of F4/80 expression35. Therefore, we sought to determine if macrophages from the SCS were capable of lipid antigen presentation by purifying them as CD169+CD11b+CD11cint F4/80− cells from mice LNs (Fig. 6f and Supplementary Fig 7). Indeed, we observed that SCS macrophages were able to present lipid antigens to co-cultured DN32.D3 iNKT cells as evidenced by IL-2 secretion (Fig. 6g).
As purified macrophages are able to present lipid antigen to iNKT cells in vitro, we assessed the ability of CD169+ cells to retain and present particulate α-GalCer in vivo. To do this, we injected animals with particles coated with α-GalCer or control lipids and 2 h later CD169+ cells were purified from draining LNs and incubated in vitro with DN32.D3 iNKT cells. Only CD169+ macrophages from animals injected with particulate α-GalCer stimulated IL-2 secretion from DN32.D3 cells (Fig. 6h). In a similar manner, CD169+ cells purified from draining nodes of WT mice injected with Gal(α1→2)α-GalCer coated particles, gave rise to IL-2 secretion from co-cultured DN32.D3 cells (Fig. 6i). CD169+ macrophages purified from draining LNs of animals injected with control particles were unable to induce IL-2 secretion by DN32.D3 cells (Fig. 6h and 6i). Furthermore, while LN DCs were capable of lipid antigen presentation in vitro, neither CD11chi DCs nor B cells purified from draining LNs 2 h after injection of particulate α-GalCer were able to stimulate IL-2 secretion from co-cultured DN32.D3 cells (Fig 6j).
Finally, to establish if CD169+ macrophages can present antigen to primary LN-iNKT cells, we performed presentation assays with CD169+ cells and CFSE-labelled primary iNKT-S1 cells purified from mouse LNs (Fig. 6k and 6l). CD169+ macrophages pulsed with particulate α-GalCer were able to induce IFN-γ secretion (Fig. 6k) and proliferation of primary LN-iNKT cells as evidenced by CFSE dilution (Fig. 6l). In the absence of macrophages or when iNKT cells were co-cultured with CD169+ cells pulsed with control lipids, no proliferation or IFN-γ secretion was detected for primary iNKT cells (Fig. 6l).
Thus, CD169+ macrophages internalize antigenic lipids both in vitro and in vivo, and can present them in association with CD1d to efficiently activate iNKT cells.
Bacterial glycolipids stimulate macrophage-dependent iNKT cell activation
To verify our findings with an iNKT cell agonist derived from bacterial glycolipids, silica particles were coated with liposomes containing α-linked galacturonic glycosphingolipid (GSL-1′) expressed by Sphingomonas yanoikuyae, which can activate iNKT cells5,37. We observed that particulate GSL-1′ induced a 1.6-fold increase in the total number of cells in the LN compared with LNs from animals injected with control particles (Fig. 7a). This enlargement was dependent on the presence of iNKT cells as it was not detected in Jα18 KO animals (lacking NKT cells) injected with GSL-1′-coated particles (Fig. 7a). While the relative proportion of DCs, B and T cells remained the same, LN iNKT cells were enriched up to 3-fold upon injection of particulate GSL-1′ (Fig. 7b). In addition we observed that iNKT cells in draining LNs up-regulate CD25 and CD69, and produce IFN-γ within few hours of particulate GSL-1′ administration (Fig. 7c-7f). To test whether iNKT cell responses to Sphingomonas glycolipids were also dependent on LN resident macrophages, we specifically depleted LN macrophages by CLL treatment. After administration of particulate GSL-1′, iNKT cells were not efficiently activated in draining LNs of CLL-treated animals, showing up to a three-fold decrease in CD25 up-regulation compared to iNKT cells in the LNs from untreated animals (Fig. 7g). Finally, we adoptively transferred labelled iNKT-S1 cells and CD4+ T cells into WT recipients to visualize the dynamics of iNKT cells in response to particulate GSL-1′ (Supplementary Movie 11). In contrast to CD4+ T cells, iNKT cells exhibited a significant decrease in speed to 7.3 + 3.6 μm/min 16 h after particulate GSL-1′ administration (Fig. 7h). This was accompanied by an increase in long-lasting arrests reflected by the altered distribution of instantaneous speeds (Fig. 7i), the increased arrest coefficient (Fig. 7j) and the decreased motility coefficient (31.2 ± 9.9 μm2/min; Fig. 7k).
