Abstract
The value of increased arterial wave reflection, usually assessed by the transit-time dependent augmentation index (AI) and augmented pressure (Pa), in the prediction of cardiovascular events may have been underestimated. We investigated whether the transit-time independent measures of reflected wave magnitude predict cardiovascular outcomes independently of arterial stiffness indexed by carotid-femoral pulse wave velocity (PWV). A total of 1272 participants (47% women, mean age 52 ±13 years old, range 30-79 years) from a community-based survey were studied. Carotid pressure waveforms derived by tonometry were decomposed into their forward wave amplitudes (Pf), backward wave amplitudes (Pb), and a reflection index (RI, = [Pb/(Pf+Pb)]), in addition to AI, Pa, and reflected wave transit time (RWTT). During a median follow-up of 15 years, 225 (17.6%) deaths occurred, including 64 (5%) cardiovascular origins. In univariate Cox proportional hazard regression analysis, PWV, Pa, and Pb predicted all-cause and cardiovascular mortality in both men and women whereas AI, RWTT, and RI were predictive only in men. In multi-variate analysis accounting for age, height and heart rate, Pb predicted cardiovascular mortality in both men and women, whereas Pa was predictive only in men. Per one standard deviation increment (6 mmHg), Pb predicted 15-year cardiovascular mortality independently of brachial but not central pressure, PWV, AI, Pa and conventional cardiovascular risk factors with hazard ratios of around 1.60 (all P values <0.05). In conclusion, Pb, a transit-time independent measure of reflected wave magnitude, predicted long term cardiovascular mortality in men and women independently of arterial stiffness.
Keywords: wave reflection, pulse wave velocity, arterial stiffness, vascular aging, mortality, epidemiology
Introduction
Cardiovascular disease may be viewed as manifestations of premature, accelerated, or early vascular aging.1 Early vascular aging and the associated target organ damage represent a mediating step between risk factors' exposure and cardiovascular events.1 Markers of early vascular aging and target organ damage reflect cumulative damaging effects from risk factors, predict cardiovascular event independently of the conventional risk factors, and may become relevant targets for aggressive intervention for the prevention and treatment of cardiovascular disease.1,2 Increased arterial stiffness is a direct manifestation of early vascular aging.1 Carotid-femoral pulse wave velocity (PWV), an index of the degree of aortic stiffness, is considered as the gold standard measurement of arterial stiffness. 3 Increased carotid-femoral PWV has been shown to be an independent predictor of cardiovascular events in patients with hypertension,4 impaired glucose tolerance and diabetes mellitus,5 end-stage renal disease,6 and in the elderly7 and general populations.8,9
Systolic (SBP) and pulse (PP) pressure levels rise progressively as the pressure wave travels in the continuously narrowing and branching system from the central aorta toward the peripheral arteries. This is the so-called phenomenon of pulse pressure amplification, mainly due to wave reflections detectable at any peripheral point.10 Early return of a large reflected wave augments central aortic SBP and PP and the reflected wave, independently of its timing, impacts adversely on the left ventricular afterload and the coronary perfusion.10,11 However, whether increased wave reflection is an effective marker of early vascular aging remains controversial.12 Specifically, wave reflection intensity gauged by the augmentation index (AI) and augmented pressure (Pa) has only been shown to be independent predictor of cardiovascular events in relatively elderly patients with end-stage renal disease13 and patients following percutaneous coronary intervention.14 On the other hand, AI and Pa failed to predict cardiovascular events in elderly female hypertensives,15 patients with chronic kidney disease,16 and relatively young non-diabetic dialysis patients.17 To date, evidence supporting the role of wave reflection in the prediction of cardiovascular events in the hypertensive and general populations remains lacking. This is intriguing because wave reflection should dominate age-related change in aortic blood pressure and contribute significantly to cardiac work throughout the human life span, while the impact of aortic stiffness on the rise in central pressure may only be detected as important beyond age 60.18
We hypothesized that the utility of increased wave reflection in the prediction of cardiovascular events may have been underestimated, because the commonly used measures of relative and absolute central pressure augmentation by wave reflection, such as AI and Pa, are affected by the reflected wave transit time (RWTT). Other timing-independent measures of the wave reflection may be more relevant than AI and Pa as markers for early vascular aging. Therefore, we tested the hypothesis by investigating whether the various measures of wave reflection predict all-cause and cardiovascular mortalities independently of carotid-femoral PWV in a homogeneous Taiwanese community-based population.
Methods
Study population
Details of the study and characteristics of participants were previously reported.19 The study cohort included 1272 normotensive and untreated hypertensive Taiwanese subjects (47% women, mean age 52 ± 13 years old, range 30-79 years), which was drawn from a previous community-based survey conducted in 1992-1993.20 All participants gave informed consent and the study was approved by the institutional review board at the Johns Hopkins University. All measurements were taken during the 1992-1993 survey.
Data collection
Measurements of brachial SBP and diastolic blood pressure (DBP) separated by at least five minutes from the right arm of subjects after they were seated for at least 5 minutes were taken manually using a mercury sphygmomanometer and a standard-sized cuff (13 cm x 50 cm) by 4 senior cardiologists. Reported blood pressures represent the average of at least two consecutive measurements. Brachial PP was calculated as [brachial SBP – brachial DBP] and brachial mean blood pressure (MBP) was calculated as [brachial DBP + (brachial PP/3)]. Subsequent cardiovascular examinations were performed in the supine position.
