Abstract
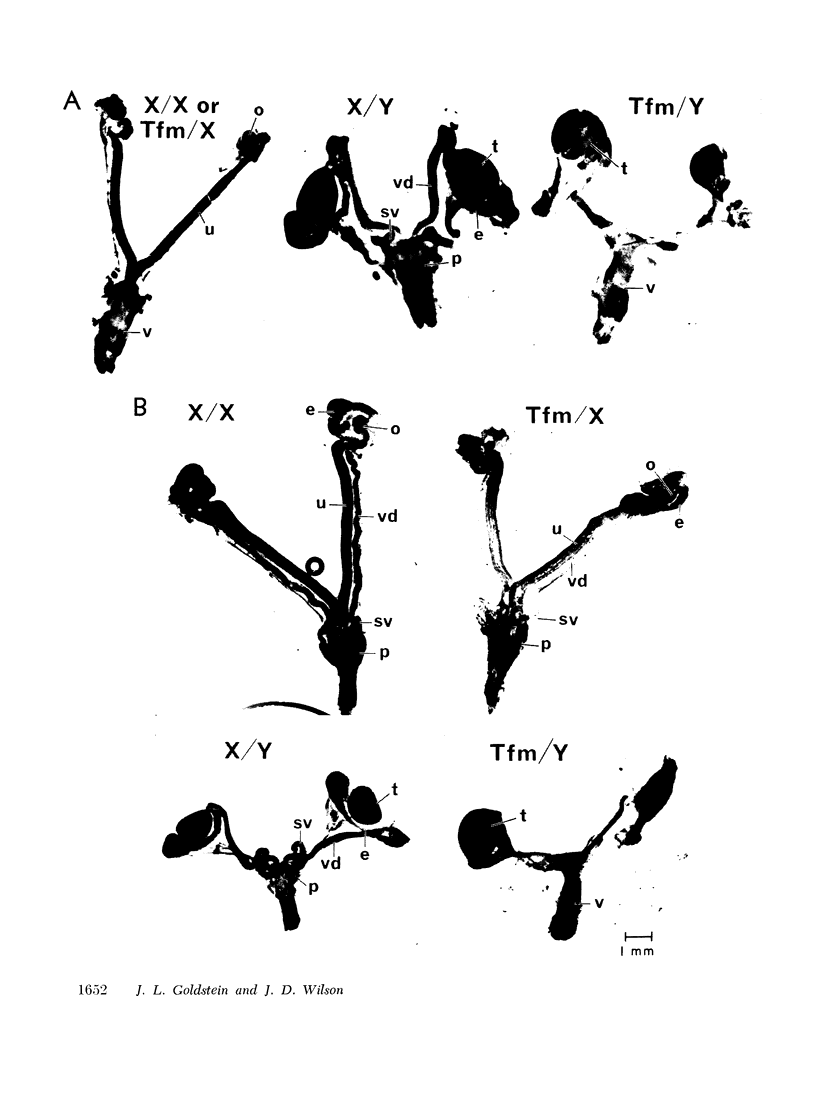
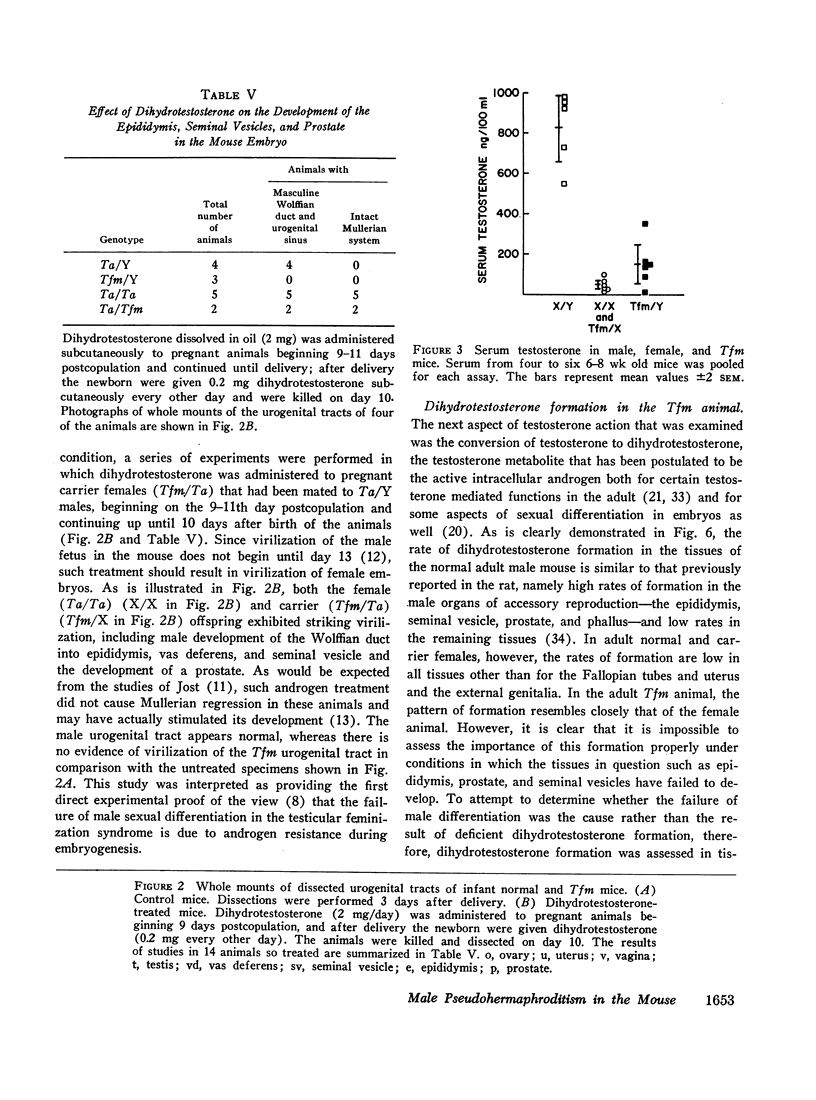
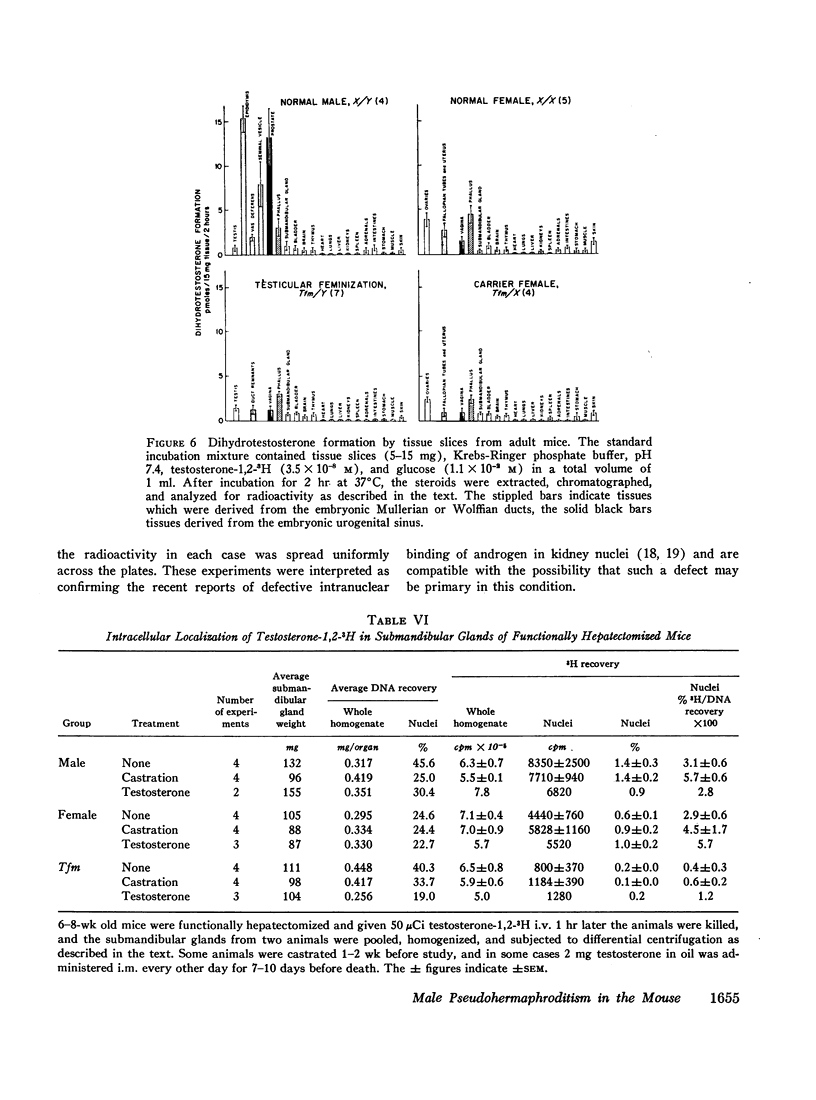
The pathogenesis of the male pseudohermaphroditism in the mouse with X-linked testicular feminization (Tfm) has been investigated by comparing testosterone formation, the effects of androgen administration, and the metabolism of testosterone-1,2-3H in normal mice and Tfm mice of varying ages. First, it was established that the adult Tfm animal, in contrast to the human with testicular feminization, has both a low serum testosterone and a low rate of testosterone formation as assessed in slices of testes utilizing a variety of precursors. However, the formation of testosterone from pregnenolone-7α-3H was shown to be normal in newborn Tfm testes, suggesting that a defect in testosterone synthesis may not be primary to this mutation. Second, to establish that the pseudohermaphroditic state is due to androgen resistance rather than to diminished androgen biosynthesis during fetal life, the effect of the administration of dihydrotestosterone to pregnant animals was studied in male, female, and Tfm offspring. Whereas normal and carrier female littermates demonstrated striking virilization of the internal genital tract after such treatment, there was no sign of virilization in the Tfm animals. This finding provides direct experimental evidence in support of the view that male pseudohermaphroditism in testicular feminization is the result of resistance to androgen action during androgen-mediated sexual differentiation in embryos. Third, the metabolism of testosterone-1,2-3H was investigated both in tissue slices and in functionally hepatectomized animals. Dihydrotestosterone formation in tissue slices of the fetal anlage of the male organs of accessory reproduction is normal in the Tfm animal, suggesting that the primary defect in this disorder involves an intracellular event subsequent to this step and