Abstract
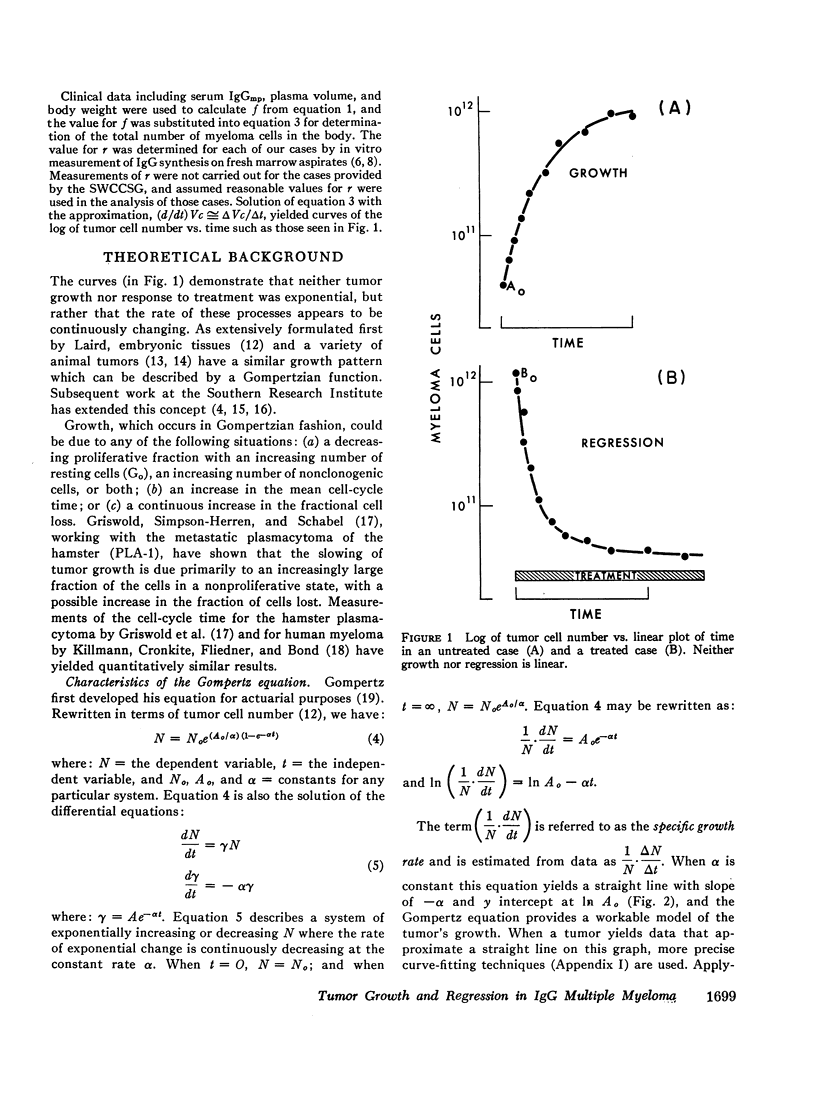
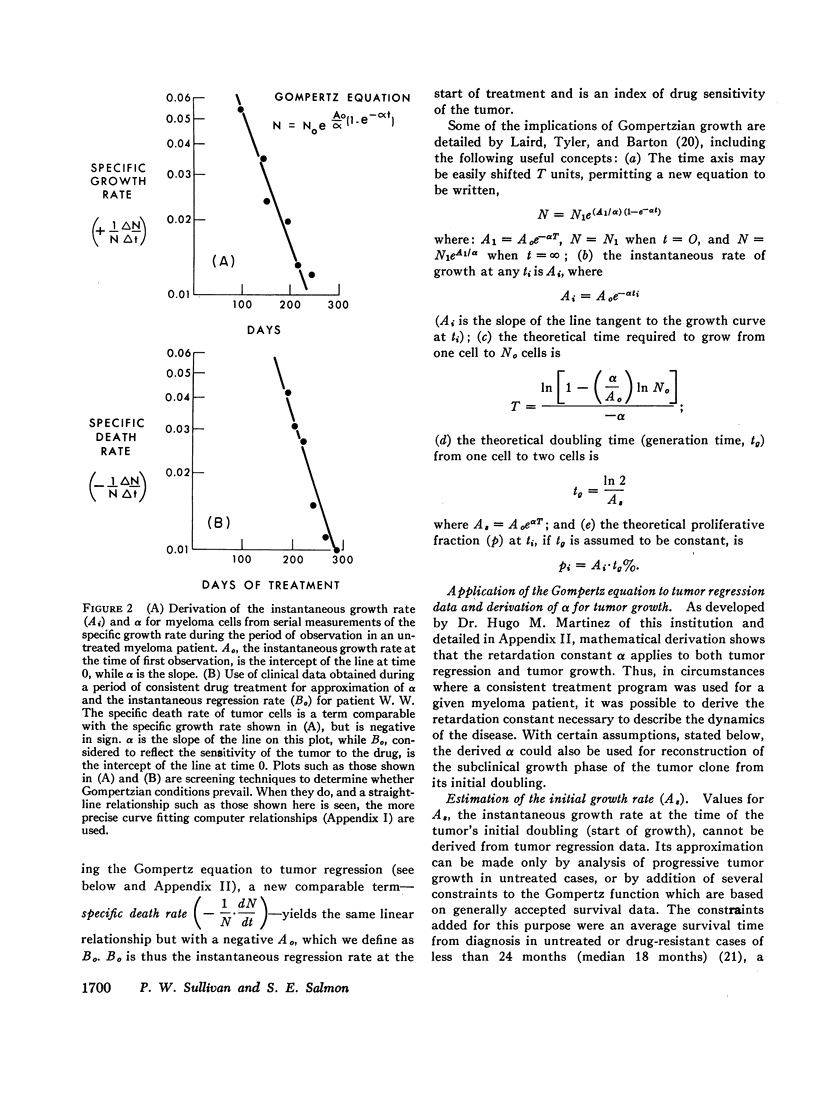
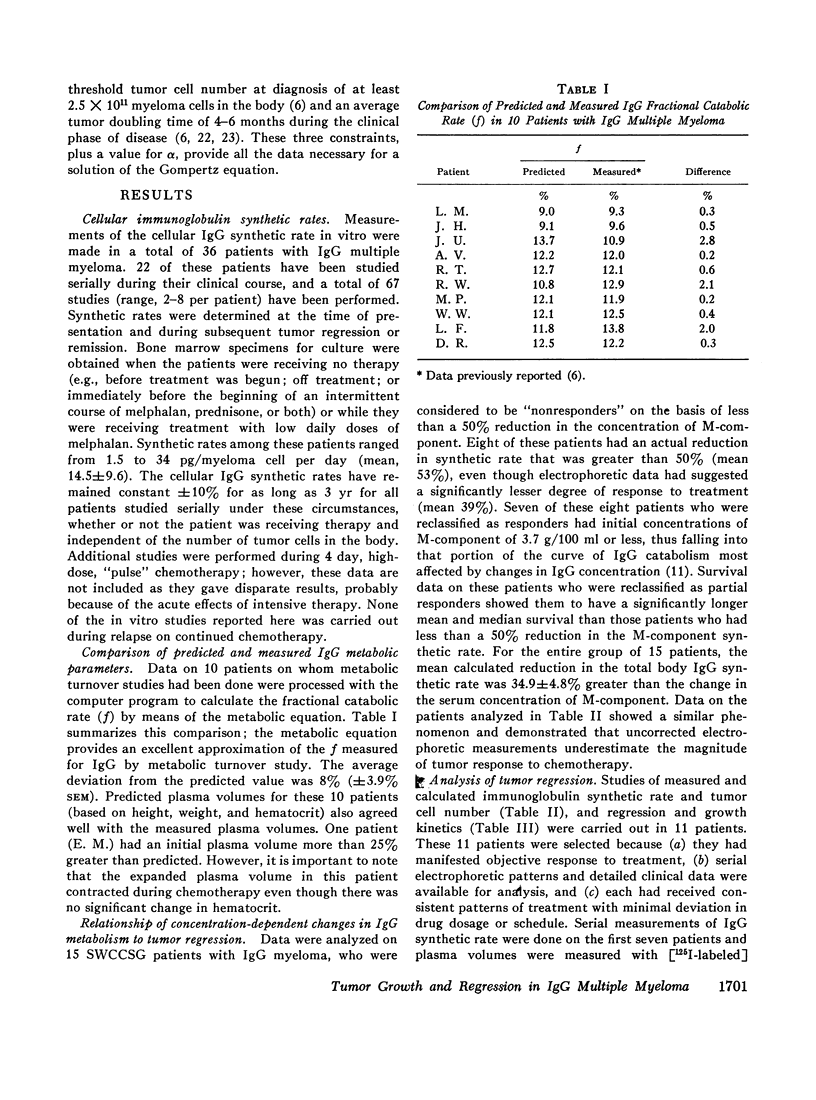
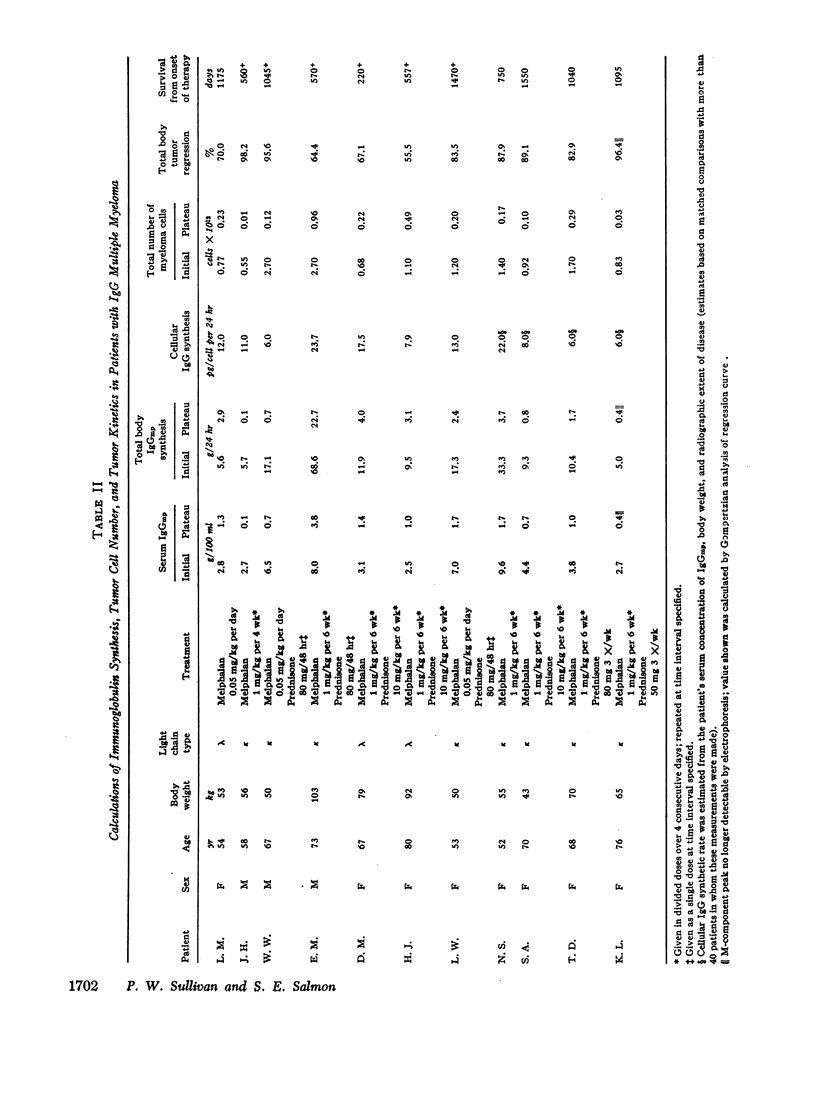
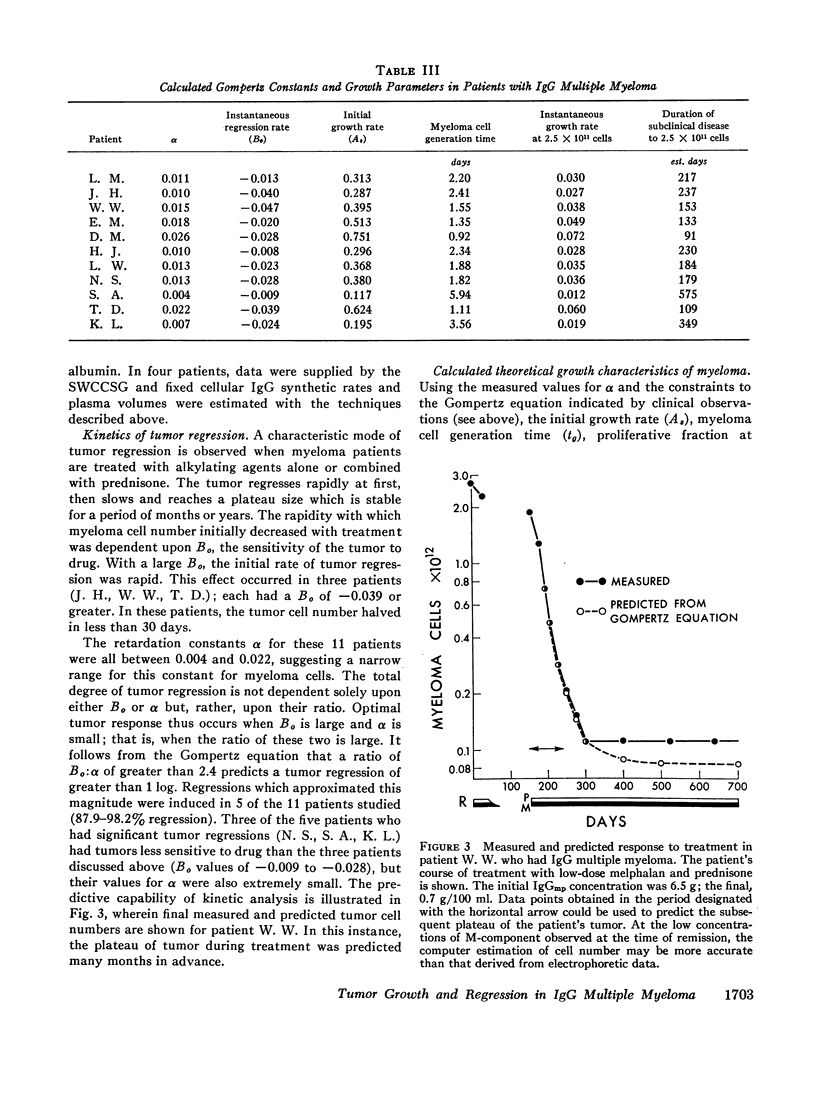
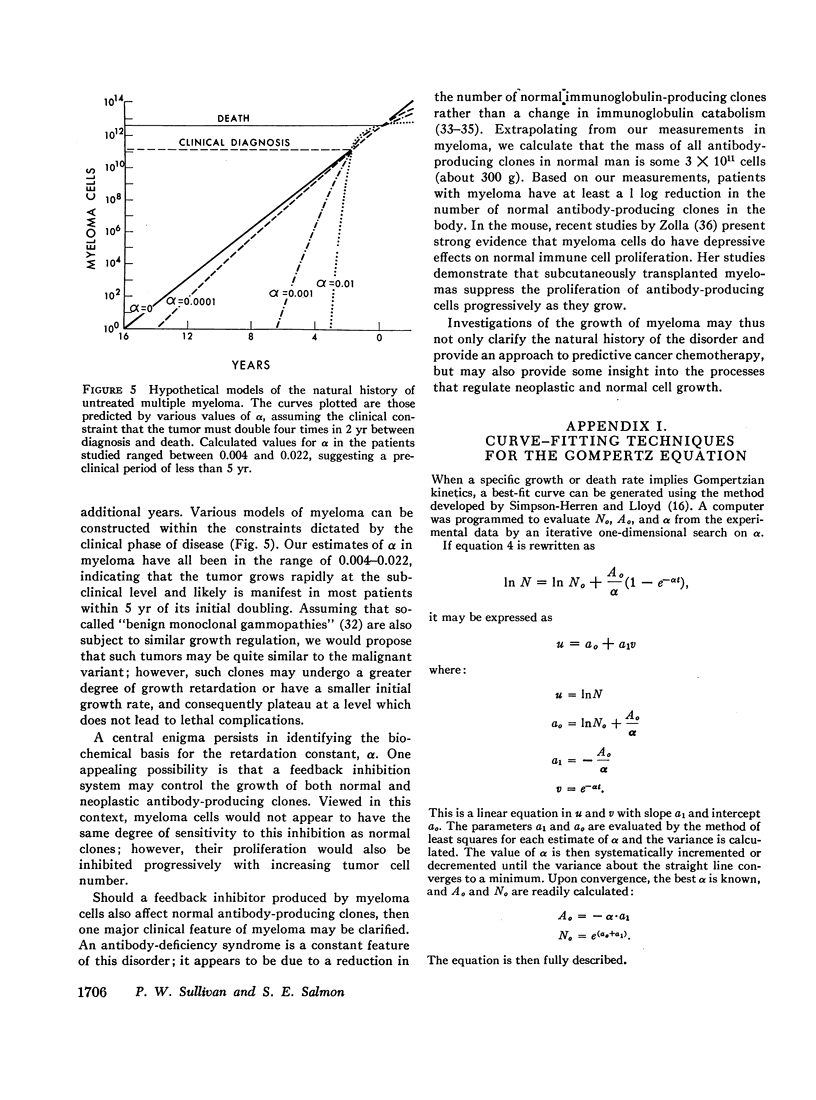
Studies of immunoglobulin synthesis, total body tumor cell number, and tumor kinetics were carried out in a series of patients with IgG multiple myeloma. The changes in tumor size associated with tumor growth or with regression were underestimated when the concentration of serum M-component was used as the sole index of tumor mass. Calculation of the total body M-component synthetic rate (corrected for concentration-dependent changes in IgG metabolism) and tumor cell number gave a more accurate and predictable estimate of changes in tumor size. Tumor growth and drug-induced tumor regression were found to follow Gompertzian kinetics, with progressive retardation of the rate of change of tumor size in both of these circumstances. This retardation effect, describable with a constant α, may be caused by a shift in the proportion of tumor cells in the proliferative cycle. Drug sensitivity of the tumor could be described quantitatively with a calculation of BO, the tumor's initial sensitivity to a given drug regimen. Of