Carotid-femoral PWV was measured by recording sequential nondirectional Doppler (Parks model 802, Parks Medical Electronics, Inc. Las Vegas, U.S.A.) flow velocity signals at the right common carotid artery and right femoral artery and a simultaneous electrocardiogram. Carotid artery pressure waveforms were registered noninvasively with a tonometer (model SPC-350, Millar Instruments, Inc. Houston, U.S.A.).
Left ventricular mass was obtained from the 2-D guided M-mode echocardiography performed by the same experienced sonographer using an echocardiographic unit with a 2.5 MHz transducer (SONOS 500, Hewlett-Packard). Left ventricular mass index (LVMI) was calculated by correcting left ventricular mass with body surface area.
Intima-media thickness (IMT) of the posterior wall of the right common carotid artery was measured on-line from frozen, digitized images obtained with a 7-MHz vascular probe incorporated in the echocardiographic unit.
Estimated glomerular filtration rate (eGFR) was calculated using age, sex, and serum levels of blood urea nitrogen, creatinine, and albumin according to the modified glomerular filtration rate estimating equations for Chinese patients.21
Carotid pulse wave analysis
The digitized carotid pressure waveform signals were analyzed using custom-designed software on a commercial software package (Matlab®, version 4.2, The MathWorks, Inc.). All processed individual signals were subject to fully automatic batch analysis to avoid inter- and intra-observer variations. Five to ten consecutive carotid pressure waves were ensemble averaged to one wave. The averaged carotid pressure waveform was then calibrated using brachial MBP and DBP taken in the seated position.
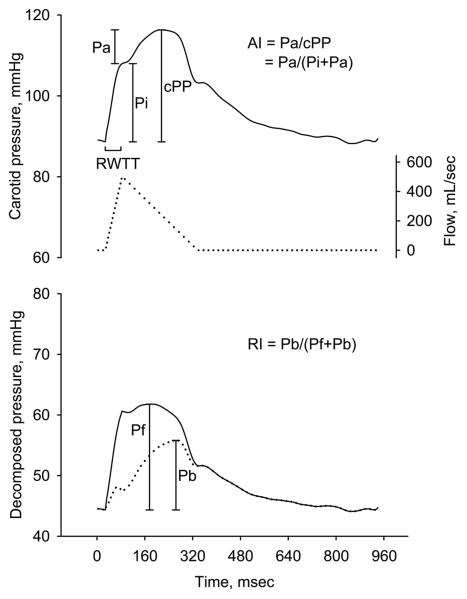
The calibrated carotid pressure waveform was analyzed to identify the inflection point resulting from the wave reflection using the zero-crossing timings of the fourth derivative of the pressure wave,22 and the incisura resulting from the aortic valve closure, and the carotid (central) SBP, PP, AI,23 incident pressure wave height (Pi), augmented pressure (Pa), systolic ejection period, and RWTT were calculated accordingly (Figure 1).18 The carotid pressure waveform was then separated into its forward and reflected components to calculate the transit-time independent parameters of wave reflection intensity using the triangulation method.24,11 This method creates a triangular-shaped flow wave by matching the start, peak and end of the flow wave to the timings of the foot, inflection point and incisura of the carotid pressure wave. Since calculation of both the forward and reflected pressure components involves the product of flow and characteristic impedance (Zc), which itself has flow in the denominator, calibration of the flow waveform is not needed. Thus, the forward and backward components of the pressure wave can be constructed using the following equations:
Where Pm(t) is the carotid pressure wave, F(t) is the approximated triangular-shaped flow wave, Pf(t) is the forward, and Pb(t) is the backward pressure component. Pf and Pb are the pressure amplitudes of Pf(t) and Pb(t), respectively. The reflection index (RI) was calculated as Pb/(Pf + Pb) (Figure 1).24,11 Pf and Pb calculated by using the triangular-shaped flow wave have been compared with those calculated by using the true aortic flow wave derived from Doppler echocardiography in another 30 subjects in our laboratory. The two Pf values were similar with a correlation coefficient of 0.904 and a mean difference of −1.9±3.4 mmHg (please see http://hyper.ahajournals.org, Figure S1, Panel A). The two Pb values were also similar with a correlation coefficient of 0.910 and a mean difference of 1.0±2.0 mmHg (please see http://hyper.ahajournals.org. Figure S1, Panel B).
Figure 1.
Definition of carotid waveform analysis parameters. Upper panel shows an example of carotid pressure wave (solid line) and the constructed triangular flow wave (dotted line) by the triangulation method. Lower panel shows the separated forward pressure wave (solid line) and backward pressure wave (dotted line). AI = augmentation index; cPP = central pulse pressure; Pi = incident pressure wave height from foot to the inflection point; Pa = augmented pressure; Pb = amplitude of the backward pressure wave; Pf = amplitude of the forward pressure wave; RI = reflection index; RWTT = reflected wave transit time.
