Abstract
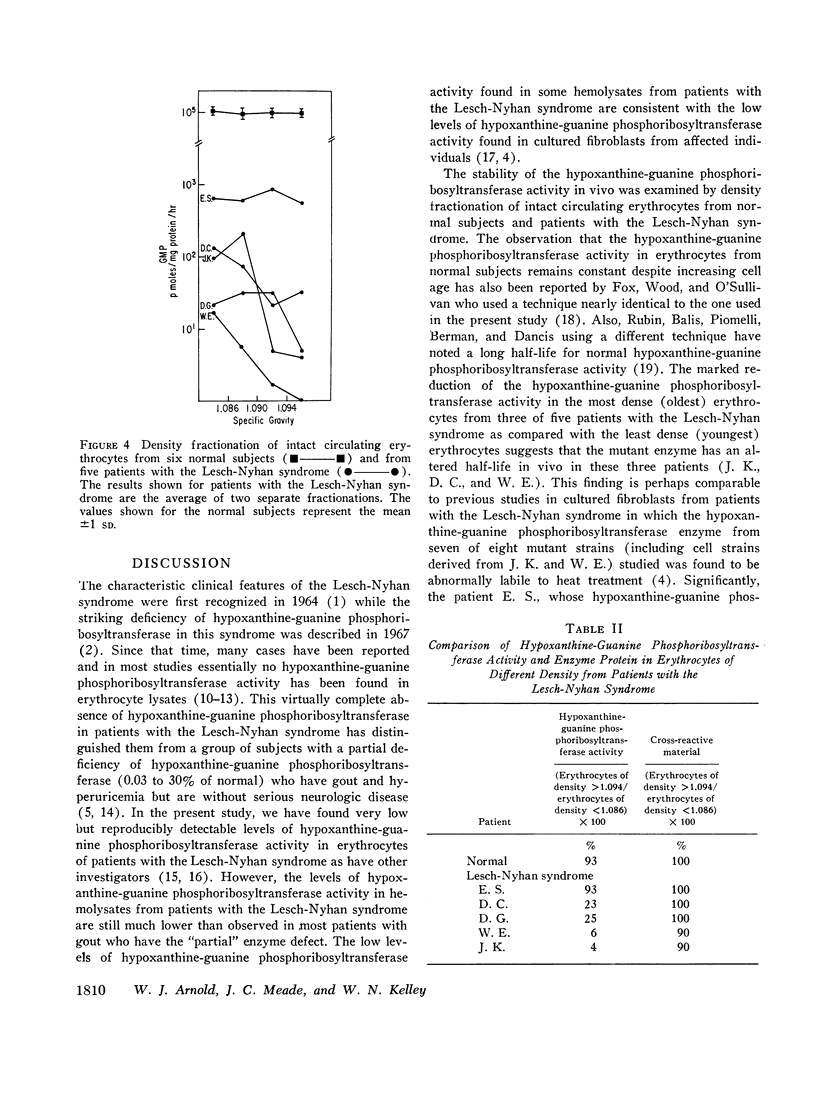
The Lesch-Nyhan syndrome is characterized clinically by choreoathetosis, spasticity, selfmutilation, and mental and growth retardation. Biochemically, there is a striking reduction of hypoxanthine-guanine phosphoribosyltransferase (HGPRT) activity in affected individuals. We have examined erythrocytes from 14 patients with the Lesch-Nyhan syndrome for the presence of hypoxanthine-guanine phosphoribosyltransferase activity and enzyme protein. In contrast to the usual finding of no detectable hypoxanthine-guanine phosphoribosyltransferase activity, we have found low levels (0.002-0.79 nmoles/mg protein per hr) of hypoxanthine-guanine phosphoribosyltransferase activity in erythrocyte lysates from five of these patients. In three of the five patients, hypoxanthine-guanine phosphoribosyltransferase activity appeared to be substantially more labile in vivo than normal using erythrocytes which had been separated according to their density (age).
