Abstract
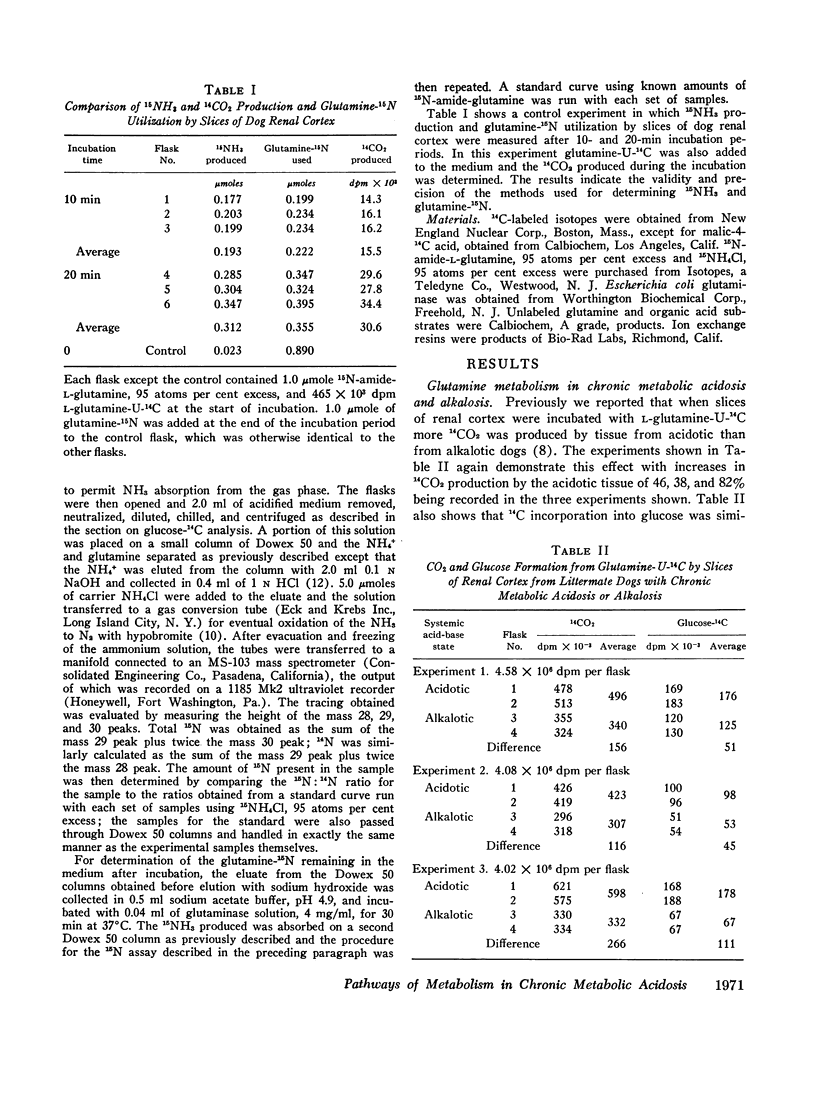
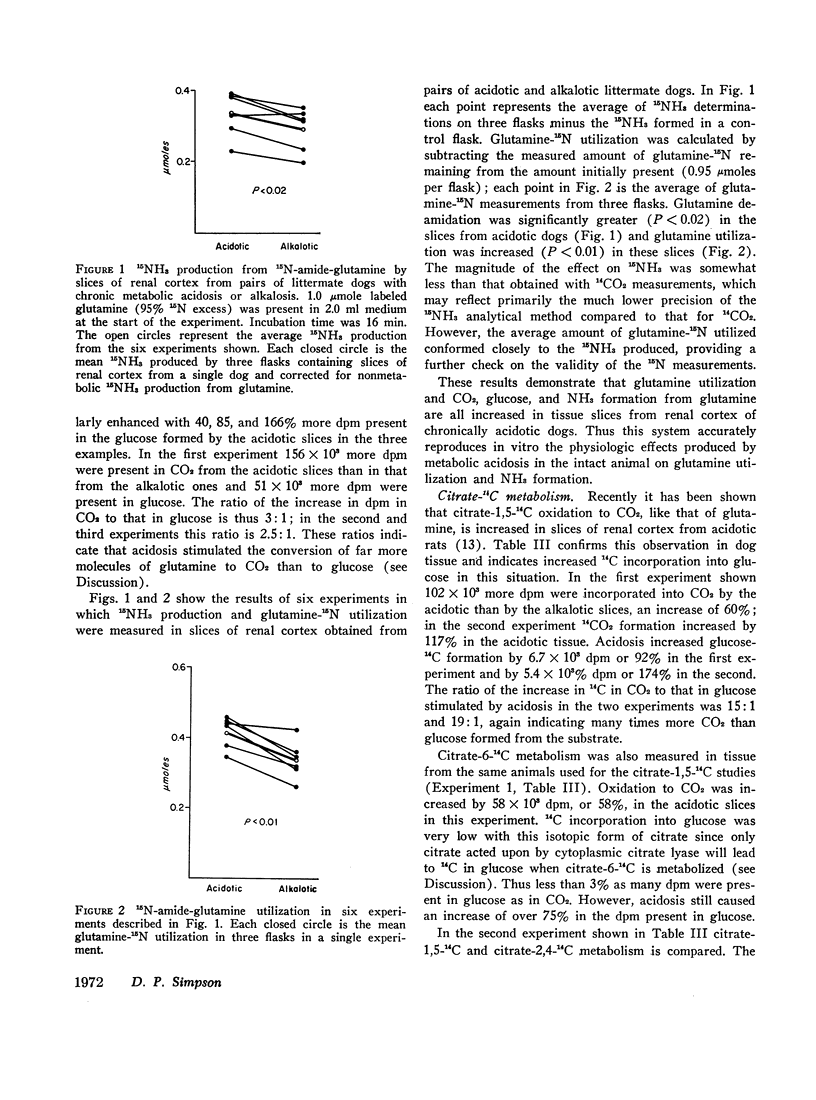
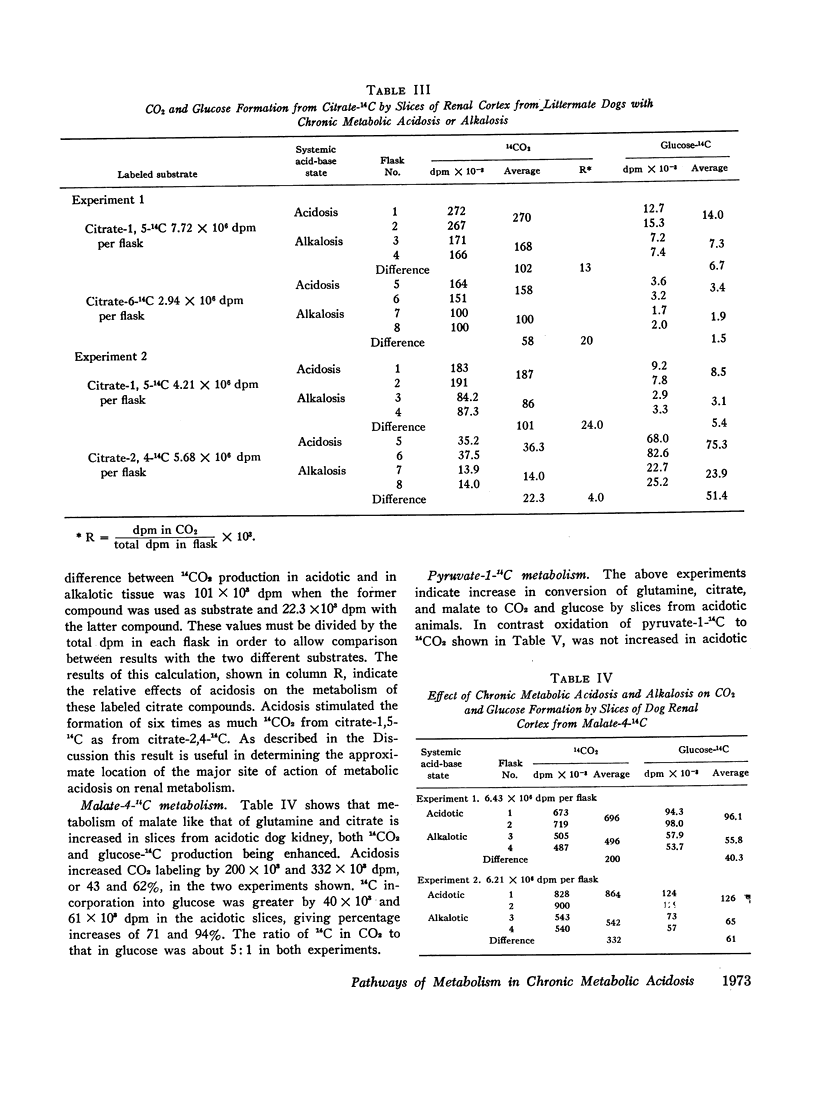
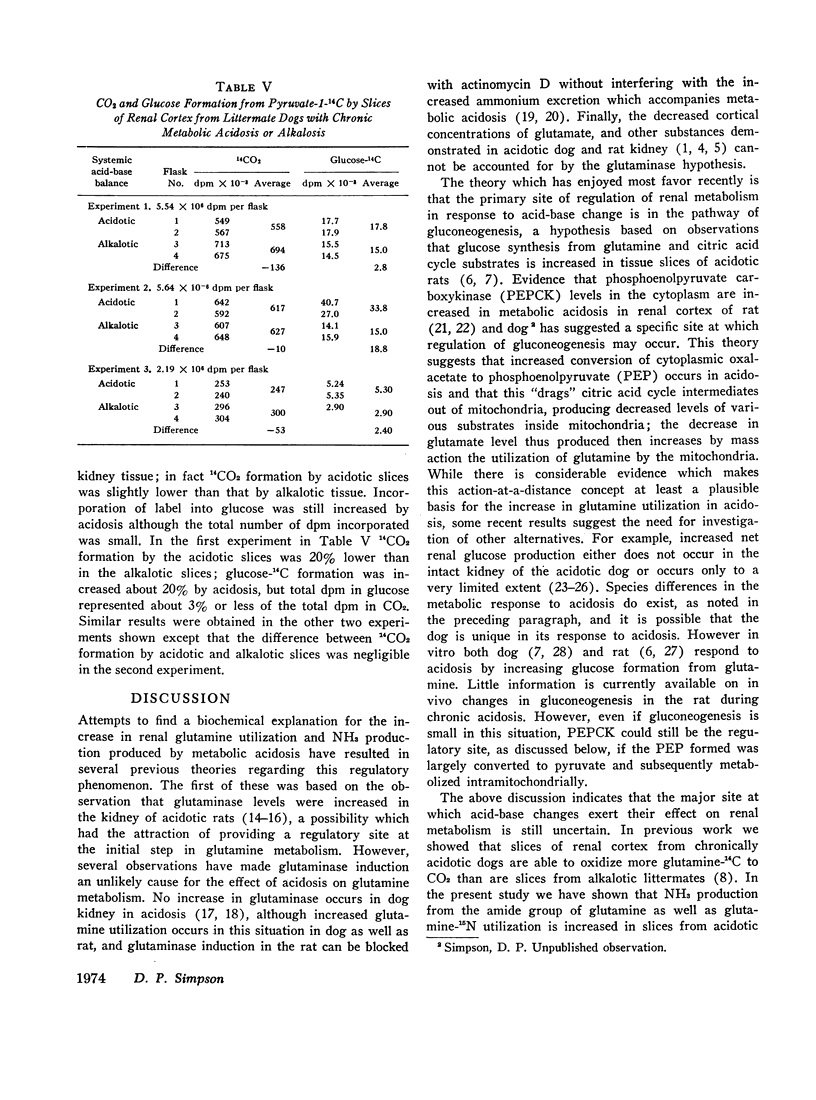
The metabolism of labeled glutamine and of several labeled organic acid anions was compared in tissue slices of renal cortex from chronically acidotic and alkalotic littermate dogs. 15NH3 formation and 15N-amideglutamine utilization were significantly increased by slices from acidotic animals providing further evidence for the similarity of the metabolic responses seen in the tissue slice system and the physiologic effects produced by chronic metabolic acidosis on renal metabolism in the intact animal. Slices from acidotic dogs formed more 14CO2 and glucose-14C than did slices from alkalotic animals when labeled glutamine, citrate, or malate was used as substrate but 14CO2 production from pyruvate-1-14C was slightly reduced in acidotic tissue. With most of the substrates used glucose-14C formation was small compared with 14CO2 formation. Using the amount of glucose-14C formed, the expected 14CO2 production was calculated based