Follow-up
The date and causes of death among the 1272 participants were obtained by linking our database with the National Death Registry. Subjects not appearing on the National Death Registry on December 31, 2007 were considered surviving. The National Death Registry database registers valid information based on the certified death certificates coded according to the International Classification of Disease, Ninth Revision (ICD-9). The ICD-9 codes used for cardiovascular death were 390-459. The accuracy of cause-of-death coding in Taiwan's National Death Registry database has been validated.25
Statistical Analysis
Data are presented as percents or mean ± standard deviation. Gender differences in means were tested by Student's t test. Sex-stratified Pearson's correlation coefficients between the target-organ indices and parameters of blood pressure, aortic stiffness and wave reflection were calculated. Comparisons between two correlation coefficients from paired measurements were carried out using the formula created by Kleinbaum et al.26 Predictors of 15-year all-cause and cardiovascular mortality were identified using the Cox proportional hazard regression model. Crude and adjusted hazard ratios and their 95% confidence intervals per one-standard deviation increment of individual predictors were estimated. To select the best among the 4 wave reflection related predictors, Pi, Pa, Pf, and Pb, hazard ratios of all-cause and cardiovascular mortality at 5-, 10-, and 15-year follow-up in both men and women were estimated, with adjustment for age, heart rate, and height.27 To further examine whether the best wave reflection predictor could predict 15-year cardiovascular mortality independently of other predictors, including brachial MBP, brachial SBP, brachial PP, central SBP, central PP, and carotid-femoral PWV, the best wave reflection predictor and the other predictor were jointly entered into the Cox models adjusting for age, sex, height, heart rate with and without other conventional cardiovascular risk factors (current smoking, fasting plasma glucose levels, and the ratio of total cholesterol to high-density lipoprotein cholesterol). Two-tailed P<0.05 was considered statistically significant. Statistical analyses were performed using the statistical package SPSS15.0 (SPSS Inc. Chicago, Illinois, USA).
Results
The baseline characteristics of the participants have been published.19 Table 1 presents selected baseline hemodynamics and results of the carotid pressure waveform analysis. Women were significantly shorter, had greater brachial PP, central SBP and PP, AI, Pa, systolic ejection period, RI, and Pb, and had shorter RWTT in comparison with men.
Table 1.
Selected baseline hemodynamics and carotid pulse wave analysis
| Parameters | Women (n= 598) | Men (n= 674) | P values |
|---|---|---|---|
| Age, years* | 52 ± 13 | 52 ± 13 | 0.599 |
| Height, cm | 153 ± 6 | 165 ± 7 | <0.001 |
| Heart rate, beats/min* | 74 ± 9 | 73 ± 10 | 0.634 |
| Brachial MBP, mmHg | 104 ± 17 | 105 ± 16 | 0.282 |
| Brachial SBP, mmHg* | 139 ± 25 | 139 ± 22 | 0.524 |
| Brachial PP, mmHg* | 52 ± 18 | 50 ± 15 | 0.003 |
| Central SBP, mmHg* | 129 ± 26 | 126 ± 22 | 0.048 |
| Central PP, mmHg* | 44 ± 17 | 39 ± 14 | <0.001 |
| PWV, m/sec* | 9.5 ± 2.5 | 9.5 ± 2.3 | 0.771 |
| AI, %* | 19 ± 14 | 7 ± 14 | <0.001 |
| Pi, mmHg | 34 ± 12 | 35 ± 11 | 0.189 |
| Pa, mmHg | 10 ± 9 | 5 ± 6 | <0.001 |
| RWTT, msec | 63 ± 25 | 87 ± 44 | <0.001 |
| SEP, msec | 310 ± 27 | 303 ± 54 | 0.003 |
| RI, % | 33 ± 5 | 30 ± 6 | <0.001 |
| Pf, mmHg | 33 ± 12 | 34 ± 11 | 0.168 |
| Pb, mmHg | 17 ± 7 | 14 ± 6 | <0.001 |
cited from J Hypertens. 2009;27:461-467.
AI = carotid augmentation index; MBP = mean blood pressure; Pi = incident pressure wave height; Pa = augmented pressure; Pb = backward pressure amplitude; Pf = forward pressure amplitude; PP = pulse pressure; PWV = carotid-femoral pulse wave velocity; RI = reflection index; RWTT = reflected wave transit time; SBP = systolic blood pressure; SEP = systolic ejection period.
Relations of measures of wave reflection to target-organ indices
Table 2 shows the correlations of brachial and central SBP and PP, AI, RWTT, RI, Pi, Pa, Pf, and Pb to LVMI, IMT, eGFR, and carotid-femoral PWV in men and women, respectively. All correlation coefficients were significant except that eGFR was not correlated with RWTT or RI in women. Among the 4 wave reflection measures, Pi, Pa, Pf, and Pb, the correlation to LVMI was significantly stronger with Pb than with the other 3 measures in women, and stronger with Pb and Pa than with the other 2 measures in men (Table 2). Overall, Pb showed the best correlation with target organ indices.
Table 2.
