Abstract
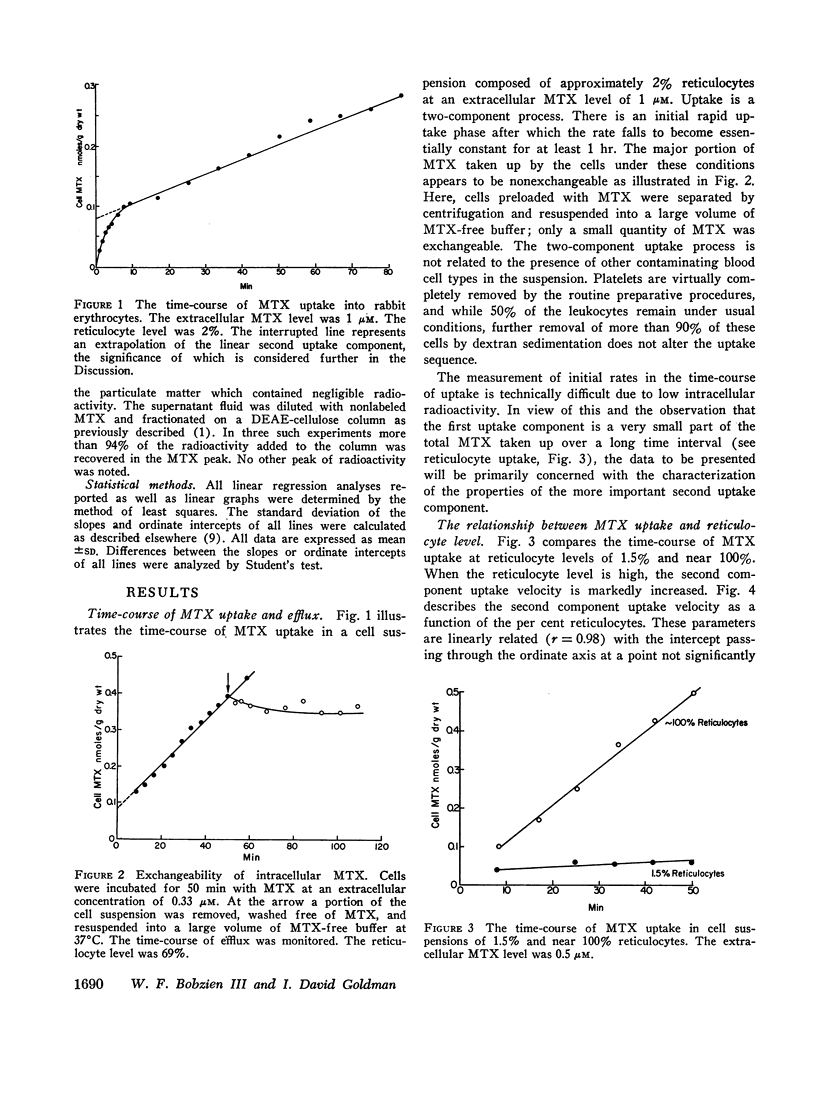
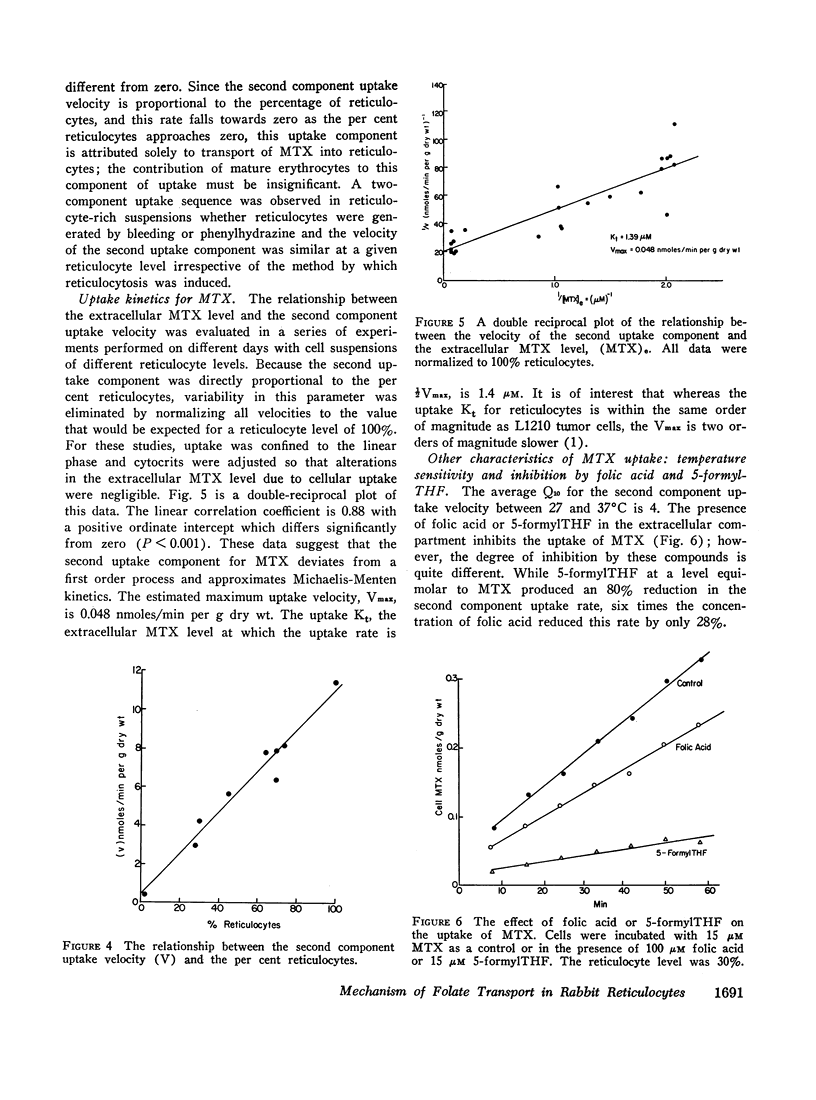
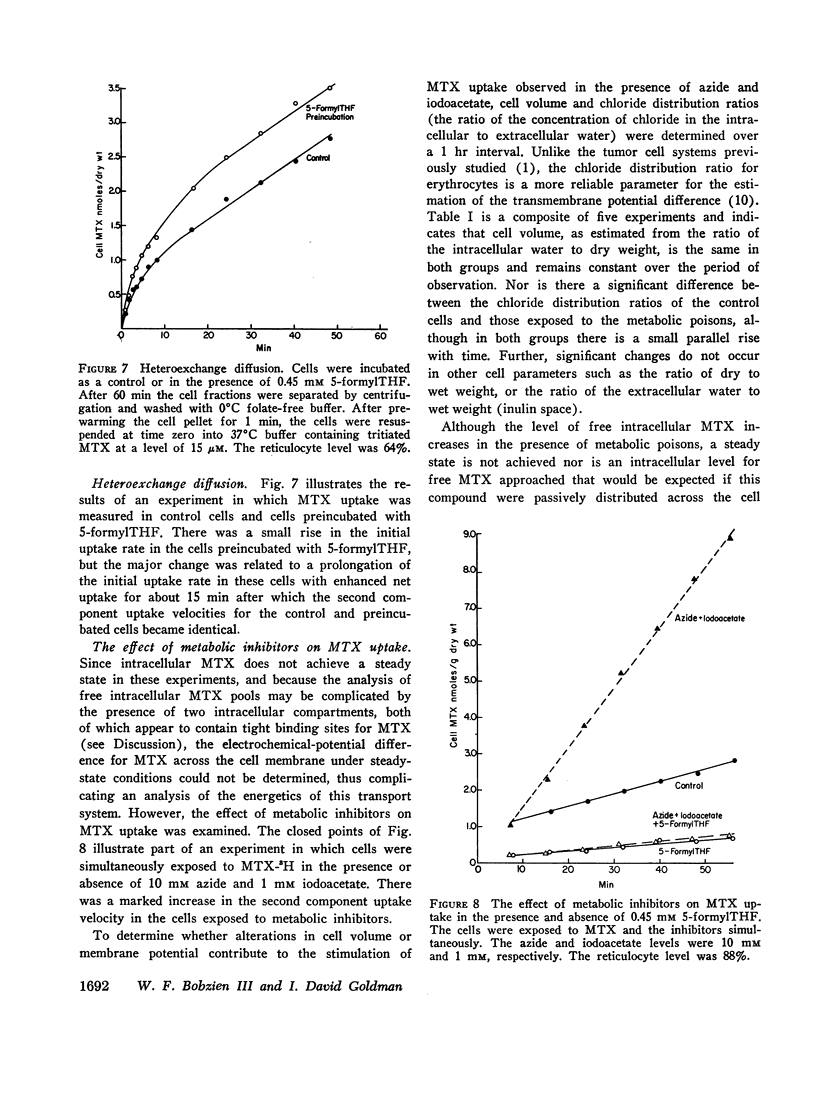
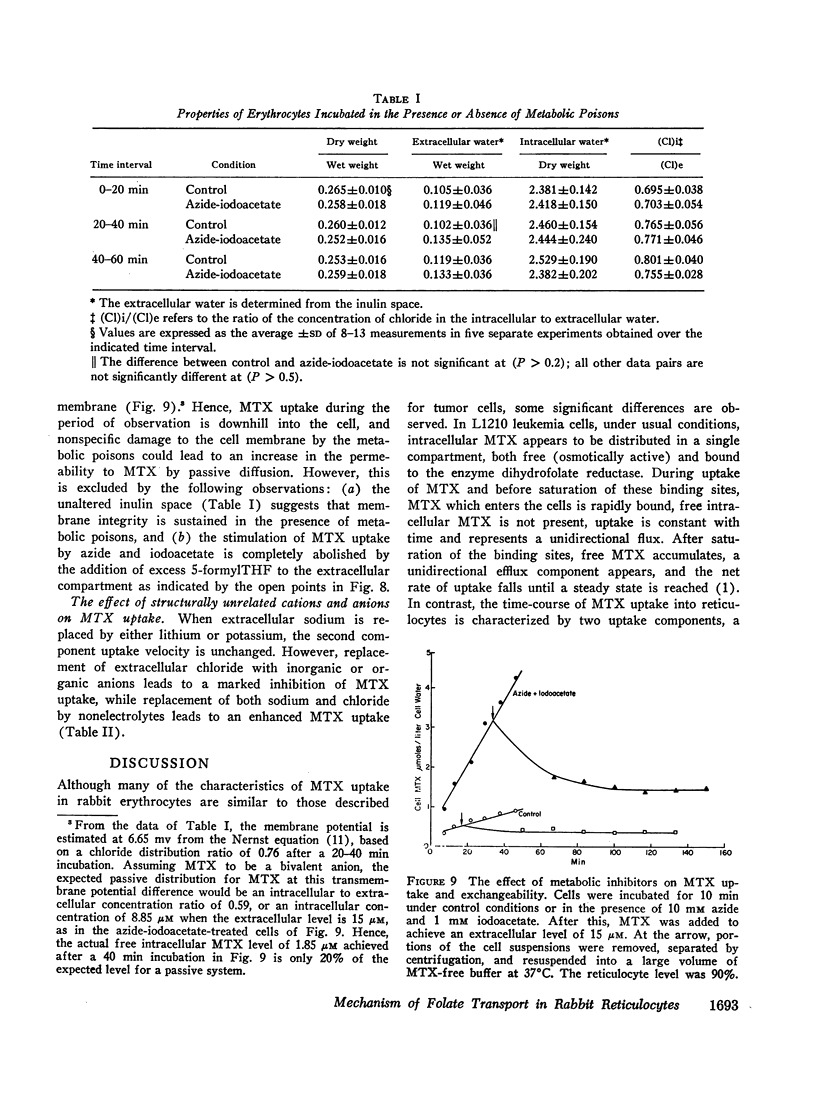
Folate transport in phenylhydrazine-induced rabbit reticulocytes was studied with the non-metabolized folate-analog, methotrexate. The time-course of methotrexate uptake into a mixed population of reticulocytes and mature erythrocytes is a two-component process consisting of a small, but rapid, initial uptake phase followed by a much slower uptake component which remains essentially constant over the period of observation. The velocity of the latter uptake component is directly proportional to the per cent reticulocytes and appears to represent a unidirectional influx of methotrexate into these cells. Uptake of methotrexate into reticulocytes was found to have the following characteristics: (a) temperature sensitivity, Q10 of 4; (b) uptake velocity as a function of the extracellular methotrexate concentration approximated Michaelis-Menten kinetics with a maximum transport velocity of 48 pmoles/min per g dry wt; the extracellular methotrexate level at which the uptake velocity was one-half maximum was 1.4 μM; (c) 5-formyltetrahydrofolate markedly inhibited methotrexate uptake but pteroylglutamic acid inhibition was weak; (d) uptake was stimulated in cells preincubated with 5-formyltetrahydrofolate, indicative of hetero-exchange diffusion; (e) uptake was independent of extracellular sodium but was inhibited by anions including nitrate, phosphate, and glucose-6-phosphate; (f) uptake was enhanced by azide plus iodoacetate.