particular clinical significance, the magnitude of a given patient's tumor regression could be predicted from the ratio of BO to α. Mathematical proof was obtained that the retardation constant determined during tumor regression also applied to the earlier period of tumor growth, and this constant was used to reconstruct the preclinical history of disease. In the average patient, fewer than 5 yr elapse from the initial tumor cell doubling to its clinical presentation with from 1011 to more than 1012 myeloma cells in the body. The reduction in total body tumor mass in most patients responding to therapy ranges from less than one to almost two orders of magnitude. Application of predictive kinetic analysis to the design of sequential drug regimens may lead to further improvement in the treatment of multiple myeloma and other tumors with similar growth characteristics.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexanian R., Haut A., Khan A. U., Lane M., McKelvey E. M., Migliore P. J., Stuckey W. J., Jr, Wilson H. E. Treatment for multiple myeloma. Combination chemotherapy with different melphalan dose regimens. JAMA. 1969 Jun 2;208(9):1680–1685. doi: 10.1001/jama.208.9.1680. [DOI] [PubMed] [Google Scholar]
- Bergsagel D. E. Total body irradiation for myelomatosis. Br Med J. 1971 May 8;2(5757):325–325. doi: 10.1136/bmj.2.5757.325-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P., Laskov R., Scharff M. D. Immunoglobulin production: method for quantitatively detecting variant myeloma cells. Science. 1970 Jan 9;167(3915):186–188. doi: 10.1126/science.167.3915.186. [DOI] [PubMed] [Google Scholar]
- DE BERGSAGEL D. E., MIGLIORE P. J., GRIFFITH K. M. MYELOMA PROTEINS AND THE CLINICAL RESPONSE TO MELPHALAN THERAPY. Science. 1965 Apr 16;148(3668):376–377. doi: 10.1126/science.148.3668.376. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., SCOGGINS R., UTZ J. P., SZWED C. F. INFECTION, ANTIBODY RESPONSE AND GAMMA GLOBULIN COMPONENTS IN MULTIPLE MYELOMA AND MACROGLOBULINEMIA. Am J Med. 1963 Nov;35:698–707. doi: 10.1016/0002-9343(63)90140-2. [DOI] [PubMed] [Google Scholar]
- Goldin A. Factors pertaining to complete drug-induced remission of tumor in animals and man. Cancer Res. 1969 Dec;29(12):2285–2291. [PubMed] [Google Scholar]
- Griswold D. P., Jr, Schabel F. M., Jr, Wilcox W. S., Simpson-Herren L., Skipper H. E. Success and failure in the treatment of solid tumors. I. Effects of cyclophosphamide (NSC-26271) on primary and metastatic plasmacytoma in the hamster. Cancer Chemother Rep. 1968 Apr;52(3):345–387. [PubMed] [Google Scholar]