Sex-stratified Pearson correlation coefficients of parameters of blood pressure, aortic stiffness and wave reflection with various target-organ indices
| Parameters | Women (n= 598) | Men (n= 674) | ||||||
|---|---|---|---|---|---|---|---|---|
| LVMI | IMT | eGFR | PWV | LVMI | IMT | eGFR | PWV | |
| Brachial SBP |
0.47† | 0.29† | −0.20† | 0.43† | 0.28† | 0.17† | −0.16† | 0.37† |
| Brachial PP |
0.36† | 0.25† | −0.18† | 0.35† | 0.22† | 0.18† | −0.14† | 0.34† |
| Central SBP |
0.51† | 0.33† | −0.23† | 0.49† | 0.32† | 0.20† | −0.19† | 0.38† |
| Central PP | 0.46† | 0.34† | −0.28† | 0.45† | 0.30† | 0.23† | −0.19† | 0.36† |
| PWV | 0.30† | 0.33† | −0.32† | 0.14† | 0.20† | −0.32† | ||
| AI | 0.33† | 0.23† | −0.17† | 0.27† | 0.27† | 0.24† | −0.24† | 0.28† |
| RWTT | −0.13* | −0.14* | −0.01 | −0.14* | −0.18† | −0.18† | 0.19† | −0.24† |
| RI | 0.21† | 0.19† | −0.03 | 0.16† | 0.19† | 0.12* | −0.18† | 0.17† |
| Pi | 0.36† | 0.28† | −0.22† | 0.39† | 0.21† | 0.14† | −0.11* | 0.29†‡ |
| Pa | 0.41† | 0.29† | −0.25† | 0.37† | 0.31†‡ | 0.27†‡ | −0.24†‡ | 0.28† |
| Pf | 0.41† | 0.28† | −0.26† | 0.40† | 0.21† | 0.16† | −0.10* | 0.26† |
| Pb | 0.47†‡ | 0.34†‡ | −0.25† | 0.44† | 0.32†‡ | 0.22† | −0.22†‡ | 0.33†‡ |
P value <0.01
P value <0.001
Among Pi, Pa, Pf and Pb, the presence of the mark indicates significantly better correlation to the target organ than the other 2 or 3 parameters without the mark. Correlation coefficients were compared using the Kleinbaum formula.26
AI = carotid augmentation index; eGFR = estimated glomerular filtration rate; IMT = intima-media thickness; LVMI = left ventricular mass index; Pi = incident pressure wave height; Pa = augmented pressure; Pb = backward pressure amplitude; Pf = forward pressure amplitude; PP = pulse pressure; PWV = carotid-femoral pulse wave velocity; RI = reflection index; RWTT = reflected wave transit time; SBP = systolic blood pressure.
Relations of measures of wave reflection to mortality
After a median of 15 years follow-up, 225 (18%) deaths occurred, including 64 (5%) cardiovascular deaths (36 were cerebrovascular deaths). Table 3 reveals the results of univariate Cox proportional hazard regression analysis in men and women. Carotid-femoral PWV, Pa, Pf, and Pb were predictive of all-cause and cardiovascular mortality in both men and women, whereas AI, RWTT, and RI were predictive only in men. On the other hand, Pi predicted all-cause mortality in men and women and cardiovascular mortality in women only.
Table 3.
Hazard ratios and 95% confidence intervals per one-standard deviation increment of each variable for 15-year all-cause and cardiovascular moralities by univariate analysis
| All-cause mortality | Cardiovascular mortality | |||
|---|---|---|---|---|
| Parameters | Women (n= 598) | Men (n= 674) | Women (n= 598) |
Men (n= 674) |
| PWV (1 SD = 2.5 m/sec in women) (1 SD = 2.3 m/sec in men) |
1.76 (1.54-2.01) | 1.50 (1.33-1.69) | 1.94 (1.56-2.42) | 1.56 (1.25-1.94) |
| AI (1 SD = 14% in women) (1 SD = 14% in men) |
1.22 (0.99-1.50) | 1.62 (1.36-1.92) | 1.37 (0.94-2.00) | 2.33 (1.67-3.26) |
| Pi (1 SD = 12 mmHg in women) (1 SD = 11 mmHg in men) |
1.99 (1.69-2.35) | 1.41 (1.24-1.61) | 2.69 (2.01-3.54) | 1.30 (0.99-1.71) |
| Pa (1 SD = 9 mmHg in women) (1 SD = 6 mmHg in men) |
1.49 (1.26-1.76) | 1.48 (1.31-1.68) | 1.70 (1.28-2.26) | 1.80 (1.47-2.21) |
| RWTT (1 SD = 25 mmHg in women) (1 SD = 44 mmHg in men) |
0.97 (0.78-1.20) | 0.72 (0.59-0.89) | 0.74 (0.41-1.35) | 0.35 (0.17-0.71) |
| RI (1 SD = 5% in women) (1 SD = 6% in men) |
1.02 (0.83-1.25) | 1.32 (1.11-1.57) | 1.43 (0.94-2.17) | 1.88 (1.31-2.69) |
| Pf (1 SD = 9 mmHg in women) (1 SD = 6 mmHg in men) |
1.96 (1.66-2.32) | 1.40 (1.22-1.61) | 2.49 (1.88-3.30) | 1.36 (1.04-1.78) |
| Pb (1 SD = 7 mmHg in women) (1 SD = 6 mmHg in men) |
1.83 (1.54-2.18) | 1.55 (1.35-1.77) | 2.48 (1.85-3.31) | 1.75 (1.37-2.22) |
Numbers in bold letters indicate statistical significance.