Figure 7.
Bacterial glycolipids stimulate macrophage-dependent iNKT cell activation (a-b) Fold increase in total cell number (a) or in the depicted populations (b) in LNs of WT (a,b) and Jα18 (NKT KO, a) mice after injection with GSL-1′ particles relative to animals injected with control particles, *p<0.05; NS, no significant (c-f) iNKT cell activation was assessed by flow cytometry after i.p. injection of GSL-1′ (black) or control particles (grey). (c,d) CD25 expression in mediastinal LN iNKT cells at various times after injection. MFI were normalized to mice injected with control particles. (e,f) Expression of CD69 (e) and intracellular IFN-γ (f) in iNKT cells from mediastinal LNs 12 h after injection. Data are representative of 3 independent experiments. (g) CD25 expression in LN iNKT cells was assessed 12 h after injection of GSL-1′ particles into CLL treated (red) or untreated (black) mice. MFI were normalized to mice injected with particles containing control lipids. Black lines indicate mean values. Data points represent one mediastinal LN from individual mice and representative from two independent experiments with at least 2 animals per experiment. Data were compared with two-tailed t-test, ***p=0.0006. (h-k) iNKT-S1 (red) and CD4+T cells (blue) were transferred into WT recipients injected i.p. with GSL-1′ particles. Average speed (h), instantaneous speed distribution (i), arrest coefficient (j) and mean displacement (± s.e.m., k) for iNKT and CD4+T cells are shown at 16 h after antigen administration. Data were obtained from at least two independent experiments and compared with a two-tailed unpaired Mann-Whitney test. **p<0.005
Error bars (a,b), s.e.m.
Thus, similar to α-GalCer, bacterial glycolipids can mediate the early activation of iNKT cells in draining LNs in a manner dependent on the presence of macrophages.
DISCUSSION
This study provides new insight into the early events of iNKT cell activation in the LNs in vivo. We have imaged iNKT cells in living lymphoid tissues showing that they patrol the LN paracortex in resting conditions. Within minutes after administration, lipid antigens arrive to the SCS of the LNs where they are retained and presented to iNKT cells. Cognate antigen recognition induces iNKT cell arrest and leads to cytokine production and expansion of the iNKT cell population. Finally, we demonstrate that CD169+ macrophages play a critical role in iNKT cell activation as they retain, internalize and present lipids to iNKT cells acting as bona fide APCs in the LNs.
Over the past decade much effort has been expended in the search for lipid antigens that bind CD1d molecules and activate iNKT cells2,14 however, very little is known about how and where iNKT cells encounter antigen in vivo. In fact, to date very few studies have achieved the visualization of iNKT cells in situ, probably due to the lack of reliable reagents that allow their identification. In this line, we and others have unsuccessfully tried to visualize iNKT cells by immunohistochemitry using CD1d-tet29. On the other hand, the use of less stringent markers (like NK1.1) results in the inclusion of other cell populations making difficult the unequivocal identification of iNKT cells. To circumvent these issues we used two complementary strategies to purify iNKT cells prior to their labelling, adoptive transfer and visualization by multi-photon microscopy. Since we were concerned that the purification of iNKT cells with CD1d-tet could occasionally induce their partial activation29, in a first approach we purified iNKT cells from spleen and liver as TCRβ+NK1.1+ cells. However, as LN iNKT cells are represented by both NK1.1+ and NK1.1− cells28 we verified our results by purifying iNKT cells from the LNs as TCRβ+CD1d-tet+ cells. iNKT cells purified by both strategies were detected in the LNs of recipient animals in similar numbers and they showed identical location and dynamic behaviours, independent of their tissue of origin or purification method. In spite of the fact that iNKT cell number is lower in the LNs compared with the spleen or liver population, in normal conditions of clonal abundance the number of iNKT cells in the LNs is between 500-5000 times higher than that for any antigen-specific T cell population11. Remarkably, the number of transferred iNKT cells observed in the LNs was in the same order as that of endogenous iNKT cells, allowing us to analyze iNKT cell behaviour under conditions of typical clonal abundance. As such, our adoptive transfer strategy avoided the abnormally high numbers of antigen specific cells frequently associated with this kind of experiments38. Using multi-photon microscopy imaging of living LNs, we observed that iNKT cells in resting conditions were predominantly located in the paracortical region where they move in random walks with average speed of around 10 μm/min. Accordingly, iNKT cells exhibit similar kinetic behaviour as observed for classical CD4+ T cells as they scan the LN in search for specific antigen20, 30.