that the deficient dihydrotestosterone formation observed in the adult genital tract of the Tfm mouse is secondary to the failure of differentiation in these tissues. Finally, deficient binding of testosterone in the nuclei of the submandibular gland of adult Tfm animals, a known testosterone target tissue, was demonstrated in functionally hepatectomized mice. This finding could either be a manifestation of the primary genetic defect in this disorder or might reflect another acquired abnormality due to incomplete differentiation of adrogen-sensitive cell lines.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin C. W., Allison J. E., Stanley A. J., Gumbrecklg Secretion of testosterone by the pseudohermaphrodite rat. Endocrinology. 1969 Feb;84(2):435–436. doi: 10.1210/endo-84-2-435. [DOI] [PubMed] [Google Scholar]
- Bardin C. W., Bullock L., Schneider G., Allison J. E., Stanley A. J. Pseudohermaphrodite rat: end organ insensitivity to testosterone. Science. 1970 Feb 20;167(3921):1136–1137. doi: 10.1126/science.167.3921.1136. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J. D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968 Apr 25;243(8):2012–2021. [PubMed] [Google Scholar]
- Bullock L. P., Bardin C. W., Ohno S. The androgen insensitive mouse: absence of intranuclear androgen retention in the kidney. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1537–1543. doi: 10.1016/s0006-291x(71)80261-9. [DOI] [PubMed] [Google Scholar]
- CHAUVEAU J., MOULE Y., ROUILLER C. Isolation of pure and unaltered liver nuclei morphology and biochemical composition. Exp Cell Res. 1956 Aug;11(2):317–321. doi: 10.1016/0014-4827(56)90107-0. [DOI] [PubMed] [Google Scholar]
- Frederiksen D. W., Wilson J. D. Partial characterization of the nuclear reduced nicotinamide adenine dinucleotide phosphate: delta 4-3-ketosteroid 5 alpha-oxidoreductase of rat prostate. J Biol Chem. 1971 Apr 25;246(8):2584–2593. [PubMed] [Google Scholar]