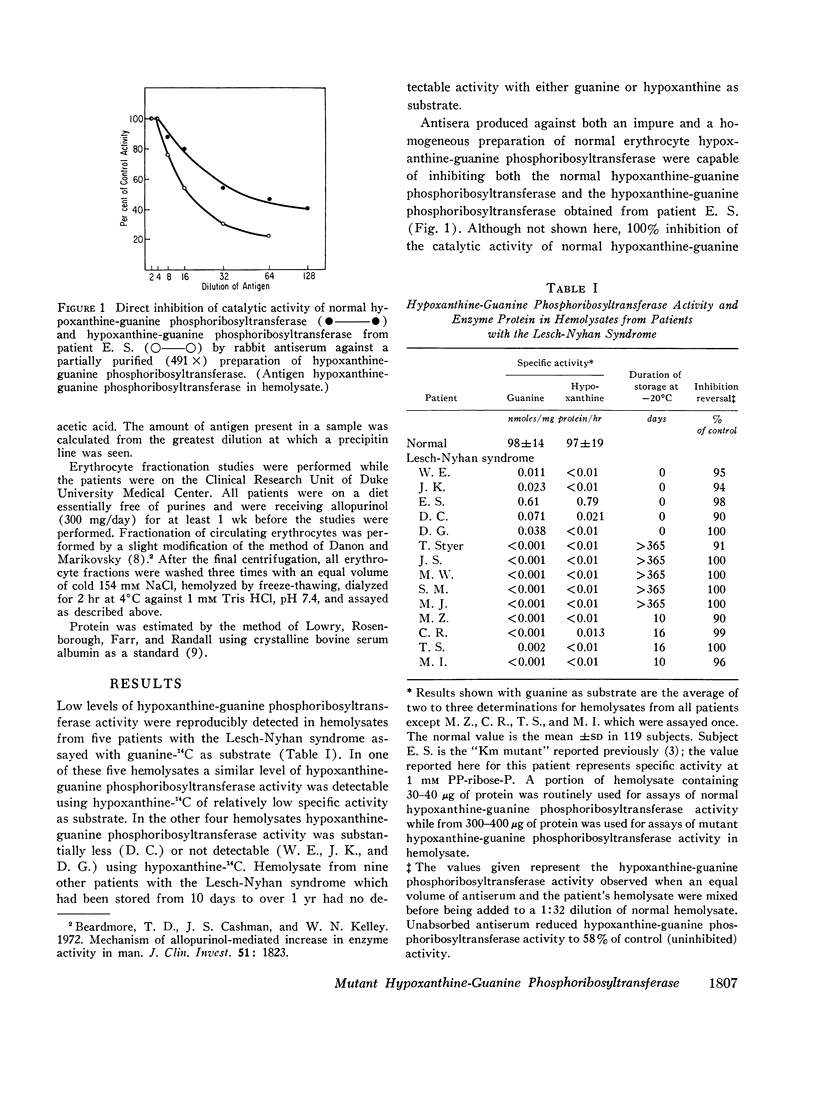
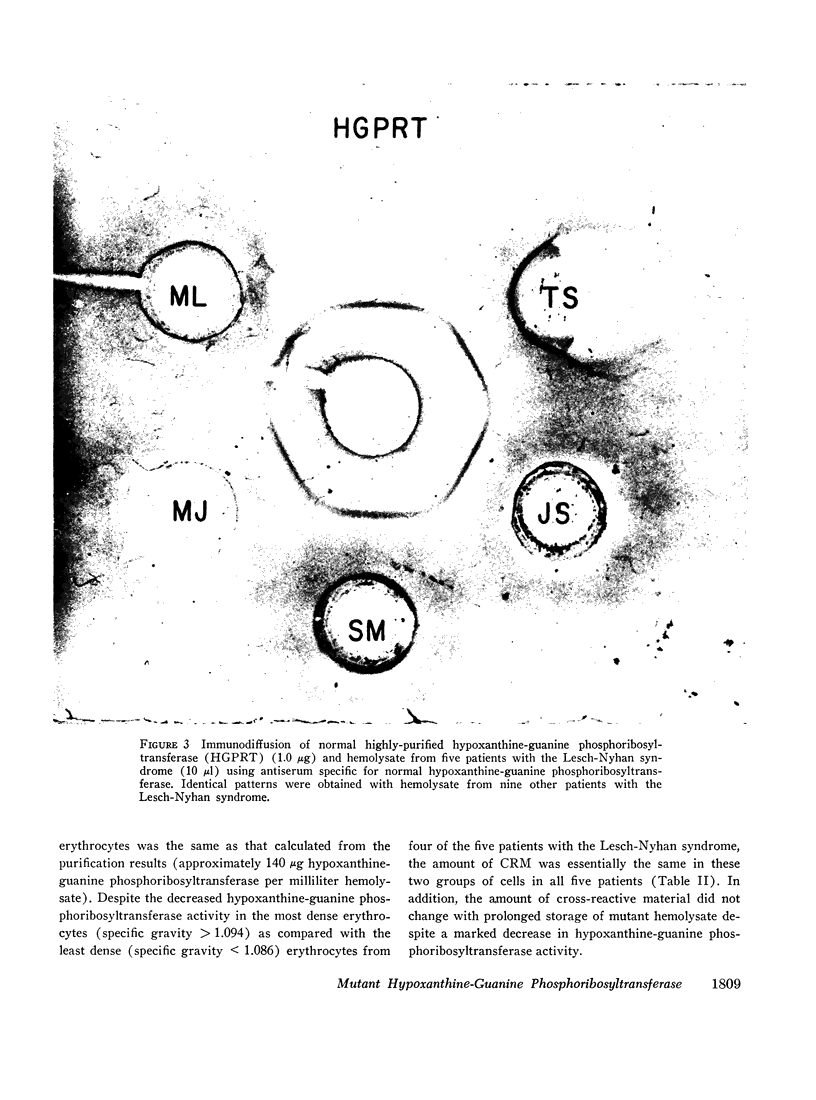
Immunochemical studies using a monospecific antiserum prepared from a homogeneous preparation of normal human erythrocyte hypoxanthine-guanine phosphoribosyltransferase revealed immunoreactive protein (CRM) in hemolysate from all 14 patients with the Lesch-Nyhan syndrome. The immunoreactive protein from each patient gave a reaction of complete identity with normal erythrocyte hypoxanthine-guanine phosphoribosyltransferase and was present in quantities equal to those observed in normal erythrocytes. In addition, a constant amount of CRM was found in erythrocytes of increasing density (age) from patients with the Lesch-Nyhan syndrome despite the decreasing hypoxanthine-guanine phosphoribosyltransferase activity.
These studies confirm previous data which indicate that the mutations leading to the Lesch-Nyhan syndrome are usually, if not always on the structural gene coding for hypoxanthine-guanine phosphoribosyltransferase. In addition, although the mutant proteins appear to be present in normal amounts, they are often very labile in vivo with respect to enzymatic activity. These observations suggest that therapy directed at stabilization or activation of enzyme activity in vivo may be of potential benefit.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. J., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Purification and subunit structure. J Biol Chem. 1971 Dec 10;246(23):7398–7404. [PubMed] [Google Scholar]
- Beardmore T. D., Cashman J. S., Kelley W. N. Mechanism of allopurinol-mediated increase in enzyme activity in man. J Clin Invest. 1972 Jul;51(7):1823–1832. doi: 10.1172/JCI106984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANON D., MARIKOVSKY V. DETERMINATION OF DENSITY DISTRIBUTION OF RED CELL POPULATION. J Lab Clin Med. 1964 Oct;64:668–674. [PubMed] [Google Scholar]