These data indicate that folate transport in rabbit reticulocytes is mediated by a carrier mechanism which disappears with reticulocyte maturation. The mechanism of folate transport in rabbit reticulocytes is qualitatively similar to tumor cells previously studied; both appear to have an energy-dependent mechanism limiting folate uptake, and influx in both is inhibited by structurally unrelated inorganic and organic anions. These studies suggest that circulating pteroylglutamic acid is of little importance in meeting the folate requirements of folate-dependent tissues and raise the possibility that clinical conditions associated with alterations in the anionic composition of the blood may be accompanied by impaired utilization of the folates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonioli J. A., Christensen H. N. Differences in schedules of regression of transport systems during reticulocyte maturation. J Biol Chem. 1969 Mar 25;244(6):1505–1509. [PubMed] [Google Scholar]
- BERTINO J. R., SIMMONS B., DONOHUE D. M. LEVELS OF DIHYDROFOLATE REDUCTASE AND THE FORMATE-ACTIVATING ENZYME ACTIVITIES IN GUINEA PIG TISSUES BEFORE AND AFTER AMETHOPTERIN ADMINISTRATION. Biochem Pharmacol. 1964 Feb;13:225–233. doi: 10.1016/0006-2952(64)90140-6. [DOI] [PubMed] [Google Scholar]
- Bird O. D., McGlohon V. M., Vaitkus J. W. Naturally occurring folates in the blood and liver of the rat. Anal Biochem. 1965 Jul;12(1):18–35. doi: 10.1016/0003-2697(65)90138-7. [DOI] [PubMed] [Google Scholar]
- Corcino J. J., Waxman S., Herbert V. Uptake of tritiated folates by human bone marrow cells in vitro. Br J Haematol. 1971 May;20(5):503–509. doi: 10.1111/j.1365-2141.1971.tb07064.x. [DOI] [PubMed] [Google Scholar]
- Das K. C., Hoffbrand A. V. Studies of folate uptake by phytohaemagglutinin-stimulated lymphocytes. Br J Haematol. 1970 Aug;19(2):203–221. doi: 10.1111/j.1365-2141.1970.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Goldman I. D. A model system for the study of heteroexchange diffusion: methotrexate-folate interactions in L1210 leukemia and Ehrlich ascites tumor cells. Biochim Biophys Acta. 1971 Jun 1;233(3):624–634. doi: 10.1016/0005-2736(71)90162-3. [DOI] [PubMed] [Google Scholar]
- Goldman I. D., Lichtenstein N. S., Oliverio V. T. Carrier-mediated transport of the folic acid analogue, methotrexate, in the L1210 leukemia cell. J Biol Chem. 1968 Oct 10;243(19):5007–5017. [PubMed] [Google Scholar]
- Goldman I. D. The characteristics of the membrane transport of amethopterin and the naturally occurring folates. Ann N Y Acad Sci. 1971 Nov 30;186:400–422. doi: 10.1111/j.1749-6632.1971.tb46996.x. [DOI] [PubMed] [Google Scholar]
- Goldman I. D. Transport energetics of the folic acid analogue, methotrexate, in L1210 leukemia cells. Enhanced accumulation by metabolic inhibitors. J Biol Chem. 1969 Jul 25;244(14):3779–3785. [PubMed] [Google Scholar]
- HEINZ E., WALSH P. M. Exchange diffusion, transport, and intracellular level of amino acids in Ehrlich carcinoma cells. J Biol Chem. 1958 Dec;233(6):1488–1493. [PubMed] [Google Scholar]
- HERBERT V., LARRABEE A. R., BUCHANAN J. M. Studies on the identification of a folate compound of human serum. J Clin Invest. 1962 May;41:1134–1138. doi: 10.1172/JCI104565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izak G., Rachmilewitz M., Grossowicz N., Galewski K., Kraus S. Folate activity in reticulocytes and the incorporation of triated pteroylglutamic acid into red cells. Br J Haematol. 1968 Apr;14(4):447–452. doi: 10.1111/j.1365-2141.1968.tb06995.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein N. S., Oliverio V. T., Goldman I. D. Characteristics of folic acid transport in the L1210 leukemia cell. Biochim Biophys Acta. 1969;193(2):456–467. doi: 10.1016/0005-2736(69)90204-1. [DOI] [PubMed] [Google Scholar]
- SCHREINER G. E. Determination of inulin by means of resorcinol. Proc Soc Exp Biol Med. 1950 May;74(1):117–120. doi: 10.3181/00379727-74-17827. [DOI] [PubMed] [Google Scholar]
- Stokstad E. L., Koch J. Folic acid metabolism. Physiol Rev. 1967 Jan;47(1):83–116. doi: 10.1152/physrev.1967.47.1.83. [DOI] [PubMed] [Google Scholar]
- WILBRANDT W., ROSENBERG T. The concept of carrier transport and its corollaries in pharmacology. Pharmacol Rev. 1961 Jun;13:109–183. [PubMed] [Google Scholar]