- Griswold D. P., Jr, Simpson-Herren L., Schabel F. M., Jr Altered sensitivity of a hamster plasmacytoma to cytosine arabinoside (NSC-63878). Cancer Chemother Rep. 1970 Oct;54(5):337–346. [PubMed] [Google Scholar]
- Hobbs J. R. Growth rates and responses to treatment in human myelomatosis. Br J Haematol. 1969 Jun;16(6):607–617. doi: 10.1111/j.1365-2141.1969.tb00441.x. [DOI] [PubMed] [Google Scholar]
- Hobbs J. R. Immunochemical classes of myelomatosis. Including data from a therapeutic trial conducted by a Medical Research Council working party. Br J Haematol. 1969 Jun;16(6):599–606. doi: 10.1111/j.1365-2141.1969.tb00440.x. [DOI] [PubMed] [Google Scholar]
- Hobbs J. R. Immunocytoma o' mice an' men. Br Med J. 1971 Apr 10;2(5753):67–72. doi: 10.1136/bmj.2.5753.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs J. R. Modes of escape from therapeutic control in myelomatosis. Br Med J. 1971 May 8;2(5757):325–325. doi: 10.1136/bmj.2.5757.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILLMANN S. A., CRONKITE E. P., FLIEDNER T. M., BOND V. P. Cell proliferation in multiple myeloma studied with tritiated thymidine in vivo. Lab Invest. 1962 Oct;11:845–853. [PubMed] [Google Scholar]