- Fox R. M., Wood M. H., O'Sullivan W. J. Studies on the coordinate activity and liability of orotidylate phosphoribosyltransferase and decarboxylase in human erythrocytes, and the effects of allopurinol administration. J Clin Invest. 1971 May;50(5):1050–1060. doi: 10.1172/JCI106576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto W. Y., Seegmiller J. E. Hypoxanthine-guanine phosphoribosyltransferase deficiency: activity in normal, mutant, and heterozygote-cultured human skin fibroblasts. Proc Natl Acad Sci U S A. 1970 Mar;65(3):577–584. doi: 10.1073/pnas.65.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon H., Gershon D. Detection of inactive enzyme molecules in ageing organisms. Nature. 1970 Sep 19;227(5264):1214–1217. doi: 10.1038/2271214a0. [DOI] [PubMed] [Google Scholar]
- Ghadimi H., Bhalla C. K., Kirchenbaum D. M. The significance of the deficiency state in Lesch-Nyhan disease. Acta Paediatr Scand. 1970 May;59(3):233–240. doi: 10.1111/j.1651-2227.1970.tb08998.x. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Greene M. L., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. Hypoxanthine-guanine phosphoribosyltransferase deficiency in gout. Ann Intern Med. 1969 Jan;70(1):155–206. doi: 10.7326/0003-4819-70-1-155. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Meade J. C. Studies on hypoxanthine-guanine phosphoribosyltransferase in fibroblasts from patients with the Lesch-Nyhan syndrome. Evidence for genetic heterogeneity. J Biol Chem. 1971 May 10;246(9):2953–2958. [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. A specific enzyme defect in gout associated with overproduction of uric acid. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1735–1739. doi: 10.1073/pnas.57.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESCH M., NYHAN W. L. A FAMILIAL DISORDER OF URIC ACID METABOLISM AND CENTRAL NERVOUS SYSTEM FUNCTION. Am J Med. 1964 Apr;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McDonald J. A., Kelley W. N. Lesch-Nyhan syndrome: altered kinetic properties of mutant enzyme. Science. 