- GRADY K. L., PHOENIX C. H., YOUNG W. C. ROLE OF THE DEVELOPING RAT TESTIS IN DIFFERENTIATION OF THE NEURAL TISSUES MEDIATING MATING BEHAVIOR. J Comp Physiol Psychol. 1965 Apr;59:176–182. doi: 10.1037/h0021824. [DOI] [PubMed] [Google Scholar]
- Gehring U., Tomkins G. M., Ohno S. Effect of the androgen-insensitivity mutation on a cytoplasmic receptor for dihydrotestosterone. Nat New Biol. 1971 Jul 28;232(30):106–107. doi: 10.1038/newbio232106a0. [DOI] [PubMed] [Google Scholar]
- Gloyna R. E., Wilson J. D. A comparative study of the conversion of testosterone to 17-beta-hydroxy-5-alpha-androstan-3-one (Dihydrotestosterone) by prostate and epididymis. J Clin Endocrinol Metab. 1969 Jul;29(7):970–977. doi: 10.1210/jcem-29-7-970. [DOI] [PubMed] [Google Scholar]
- Grüneberg H. The glandular aspects of the tabby syndrome in the mouse. J Embryol Exp Morphol. 1971 Feb;25(1):1–19. [PubMed] [Google Scholar]
- HOTTA S., CHAIKOFF I. L. The role of the liver in the turnover of plasma cholesterol. Arch Biochem Biophys. 1955 May;56(1):28–37. doi: 10.1016/0003-9861(55)90330-1. [DOI] [PubMed] [Google Scholar]
- Hall P. F., Irby D. C., De Kretser D. M. Conversion of cholesterol to androgens by rat testes: comparison of interstitial cells and seminiferous tubules. Endocrinology. 1969 Mar;84(3):488–496. doi: 10.1210/endo-84-3-488. [DOI] [PubMed] [Google Scholar]
- JUNQUEIRA L. C., FAJER A. Biochemical and histochemical observations on the sexual dimorphism of mice submaxillary glands. J Cell Physiol. 1949 Aug;34(1):129-58, incl 6 pl. doi: 10.1002/jcp.1030340109. [DOI] [PubMed] [Google Scholar]
- KASE N., MORRIS J. M. STEROID SYNTHESIS IN THE CRYPTORCHID TESTES OF THREE CASES OF THE "TESTICULAR FEMINIZATION" SYNDROME. Am J Obstet Gynecol. 1965 Jan 1;91:102–105. doi: 10.1016/0002-9378(65)90593-4. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., ANGELETTI P. U. HORMONAL CONTROL OF THE NGF CONTENT IN THE SUBMAXILLARY GLANDS OF MICE. Int Ser Monogr Oral Biol. 1964;3:129–141. doi: 10.1016/b978-1-4832-2871-6.50016-3. [DOI] [PubMed] [Google Scholar]