Although α-GalCer is derived from a marine sponge it has proven to be a very valuable tool for dissecting the pivotal role of iNKT cells in autoimmunity, cancer and host defence2. Indeed, the wide availability of this glycolipid allowed us to understand the uptake and presentation of lipid antigens in the LNs by CD169+ macrophages that will lead to subsequent iNKT cell activation. Our dynamic imaging approach, in concert with our flow cytometry data, demonstrates that iNKT cell activation occurs within a few hours of antigen arrival at the draining LN. Furthermore, we validated these results showing that Sphingomonas glycolipids can rapidly activate LN iNKT cells inducing IFN-γ secretion, LN enlargement and altering the dynamic behaviour of iNKT cells in the LNs. In line with the fact that GSL-1′ is a weaker iNKT cell agonist compared with α-GalCer5, its effect on LN enlargement and iNKT cell dynamics was less marked than that observed for α-GalCer. However, GSL-1′ was efficient in inducing activation of LN iNKT cells as measured by CD25 and CD69 up-regulation and IFN-γ secretion. In agreement with these results, the work from Lee et al. (in the current issue) shows how liver iNKT cells are activated in response to the administration of Borrelia burdorgferi as reflected by the alteration in their dynamic behaviour. Therefore, our results point to a pivotal role of iNKT cells in the initiation of immune responses in the LNs against a variety of infectious agents.
Traditionally, iNKT cell activation has been studied using soluble lipids or lipid-pulsed DCs. However, during the course of an infection, antigenic lipids would be more likely present in the capsule of different pathogens like Sphingomonas, Borrelia or Erlichia4-6. On arrival to the LN, particulate antigens are retained in the SCS region where they can be directly recognized by specific lymphocytes21-25,35. In agreement with this, we observed that particulate lipids localised within the SCS very rapidly following administration. Subsequently iNKT cells arrest in the SCS region of the LNs within few hours after antigen administration. In line with this, naïve and memory CD8+ T cells relocate close to the SCS region within few hours after antigen arrival allowing T cell activation in the LN periphery22,25. Although the low numbers of iNKT cells in the LNs prevent examination of the kinetic of relocation of iNKT cells to the LN periphery, it is conceivable that they will follow a similar behaviour to that described for classical αβ T cells. On the other hand, lipid antigens could also be presented to iNKT cells in the LN cortex by DCs immigrating from peripheral tissues and, in fact, skin DCs are able to present antigen to iNKT cells after subcutaneous injection of soluble αGalCer13. However, DC migration to draining LNs takes place several hours after antigen administration and is unlikely to play a significant role in initiating very early immune responses26,33. Nevertheless, it is conceivable that both mechanisms of antigen presentation mediated by resident and migratory APCs collaborate to activate iNKT cells in different LN locations at various stages during the immune response.
CD169+ macrophages can be classified as SCS or medullary based on F4/80 expression and they exhibit different endocytic and degradative capabilities that might influence their function as APCs35. Several studies have identified a role for CD169+ macrophages in the SCS in presenting native antigen to follicular B cells23, 24,35 and, indeed SCS cells seem to have a higher ability to retain antigen on their surface than medullary macrophages35. However, the ability of SCS macrophages to present processed antigen to T cells is still to be established23-25,35. Our data provide formal proof that SCS CD169+ macrophages can act as bona fide APCs, internalizing and presenting lipid antigen to iNKT cells, although we do not exclude that medullary macrophages might also play a role in antigen presentation. Accordingly, depletion of LN macrophages by treating with clodronate liposomes severely impairs iNKT cell activation and avoids further proliferation. Although the mechanism governing the decision for antigen retention or internalization by SCS macrophages has not been characterized, this may depend on the cell surface receptors responsible for antigen binding. Indeed, in DCs, antigen binding through FcγRIIB leads to retention and/or recycling of intact antigen to the surface whereas binding through FcγRI and FcγRIII targets antigen for intracellular degradation39. Thus, DCs are capable of presenting antigen to both T and B cells40,41. Recognition of microbial lipids by iNKT cells in the context of an infection may require internalization of the pathogen through different sets of surface receptors and the release of the microbial glycolipids in intracellular compartments before loading in CD1d. In line with this, it has been suggested that CD169 (also known as sialoadhesin) could be involved in regulating the function of different immune cells, either by favouring cell-cell interactions or by functioning as a phagocytic receptor to clear sialylated pathogens42. However, the identification of the molecules expressed on the surface of macrophages that could play a direct role in modulating iNKT cell responses will require further investigation.