AI = carotid augmentation index; Pi = incident pressure wave height; Pa = augmented pressure; Pb = backward pressure amplitude; Pf = forward pressure amplitude; PWV = carotid-femoral pulse wave velocity; RI = reflection index; RWTT = reflected wave transit time; SD = standard deviation.
When the study population was stratified by age (≥ 55 and <55 years), only carotid-femoral PWV, Pa and Pb were predictive of cardiovascular mortality in both subgroups (please see http://hyper.ahajournals.org. Table S1). Carotid-femoral PWV and Pb were also predictive of all-cause mortality in both subgroups.
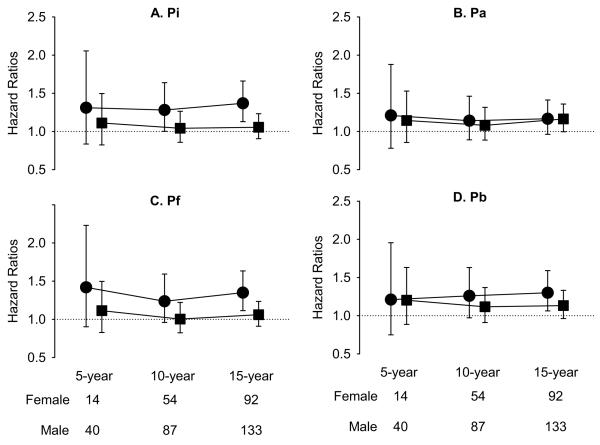
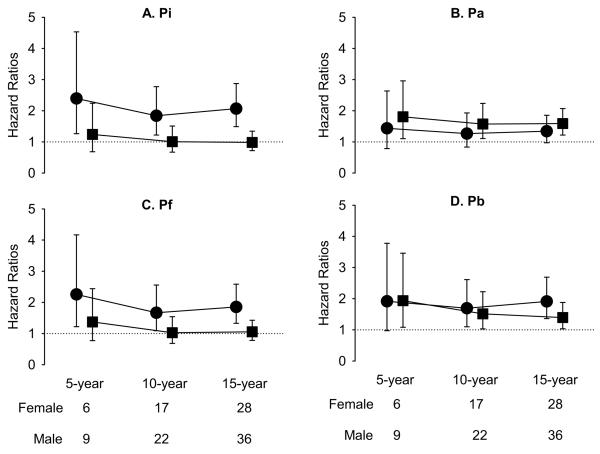
Figure 2 and 3 display the sex-stratified hazard ratios and 95% confidence intervals for Pi, Pa, Pf, and Pb in the prediction of all-cause and cardiovascular mortalities, respectively, at 5-, 10-, and 15-year follow-up, with adjustment for age, height, and heart rate. In women, Pi, Pf, Pb but not Pa predicted the 15-year all-cause mortality (Figure 2). On the other hand, Pi, Pf, Pb but not Pa predicted the 10- and 15-year cardiovascular mortality in women (Figure 3). In contrast, none of the 4 parameters predicted all-cause mortality in men (Figure 2). On the other hand, Pa and Pb predicted the 5-, 10-, and 15-year cardiovascular mortality in men (Figure 3). Therefore, only Pb consistently predicted the 10- and 15-year cardiovascular mortality independently of age, height, and heart rate in both men and women (Figure 3).
Figure 2.
Hazard ratios per increment of one-standard deviation of Pi (A), Pa (B), Pf (C) and Pb (D) for 5-year, 10-year and 15-year all-cause mortality, accounting for age, heart rate, and height. Circles indicate women and squares indicate men.
Pi = incident pressure wave height from foot to the inflection point; Pa = augmented pressure; Pb = amplitude of the backward pressure; Pf = amplitude of the forward pressure.
Figure 3.
Hazard ratios per increment of one-standard deviation of Pi (A), Pa (B), Pf (C) and Pb (D) for 5-year, 10-year and 15-year cardiovascular mortality, accounting for age, heart rate, and height. Circles indicate women and squares indicate men.
Abbreviations are the same as for Figure 2.
Table 4 presents the hazard ratios and 95% confidence intervals for cardiovascular mortality when Pb was entered into the multivariate Cox regression models jointly with each central or brachial blood pressure variable, carotid-femoral PWV, AI or Pa, with adjustment for age, sex, height, and heart rate, with or without other conventional risk factors. Pb significantly predicted cardiovascular mortality independently of brachial blood pressure, carotid-femoral PWV, AI or Pa. However, Pb was not significantly predictive of cardiovascular mortality when either central SBP or PP was in the models.
Table 4.