1971 Feb 19;171(3972):689–691. doi: 10.1126/science.171.3972.689. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Segawa M., Kurumada T., Maruyama H., Onisawa J. Clinical and therapeutic aspects of the Lesch-Nyhan syndrome in Japanese children. Neuropadiatrie. 1970 Aug;2(1):38–52. doi: 10.1055/s-0028-1091839. [DOI] [PubMed] [Google Scholar]
- Piomelli S., Corash L. M., Davenport D. D., Miraglia J., Amorosi E. L. In vivo lability of glucose-6-phosphate dehydrogenase in GdA- and GdMediterranean deficiency. J Clin Invest. 1968 Apr;47(4):940–948. doi: 10.1172/JCI105786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Balis M. E., Piomelli S., Berman P. H., Dancis J. Elevated AMP pyrophosphorylase activity in congenital IMP pyrophosphorylase deficiencey (Lesch-Nyhan disease). J Lab Clin Med. 1969 Nov;74(5):732–741. [PubMed] [Google Scholar]
- Rubin C. S., Dancis J., Yip L. C., Nowinski R. C., Balis M. E. Purification of IMP:pyrophosphate phosphoribosyltransferases, catalytically incompetent enzymes in Lesch-Nyhan disease. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1461–1464. doi: 10.1073/pnas.68.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Sorensen L. B. Mechanism of excessive purine biosynthesis in hypoxanthine-guanine phosphoribosyltransferase deficiency. J Clin Invest. 1970 May;49(5):968–978. doi: 10.1172/JCI106316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling O., Eilam G., Schmidt R., Mundel G., De Vries A. Purine base incorporation into erythrocyte nucleotides in hypoxanthine-guanine phosphoribosyltransferase deficiency. Biochem Med. 1971 Apr;5(2):173–176. doi: 10.1016/0006-2944(71)90085-8. [DOI] [PubMed] [Google Scholar]
- WRIGHT S. T. A quantitative serum-agar technique. Nature. 1959 May 2;183(4670):1282–1283. doi: 10.1038/1831282b0. [DOI] [PubMed] [Google Scholar]