Activation of iNKT cells may have important implications for the outcome of immune responses as these cells have been described as a bridge between adaptive and innate immune responses for their capacity to make rapid responses that lead to downstream activation of many other cell types1,16,43. Indeed, mice lacking iNKT cells have a reduced clearance after infection with several pathogens like Sphingomonas or Erlichia5,6,14. Moreover, iNKT-deficient animals exhibit enhanced mortality in response to infection with influenza virus, as a result of a reduction in specific T and B cell responses and of an expansion of myeloid-derived suppressor cells44. Thus, while we have demonstrated that CD169+ macrophages are able to present antigen to iNKT cells at the start of an immune response, it is conceivable that iNKT cells interact in an antigen-dependent manner with other cell types during the initiation or at later stages of an immune reaction. In this line, it is known that several hours after injection antigen-loaded DCs arrive to the LNs where they are able to interact with antigen-specific T cells26. iNKT cells could therefore interact with lipid-loaded DCs in the LNs which can lead to an increase in CD4+ and CD8+ T cell responses16,43. In addition, the role of iNKT cells in helping antigen specific B cell proliferation and antibody production is well-established31,45,46. Specific B cells take up antigen on its arrival to the LNs and subsequently migrate towards the border between the B cell and the T cell area where they form stable conjugates with antigen specific T cells47. Our preliminary data suggest that B cell-iNKT cell interactions can take place in the LNs within 1-2 days of antigen administration and result in B cell activation and proliferation (P.B. and F.D.B, unpublished data). Consequently, it is conceivable that SCS macrophages act as a platform to allow antigen presentation to iNKT cells at the beginning of an immune response, whereas later B cell-iNKT cell interactions will favour the development of adaptive immune responses. Accordingly, this adjuvant-like feature of iNKT cells make them great candidates for the design of new vaccination strategies1. Further studies to target antigen to SCS macrophages facilitating fast iNKT cell activation might allow improved vaccination approaches.
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded by: Cancer Research UK; EMBO YIP Award (F.D.B); Royal Society Wolfson Research Merit Award (F.D.B.); Cancer Research UK (C399/A2291 and C5255/A10339) (V.C.) and The Wellcome Trust (Programme Grant 084923 V.C., G.B. and F.D.B.). P.B. was supported by a postdoctoral fellowship from the Ministerio de Education from Spain (2007-0148) and by a Marie Curie Intra-European Fellowship within the seventh European Community Framework Programme (PIEF-GA-2008-220863). P.P. was supported by a Marie Curie Fellowship, Immunanomap (MRTN-CT-2006-035946). We thank Mitchell Kronenberg (La Jolla Institute for Allergy & Immunology) and Chi-Huey Wong (Scripps Laboratories) for providing GSL-1′.
Appendix
MATERIALS AND METHODS
Mice and injections
Vα14 transgenic, CD1d KO, Jα18 KO and CD45.1 C57BL/6 mice were bred and maintained at the animal facility of the John Radcliffe Hospital, Oxford. CD1d-KO animals were provided by L. Van Kaer (Vanderbilt University School of Medicine, Nashville, USA). C57BL/6 WT mice were purchased from Charles River. All experiments were approved by the Cancer Research UK, Animal Ethics Committee and the United Kingdom Home Office.
For depletion of macrophages mice were injected with a standard suspension clodronate-loaded liposomes48 six days before analysis. Clodronate was a gift of Roche Diagnostics GmbH.