Hazards ratios and 95% confidence intervals per one-standard deviation increment of Pb (1 SD = 6 mmHg) for 15-year cardiovascular mortality by multi-variate analysis
| Parameters | A | B |
|---|---|---|
| Brachial MBP (1 SD = 16 mmHg) | 1.53 (1.18-1.98) | 1.59 (1.21-2.09) |
| Brachial SBP (1 SD = 24 mmHg) | 1.55 (1.18-2.03) | 1.61 (1.21-2.14) |
| Brachial PP (1 SD = 17 mmHg) | 1.64 (1.26-2.13) | 1.68 (1.28-2.19) |
| Central SBP (1 SD = 24 mmHg) | 0.98 (0.63-1.52) | 1.03 (0.64-1.64) |
| Central PP (1 SD = 16 mmHg) | 1.89 (0.87-4.08) | 1.82 (0.85-3.87) |
| PWV (1 SD = 2.4 m/sec) | 1.57 (1.24-1.98) | 1.60 (1.24-2.06) |
| AI (1 SD = 15%) | 1.52 (1.18-1.97) | 1.58 (1.20-2.07) |
| Pa (1 SD = 8 mmHg) | 1.54 (1.08-2.18) | 1.61 (1.13-2.31) |
Numbers in bold letters indicate statistical significance.
A: adjusted for age, sex, height, heart rate and the variable at the leftmost column.
B: adjusted for age, sex, height, heart rate, current smoking, fasting plasma glucose levels, cholesterol/HDL ratio and the variable in the leftmost column.
AI = carotid augmentation index; HDL = high-density lipoprotein cholesterol; MBP = mean blood pressure; Pb = backward pressure amplitude; PP = pulse pressure; PWV = carotid-femoral pulse wave velocity; SBP = systolic blood pressure; SD = standard deviation.
Discussion
The present study presents the first epidemiologic evidence that increased wave reflection independent of its timing, predicts cardiovascular mortality independently of arterial stiffness in men and women. Specifically, both of the RWTT dependent measures of wave reflection intensity failed to predict cardiovascular mortality in women (AI in the univariate analysis, Table 3, and Pa in the multivariate analysis, Figure 3) whereas Pb significantly and independently predicted cardiovascular mortality regardless of gender. These results support the hypothesis that the utility of increased wave reflection in the prediction of cardiovascular events has been underestimated by using AI and Pa as measures of wave reflection. Thus, Pb, the absolute magnitude of the reflected wave independent of timing, appears to be more relevant than AI and Pa as a marker for early vascular aging.
AI and Pa underestimate the impact of increased wave reflection on the pathogenesis of cardiovascular disease
Wave reflections occur when the propagating pressure wave encounters bifurcations of conducting arteries, high resistance from smaller muscular arteries and arterioles, and increasing stiffening from central to peripheral arteries.3 Thus, both small and large arteries are involved in the generation of wave reflections in the normal arterial tree and the amplification of wave reflections in the presence of pathological conditions, such as hypertension, diabetes mellitus, and aging.3 Increased wave reflections are recognized as the major cause for the elevation of central aortic pressure and the reduction of the pressure amplification phenomenon with vascular aging.28,10 The adverse impacts of increased wave reflections had mainly been focused on the hemodynamic burden on the left ventricle,29 and AI and Pa were therefore developed from the analysis of central aortic pressure wave, based on the concept that early and large reflected wave arrives in early systole, superimposes on the forward wave, and augments the central SBP and PP.3
Although AI and Pa represent the integrated effect of the magnitude and timing of the reflected wave on the augmentation of central aortic SBP and PP, they may underestimate the hemodynamic load imposed by the increased wave reflection because central aortic pressure represents only part of the left ventricular afterload.30 In addition, high AI and Pa may result from earlier return of reflected wave due to reduced RWTT. RWTT has been thought to be an estimate of travel time that is simply the sum of travel times from ascending aorta to an assumed effective reflection site at the lower abdominal aorta and back.31 The reflection site is relatively closer to the ascending aorta with resultant reduced RWTT and increased AI and Pa in females and subjects with shorter height but without other apparent causes for increased wave reflection. With aging, a decrease in RWTT along with a disproportionate increase in carotid-femoral PWV may imply that the reflection site moves toward the periphery32 or closer to the heart.33 Furthermore, a time delay may occur at the reflection site between forward and reflected waves, because the two waves are not necessarily in phase in the distal aorta.31 Therefore, the determinants of RWTT are complex and may include wave speed, distance of travel, and the time shift at the reflection site.31 Thus, high values of AI and Pa secondary to reduced RWTT do not necessarily indicate an increase in wave reflection intensity.
The value of AI and Pa in the prediction of cardiovascular events may further be reduced due to limitations of the current noninvasive measurement methods. Central aortic AI and Pa are usually estimated indirectly from the analysis of the noninvasively derived carotid pressure wave or aortic pressure wave reconstructed from a radial pressure wave using a generalized transfer function. We have shown that both the carotid AI and AI derived from the reconstructed aortic pressure wave substantially underestimate the directly measured central aortic AI.23,34
Aortic stiffness, wave reflection, and early vascular aging
Vascular aging is characterized by arterial stiffening in both the central aortic and peripheral muscular arteries.27 Normal vascular aging exerts differential non-linear effects on carotid-femoral PWV and AI, with AI increasing more with age in younger and carotid-femoral PWV in older individuals.27 After age 60 years, aortic stiffness increases carotid-femoral PWV similarly in both men and women, which enhances the propagation of the incident wave and return of the reflected wave, and contributes to the augmentation of aortic SBP and PP.35,27,18 Because central aortic SBP and PP increase linearly with age27 and the reflected wave dominates age-related changes in aortic SBP and PP across the human life span,18 a pure measure of the intensity of wave reflection would be expected to show a linear relationship with age without sex difference.