- LAIRD A. K. DYNAMICS OF TUMOR GROWTH. Br J Cancer. 1964 Sep;13:490–502. doi: 10.1038/bjc.1964.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAIRD A. K. DYNAMICS OF TUMOUR GROWTH: COMPARISON OF GROWTH RATES AND EXTRAPOLATION OF GROWTH CURVE TO ONE CELL. Br J Cancer. 1965 Jun;19:278–291. doi: 10.1038/bjc.1965.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A. K. Dynamics of relative growth. Growth. 1965 Sep;29(3):249–263. [PubMed] [Google Scholar]
- Laird A. K., Tyler S. A., Barton A. D. Dynamics of normal growth. Growth. 1965 Sep;29(3):233–248. [PubMed] [Google Scholar]
- Laster W. R., Jr, Mayo J. G., Simpson-Herren L., Griswold D. P., Jr, Lloyd H. H., Schabel F. M., Jr, Skipper H. E. Success and failure in the treatment of solid tumors. II. Kinetic parameters and "cell cure" of moderately advanced carcinoma 755. Cancer Chemother Rep. 1969 Jun;53(3):169–188. [PubMed] [Google Scholar]
- OSGOOD E. E. The survival time of patients with plasmocytic myeloma. Cancer Chemother Rep. 1960 Nov;9:1–10. [PubMed] [Google Scholar]
- OSSERMAN E. F., TAKATSUKI K. PLASMA CELL MYELOMA: GAMMA GLOBULIN SYNTHESIS AND STRUCTURE. A REVIEW OF BIOCHEMICAL AND CLINICAL DATA, WITH THE DESCRIPTION OF A NEWLY-RECOGNIZED AND RELATED SYNDROME, "H-GAMMA-2-CHAIN (FRANKLIN'S) DISEASE. Medicine (Baltimore) 1963 Nov;42:357–384. [PubMed] [Google Scholar]