Lipids and microsphere coating
Liposomes were prepared as described31 by using: 1,2-Dioleoyl-sn-Glycero-3-Phosphocholine (Avanti Polar Lipids), α-GalCer (Alexis Biochemical), Gal(α1→2)α-GalCer (produced as described49) and/or GSL-1′ (kindly provided by Dr. Mitchell Kronenberg; La Jolla Institute for Allergy and Immunology, La Jolla, USA). Silica microspheres (200 nm, Kisker GbR) were coated with liposomes as described31. CD1d-tet was produced50 or purchased from Proimmune Ltd.
Flow cytometry
For flow cytometry staining LN single-cell suspensions were prepared as described23 and anti-mouse CD16/32 (2.4G2, BD Biosciences) was used to block non-specific antibody binding. For staining of macrophages and DCs the following anti-mouse antibodies were used: CD11b-PE (M1/70), CD45/B220-pacific blue (RA3-6B2), CD11c-APC (HL3) from BD Biosciences; CD3-pacific blue (500-A2, Caltag); CD169-FITC (3D6.112, AbDSerotec) and CD1d-biotin (1B1) followed by streptavidin-PerCP (BD Biosciences). Alternatively cells were stained with: CD45/B220-pacific blue (RA3-6B2), TCRβ-FITC (H57-597), CD3-PerCP (145-2C11), CD69-PECy7 (H1.2F3) from BD Biosciences; CD25-PE (PC61.5), NK1.1-FITC (PK136) from eBiosciences; and Cd1d-tet-APC. Dead cells were excluded by staining with DAPI (2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride, Sigma). For intracellular IFN-γ staining cells were stained with CD45/B220-pacific blue, TCRβ-FITC and CD1d-tet-APC before fixation and permeabilization with Cytofix/Cytoperm (BD Biosciences) and staining with IFN-γ-PE (XMG1.2, BD Biosciences). Data were collected on a LSRII flow cytometer (Becton Dickinson) and analyzed with FlowJo (TreeStar).
Purification of LN macrophages and DCs
For purification, CD11b+ cells were enriched from LN single-cell suspensions with CD11b-magnetic beads (Miltenyi Biotec) before sorting with anti-CD11b-APC (M1/70) and CD169-FITC (3D6.112,) on a FACSAria (Becton Dickinson).
For purification of SCS macrophages and DCs, B, T and F4/80+ cells were depleted by incubation with anti-B220 (RA3-6B2, BD Biosciences), anti-CD3 (145-2C11, eBiosciences) and anti-F4/80 (BM8, Biolegend) biotinylated antibodies followed by streptavidin-magnetic beads (Dynabeads-Invitrogen). SCS macrophages and DCs were sorted as CD169+CD11b+CD11cint and CD11chiCD169−CD11b+ respectively by staining with CD11b-APC (M1/70), CD169-FITC (3D6.112) and CD11c-PE (HL3) antibodies.
Purification of iNKT, CD4+ T and B cells
iNKT cells were purified from liver, spleen and LNs from Vα14 transgenic mice. Liver and spleen single-cell suspensions were prepared44 and iNKT cells sorted with TCRβ-PE (H57-597) and NK1.1-FITC (PK136) antibodies (eBioscience). Purified cells were stained with CD1d-tet-APC (>95% purity). LN iNKT cells were isolated by depletion of B cells with B220-magnetic beads (Dynabeads-Invitrogen) and sorting with TCRβ-PE (H57-597) and CD1d-tet-APC (>97% purity).
B and CD4+ T cells were purified from spleen or LNs by using magnetic separation B or CD4+ T cell isolation kits (Miltenyi Biotec).