In the present study, a nonlinear relationship with age for carotid-femoral PWV and AI was confirmed (please see http://hyper.ahajournals.org. Figure S2, Panel A and B). Pa had a linear relationship with age in men, and a nonlinear relationship in women (Panel C). In contrast, a linear relationship between Pb and age was observed in both men and women (Panel D). Pb is the magnitude of the reflected wave that depends on both the intensity of the forward pressure wave propagating along the arterial tree and the geometry and stiffness of the peripheral muscular arteries and arterioles at the major reflecting sites. Because Pb was independent of carotid-femoral PWV in the prediction of cardiovascular mortality in the present community-based population, Pb may become an effective marker for the early vascular aging.
In our low event rate cohort with cardiovascular mortality (56.3% due to stroke) driven mainly by high blood pressure,36 women had significantly higher Pb and central SBP and PP than men yet women and men had similar 15-year cardiovascular mortality (4.7% vs. 5.3%, P=0.610). The potential protection from lower Pb and central SBP and PP in men was probably cancelled by damages from higher prevalence of smoking, higher ratio of total cholesterol to high-density lipoprotein cholesterol, thicker IMT, and lower eGFR in men than in women.19
Limitations of the present study
The carotid pressure waveforms were calibrated using MBP and DBP derived from the seated brachial blood pressure taken by 4 senior cardiologists who had been informed of the standard procedures for blood pressure measurement. Albeit the potential variation among the 4 observers, the measured brachial SBP and PP and the derived central SBP and PP were predictive of all-cause and cardiovascular mortality in both the univariate and multivariate analysis.19 Pb was estimated by the triangulation method. The triangular wave shape assumed for the flow may differ from the actual flow wave shape and therefore the actual impact of Pb on cardiovascular mortality observed in the present study may have been underestimated.
Perspectives
The intensity of wave reflection indexed by Pb but not AI or Pa, is independently associated with long term cardiovascular mortality in both men and women in the community. Although aortic stiffness indexed by carotid-femoral PWV is the gold standard measurement of arterial stiffness, measurement of the intensity of wave reflection is also relevant to identify subjects with early vascular aging for aggressive modification of atherosclerosis because wave reflection involves aortic and peripheral arterial stiffness and may influence on vascular aging through human life span.18,1 The potential impact of our results is further heightened by the finding that reflection magnitude but not PWV was associated with left ventricular mass regression in treated hypertensives.11 Further studies are required to confirm that Pb, or another appropriate index of the intensity of wave reflection, is a good marker of early vascular aging, and is a relevant target for cardiovascular preventive intervention.
Supplementary Material
Acknowledgments
Source(s) of Funding: This work was supported in part by a grant from the National Science Council (NSC 96-2314-B-010 -035 -MY3), an intramural grant from the Taipei Veterans General Hospital (Grant No. V98C1-028), grants in aid from the Research Foundation of Cardiovascular Medicine, Taipei, Taiwan, ROC, and Research and Development contract NO1-AG-1-2118 and the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Conflict(s) of Interest/Disclosure(s)
Conflict of interest disclosures: NONE
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: A tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension. 2009;54:3–10. doi: 10.1161/HYPERTENSIONAHA.109.129114. [DOI] [PubMed] [Google Scholar]
- 2.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 3.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 5.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 6.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 7.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 8.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 9.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 10.Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD, Roman MJ, Safar ME, Segers P, Smulyan H. Role of pulse pressure amplification in arterial hypertension. Experts' opinion and review of the data. Hypertension. 2009;54:375–383. doi: 10.1161/HYPERTENSIONAHA.109.134379. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O'Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008;26:1017–1024. doi: 10.1097/HJH.0b013e3282f62a9b. [DOI] [PubMed] [Google Scholar]
- 12.Hope SA, Antonis P, Adam D, Cameron JD, Meredith IT. Arterial pulse wave velocity but not augmentation index is associated with coronary artery disease extent and severity: implications for arterial transfer function applicability. J Hypertens. 2007;25:2105–2109. doi: 10.1097/HJH.0b013e3282a9be41. [DOI] [PubMed] [Google Scholar]