- Park C. H., Bergsagel D. E., McCulloch E. A. Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst. 1971 Feb;46(2):411–422. [PubMed] [Google Scholar]
- SKIPPER H. E., SCHABEL F. M., Jr, WILCOX W. S. EXPERIMENTAL EVALUATION OF POTENTIAL ANTICANCER AGENTS. XIII. ON THE CRITERIA AND KINETICS ASSOCIATED WITH "CURABILITY" OF EXPERIMENTAL LEUKEMIA. Cancer Chemother Rep. 1964 Feb;35:1–111. [PubMed] [Google Scholar]
- SOLOMON A., WALDMANN T. A., FAHEY J. L. Clinical and experimental metabolism of normal 6.6s gamma-globulin in normal subjects and in patients with macroglobulinemia and multiple myeloma. J Lab Clin Med. 1963 Jul;62:1–17. [PubMed] [Google Scholar]
- Salmon S. E., Mackey G., Fudenberg H. H. "Sandwich" solid phase radioimmunoassay for the quantitative deterimination of human immunoglobulins. J Immunol. 1969 Jul;103(1):129–137. [PubMed] [Google Scholar]
- Salmon S. E., McIntyre O. R., Ogawa M. IgE myeloma: total body tumor cell number and synthesis of IgE and DNA. Blood. 1971 Jun;37(6):696–705. [PubMed] [Google Scholar]
- Salmon S. E., Samal B. A., Hayes D. M., Hosley H., Miller S. P., Schilling A. Role of gamma globulin for immunoprophylaxis in multiple myeloma. N Engl J Med. 1967 Dec 21;277(25):1336–1340. doi: 10.1056/NEJM196712212772503. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Smith B. A. Immunoglobulin synthesis and total body tumor cell number in IgG multiple myeloma. J Clin Invest. 1970 Jun;49(6):1114–1121. doi: 10.1172/JCI106327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon S. E., Smith B. A. Sandwich solid phase radioimmunoassays for the characterization of human immunoglobulins synthesized in vitro. J Immunol. 1970 Mar;104(3):665–672. [PubMed] [Google Scholar]
- Schabel F. M., Jr The use of tumor growth kinetics in planning "curative" chemotherapy of advanced solid tumors. Cancer Res. 1969 Dec;29(12):2384–2389. [PubMed] [Google Scholar]
- Simpson-Herren L., Lloyd H. H. Kinetic parameters and growth curves for experimental tumor systems. Cancer Chemother Rep. 1970 Jun;54(3):143–174. [PubMed] [Google Scholar]
- Skipper H. E., Schabel F. M., Jr, Mellett L. B., Montgomery J. A., Wilkoff L. J., Lloyd H. H., Brockman R. W. Implications of biochemical, cytokinetic, pharmacologic, and toxicologic relationships in the design of optimal therapeutic schedules. Cancer Chemother Rep. 1970 Dec;54(6):431–450. [PubMed] [Google Scholar]
- Waldmann T. A., Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- Zolla S. The effect of plasmacytomas on the immune response of mice. J Immunol. 1972 Apr;108(4):1039–1048. [PubMed] [Google Scholar]