Lipid presentation assays and ELISA
Purified macrophages, DCs or B cells were incubated with particulate lipids (2 h), washed 3 times and cultured 1-5 × 104 cells/well with 5 × 104 DN32.D3 (kindly provided by A. Bendelac; University of Chicago, IL) or primary LN-iNKT cells in 96 well U-bottom plates with 200 μl of RPMI 1640,10% FCS. For in vivo priming experiments, mice were injected with particulate lipids in the foot-pad and 2 h later CD169+ cells were sorted from popliteal LNs and incubated with DN32.D3 cells as described above. IL-2 was measured by sandwich ELISA in the supernatant of the cultures, using anti-IL-2 (JES6-1A12) and biotinylated anti-IL-2 (JES6-5H4) antibodies (BD Biosciences). When primary iNKT cells were used IFN-γ was measured using anti-IFN-γ (R4-6A2) and biotinylated anti-IFN-γ (XMG1.2) antibodies (BD Biosciences).
Adoptive transfer
iNKT, CD4+ T and B cells (3-4 × 106) were labelled with 2 μM CFSE, 2 μM SNARF-1 or 50 μM of CMAC (Molecular Probes) as described21 and injected in the tail vein of 4-6 weeks recipients. To investigate iNKT cell homing, CD45.1 mice were used as recipients and transferred iNKT cells detected by flow cytometry staining with CD45.2-APC (104; Biolegend), CD45/B220-pacific blue (RA3-6B2, BD Biosciences) and NK1.1-FITC (PK136, eBiosciences).
To investigate iNKT cell proliferation in vivo, single-cell suspensions from Vα14 mice spleens were prepared, B cells depleted with B220-magnetic beads and cells (~30% iNKT) labeled with CFSE and transferred into WT recipients injected afterwards with α-GalCer or control particles. LNs were removed 2 days later and transferred iNKT cells stained with TCRβ-PE (H57-597, eBioscience), CD1d-tet-APC and CD45/B220-pacific blue (RA3-6B2, BD Biosciences).
Multi-photon microscopy
Mediastinal and popliteal LNs were prepared for multi-photon imaging as previously reported21. Image acquisition was performed through the capsule of the LN with an upright multi-photon microscope (Olympus Fluoview FV1000 MPE2 Twin system) using a water-immersion 25× 1.05 NA objective (Olympus XLPLNWMP) and a pulsed Ti:sapphire laser (Spectra Physics MaiTai HP DeepSee) tuned to 800 nm. For four-dimensional imaging stacks of 11-20 square xy planes spanning 508 μm by 508 μm with 5 μm z spacing were acquired over 20-30 min (30-40 time-points). Emission wavelengths were detected through 420–500 nm (CMAC), 515–560 nm (CFSE, green particles), and 590–650 nm (SNARF-1, red particles) band-pass filters. Sequences of image stacks were transformed into volume-rendered four-dimensional movies and analyzed with Imaris 6.3 × 64 software (Bitplane). Annotation and final movie compilation was performed with Adobe Photoshop CS3.0 and ImageJ. Tracks normalized at the same departure point were plotted with MatLab (The Mathworks Inc.). All statistical analysis was performed using Prism (GraphPad Software).
Immunofluorescence
Tissue sections (10 μm thick) were fixed in 4% paraformaldehyde and blocked with PBS, 1% BSA, 10% goat serum before incubation with rat anti-mouse CD45R/B220 (RA3-6B2, BD Biosciences), rat anti-mouse LyVE-1 (223322, R&D Systems) or hamster anti-mouse CD11c (N418, Caltag), followed by Alexa 633-conjugated goat anti-rat IgG or Alexa 647-conjugated goat anti-hamster IgG (Molecular Probes). Alternatively, sections were incubated with rat anti-mouse CD45R/B220-Alexa 647 (RA3-6B2, BD Biosciences), rat anti-mouse F4/80-Alexa 647 (CI:A3-1, Biolegend), rat anti-mouse CD45R/B220-FITC (RA3-6B2, BD Biosciences), rat anti-mouse CD169-FITC (3D6.112, AbDSerotec) or hamster anti-mouse CD3-FITC (145-2C11, BD Biosciences) followed by Alexa 488-conjugated anti-flourescein/Oregon green rabbit IgG (Molecular Probes) when FITC-labeled antibodies were used. Imaging was carried out on an Axiovert LSM 510-META (Zeiss) inverted microscope except for tiled images that were captured an assembled on a Fluoview FV1000 microscope (Olympus).
Footnotes
The authors declare that they have no competing financial interests.