- 13.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 14.Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Lamm G, Stark N, Rammer M, Eber B. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J. 2005;26:2657–2663. doi: 10.1093/eurheartj/ehi504. [DOI] [PubMed] [Google Scholar]
- 15.Dart AM, Gatzka CD, Kingwell BA, Willson K, Cameron JD, Liang YL, Berry KL, Wing LM, Reid CM, Ryan P, Beilin LJ, Jennings GL, Johnston CI, McNeil JJ, MacDonald GJ, Morgan TO, West MJ. Brachial blood pressure but not carotid arterial waveforms predict cardiovascular events in elderly female hypertensives. Hypertension. 2006;47:785–790. doi: 10.1161/01.HYP.0000209340.33592.50. [DOI] [PubMed] [Google Scholar]
- 16.Zoungas S, Cameron JD, Kerr PG, Wolfe R, Muske C, McNeil JJ, McGrath BP. Association of carotid intima-medial thickness and indices of arterial stiffness with cardiovascular disease outcomes in CKD. Am J Kidney Dis. 2007;50:622–630. doi: 10.1053/j.ajkd.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Covic A, Mardare N, Gusbeth-Tatomir P, Prisada O, Sascau R, Goldsmith DJ. Arterial wave reflections and mortality in haemodialysis patients--only relevant in elderly, cardiovascularly compromised? Nephrol Dial Transplant. 2006;21:2859–2866. doi: 10.1093/ndt/gfl307. [DOI] [PubMed] [Google Scholar]
- 18.Namasivayam M, McDonnell BJ, McEniery CM, O'Rourke MF. Does wave reflection dominate age-related change in aortic blood pressure across the human life span? Hypertension. 2009;53:979–985. doi: 10.1161/HYPERTENSIONAHA.108.125179. [DOI] [PubMed] [Google Scholar]
- 19.Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FCP, Chou P, Chen CH. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27:461–467. doi: 10.1097/hjh.0b013e3283220ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CH, Ting CT, Lin SJ, Hsu TL, Ho SJ, Chou P, Chang MS, O'Connor F, Spurgeon H, Lakatta E, Yin FCP. Which arterial and cardiac parameters best predict left ventricular mass? Circulation. 1998;98:422–428. doi: 10.1161/01.cir.98.5.422. [DOI] [PubMed] [Google Scholar]
- 21.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 22.Takazawa K, Tanaka N, Takeda K, Kurosu F, Ibukiyama C. Underestimation of vasodilator effects of nitroglycerin by upper limb blood pressure. Hypertension. 1995;26:520–523. doi: 10.1161/01.hyp.26.3.520. [DOI] [PubMed] [Google Scholar]
- 23.Chen CH, Ting CT, Nussbacher A, Nevo E, Kass DA, Pak P, Wang SP, Chang MS, Yin FCP. Validation of carotid artery tonometry as a means of estimating augmentation index of ascending aortic pressure. Hypertension. 1996;27:168–175. doi: 10.1161/01.hyp.27.2.168. [DOI] [PubMed] [Google Scholar]
- 24.Westerhof BE, Guelen I, Westerhof N, Karemaker JM, Avolio A. Quantification of wave reflection in the human aorta from pressure alone: a proof of principle. Hypertension. 2006;48:595–601. doi: 10.1161/01.HYP.0000238330.08894.17. [DOI] [PubMed] [Google Scholar]
- 25.Lu TH, Lee MC, Chou MC. Accuracy of cause-of-death coding in Taiwan: types of miscoding and effects on mortality statistics. Int J Epidemiol. 2000;29:336–343. doi: 10.1093/ije/29.2.336. [DOI] [PubMed] [Google Scholar]
- 26.Kleinbaum DG, Kupper LL, Muller KE, Nizam A. Applied Regression Analysis and Multivariable Methods. 3rd ed. Duxburry Press; New York: 1997. [Google Scholar]
- 27.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J Am Coll Cardiol. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 28.Segers P, Mahieu D, Kips J, Rietzschel E, De Buyzere M, De Bacquer D, Bekaert S, De Backer G, Gillebert T, Verdonck P, Van Bortel L. Amplification of the pressure pulse in the upper limb in healthy, middle-aged men and women. Hypertension. 2009;54:414–420. doi: 10.1161/HYPERTENSIONAHA.109.133009. [DOI] [PubMed] [Google Scholar]
- 29.O'Rourke MF. From theory into practice: arterial haemodynamics in clinical hypertension. J Hypertens. 2002;20:1901–1915. doi: 10.1097/00004872-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell GF. Clinical achievements of impedance analysis. Med Biol Eng Comput. 2009;47:153–163. doi: 10.1007/s11517-008-0402-3. [DOI] [PubMed] [Google Scholar]
- 31.Westerhof BE, van den Wijngaard JP, Murgo JP, Westerhof N. Location of a reflection site is elusive: consequences for the calculation of aortic pulse wave velocity. Hypertension. 2008;52:478–483. doi: 10.1161/HYPERTENSIONAHA.108.116525. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 33.Segers P, Rietzschel ER, De Buyzere ML, De Bacquer D, Van Bortel LM, De Backer G, Gillebert TC, Verdonck PR. Assessment of pressure wave reflection: getting the timing right! Physiol Meas. 2007;28:1045–1056. doi: 10.1088/0967-3334/28/9/006. [DOI] [PubMed] [Google Scholar]
- 34.Kass DA, Chen CH, Nevo E, Fetics B, Pak PH, Maughan WL, Yin FCP. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure data - response. Circulation. 1998;98:186–187. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 35.Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol. 2001;37:1374–1380. doi: 10.1016/s0735-1097(01)01166-4. [DOI] [PubMed] [Google Scholar]
- 36.Sasayama S. Heart disease in Asia. Circulation. 2008;118:2669–2671. doi: 10.1161/CIRCULATIONAHA.108.837054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.