- Lyon M. F., Hawkes S. G. X-linked gene for testicular feminization in the mouse. Nature. 1970 Sep 19;227(5264):1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- MORRIS J. M. The syndrome of testicular feminization in male pseudohermaphrodites. Am J Obstet Gynecol. 1953 Jun;65(6):1192–1211. doi: 10.1016/0002-9378(53)90359-7. [DOI] [PubMed] [Google Scholar]
- Northcutt R. C., Island D. P., Liddle G. W. An explanation for the target organ unresponsiveness to testosterone in the testicular feminization syndrome. J Clin Endocrinol Metab. 1969 Mar;29(3):422–425. doi: 10.1210/jcem-29-3-422. [DOI] [PubMed] [Google Scholar]
- SCHULTZ M. G. Male pseudohermaphroditism diagnosed with aid of sex chromatin technique. J Am Vet Med Assoc. 1962 Feb 1;140:241–244. [PubMed] [Google Scholar]
- Southren A. L. The syndrome of testicular feminization. Adv Metab Disord. 1965;2:227–255. doi: 10.1016/b978-1-4831-6750-3.50010-x. [DOI] [PubMed] [Google Scholar]
- WOLFF E. Le déterminisme de l'atrophie d'un organe rudimentaire; le canal de Müller des embryons males d'oiseaux. Experientia. 1953 Apr 15;9(4):121–133. doi: 10.1007/BF02154578. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Gloyna R. E. The intranuclear metabolism of testosterone in the accessory organs of reproduction. Recent Prog Horm Res. 1970;26:309–336. doi: 10.1016/b978-0-12-571126-5.50012-1. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Lasnitzki I. Dihydrotestosterone formation in fetal tissues of the rabbit and rat. Endocrinology. 1971 Sep;89(3):659–668. doi: 10.1210/endo-89-3-659. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Walker J. D. The conversion of testosterone to 5 alpha-androstan-17 beta-ol-3-one (dihydrotestosterone) by skin slices of man. J Clin Invest. 1969 Feb;48(2):371–379. doi: 10.1172/JCI105994. [DOI] [PMC free article] [PubMed] [Google Scholar]