REFERENCES
- 1.Cerundolo V, Silk J, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 3.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 4.Kinjo Y, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 5.Kinjo Y, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 6.Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 7.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 8.Paget C, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Salio M, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci USA. 2007;104:20490–20495. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyznik AJ, et al. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon J, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laloux V, Beaudoin L, Ronet C, Lehuen A. Phenotypic and functional differences between NKT cells colonizing splanchnic and peripheral lymph nodes. J Immunol. 2002;168:3251–3258. doi: 10.4049/jimmunol.168.7.3251. [DOI] [PubMed] [Google Scholar]
- 13.Tripp C, Sparber F, Hermans I, Romani N, Stoitzner P. Glycolipids Injected into the Skin Are Presented to NKT Cells in the Draining Lymph Node Independently of Migratory Skin Dendritic Cells. The Journal of Immunology. 2009;182:7644–7654. doi: 10.4049/jimmunol.0900134. [DOI] [PubMed] [Google Scholar]
- 14.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 15.Silk JD, Salio M, Brown J, Jones EY, Cerundolo V. Structural and functional aspects of lipid binding by CD1 molecules. Annu Rev Cell Dev Biol. 2008;24:369–395. doi: 10.1146/annurev.cellbio.24.110707.175359. [DOI] [PubMed] [Google Scholar]
- 16.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmieg J, Yang G, Franck RW, van Rooijen N, Tsuji M. Glycolipid presentation to natural killer T cells differs in an organ-dependent fashion. Proc Natl Acad Sci USA. 2005;102:1127–1132. doi: 10.1073/pnas.0408288102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geissmann F, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velázquez P, et al. Cutting edge: activation by innate cytokines or microbial antigens can cause arrest of natural killer T cell patrolling of liver sinusoids. J Immunol. 2008;180:2024–2028. doi: 10.4049/jimmunol.180.4.2024. [DOI] [PubMed] [Google Scholar]
- 20.Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. doi: 10.1146/annurev.immunol.24.021605.090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Hickman HD, et al. Direct priming of antiviral CD8(+) T cells in the peripheral interfollicular region of lymph nodes. Nat Immunol. 2008 doi: 10.1038/ni1557. [DOI] [PubMed] [Google Scholar]
- 23.Junt T, et al. Subcapsular sinus macrophages in lymph nodes clear lymphborne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 24.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 25.Chtanova T, et al. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity. 2009;31:342–355. doi: 10.1016/j.immuni.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itano AA, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 27.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 28.Doisne J-M, et al. Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor (gamma)t+ and respond preferentially under inflammatory conditions. J Immunol. 2009;183:2142–2149. doi: 10.4049/jimmunol.0901059. [DOI] [PubMed] [Google Scholar]
- 29.Berzins SP, Smyth MJ, Godfrey DI. Working with NKT cells--pitfalls and practicalities. Curr Opin Immunol. 2005;17:448–454. doi: 10.1016/j.coi.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 31.Barral P, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tilney NL. Patterns of lymphatic drainage in the adult laboratory rat. J Anat. 1971;109:369–383. [PMC free article] [PubMed] [Google Scholar]
- 33.Kool M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chtanova T, et al. Dynamics of neutrophil migration in lymph nodes during infection. Immunity. 2008;29:487–496. doi: 10.1016/j.immuni.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phan T, Green J, Gray E, Xu Y, Cyster J. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol. 2009;10:786–793. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prigozy T, et al. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291:664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 37.Kinjo Y, et al. Natural Sphingomonas glycolipids vary greatly in their ability to activate natural killer T cells. Chem Biol. 2008;15:654–664. doi: 10.1016/j.chembiol.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badovinac V, Haring J, Harty J. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergtold A, Desai D, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 41.Qi H, Egen JG, Huang AY, Germain R. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 42.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 43.Hermans IF, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 44.De Santo C, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galli G, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leadbetter, et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada T, et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 49.Veerapen N, et al. Synthesis and biological activity of alpha-galactosyl ceramide KRN7000 and galactosyl (alpha1-->2) galactosyl ceramide. Bioorg Med Chem Lett. 2009;19:4288–4291. doi: 10.1016/j.bmcl.2009.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karadimitris A, et al. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc Natl Acad Sci USA. 2001;98:3294–3298. doi: 10.1073/pnas.051604498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.