on the hypothesis that the primary site of action of metabolic acidosis is on a cytoplasmic step in gluconeogenesis. The actual difference in 14CO2 production between slices from acidotic and alkalotic animals always greatly exceeded this predicted amount, indicating that stimulation of gluconeogenesis represents a minor metabolic response to chronic metabolic acidosis. Evidence from experiments with citrate labeled in various positions showed that metabolic acidosis has its principal effect on an early step in substrate metabolism which must be intramitochondrial in location.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S., Anderson B., Zemotel L. Metabolic acid-base effects on tissue citrate content and metabolism in the rat. Am J Physiol. 1971 Apr;220(4):986–992. doi: 10.1152/ajplegacy.1971.220.4.986. [DOI] [PubMed] [Google Scholar]
- Alleyne G. A. Concentrations of metabolic intermediates in kidneys of rats with metabolic acidosis. Nature. 1968 Mar 2;217(5131):847–848. doi: 10.1038/217847a0. [DOI] [PubMed] [Google Scholar]
- Alleyne G. A., Scullard G. H. Renal metabolic response to acid base changes. I. Enzymatic control of ammoniagenesis in the rat. J Clin Invest. 1969 Feb;48(2):364–370. doi: 10.1172/JCI105993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAIR A., SEGAL S. The isolation of blood glucose as potassium gluconate. J Lab Clin Med. 1960 Jun;55:959–964. [PubMed] [Google Scholar]
- Bignall M. C., Elebute O., Lotspeich W. D. Renal protein and ammonia biochemistry in NH4Cl acidosis and after uninephrectomy. Am J Physiol. 1968 Aug;215(2):289–295. doi: 10.1152/ajplegacy.1968.215.2.289. [DOI] [PubMed] [Google Scholar]
- Cassin S., Bunnel C. E. An in vivo study of the effect of chronic metabolic acidosis on renal gluconeogenesis. Experientia. 1969 Feb 15;25(2):160–161. doi: 10.1007/BF01899098. [DOI] [PubMed] [Google Scholar]
- Churchill P. C., Malvin R. L. Relation of renal gluconeogenesis to ammonia production in the dog. Am J Physiol. 1970 Jan;218(1):241–245. doi: 10.1152/ajplegacy.1970.218.1.241. [DOI] [PubMed] [Google Scholar]
- Churchill P. C., Malvin R. L. Relation of renal gluconeogenesis to ammonia production in the rat. Am J Physiol. 1970 Feb;218(2):353–357. doi: 10.1152/ajplegacy.1970.218.2.353. [DOI] [PubMed] [Google Scholar]
- DAVIES B. M. A., YUDKIN J. Studies in biochemical adaptation; the origin or urinary ammonia as indicated by the effect of chronic acidosis and alkalosis on some renal enzymes in the rat. Biochem J. 1952 Nov;52(3):407–412. doi: 10.1042/bj0520407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R., BAUDHUIN P. Distribution of enzymes between subcellular fractions in animal tissues. Adv Enzymol Relat Subj Biochem. 1962;24:291–358. doi: 10.1002/9780470124888.ch6. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Relation of glutamate to ammonia production in the rat kidney. Am J Physiol. 1966 Mar;210(3):661–666. doi: 10.1152/ajplegacy.1966.210.3.661. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorno W. E., Rector F. C., Jr, Seldin D. W. Relation of renal gluconeogenesis to ammonia production in the dog and rat. Am J Physiol. 1967 Oct;213(4):969–974. doi: 10.1152/ajplegacy.1967.213.4.969. [DOI] [PubMed] [Google Scholar]
- LEONARD E., ORLOFF J. Regulation of ammonia excretion in the rat. Am J Physiol. 1955 Jul;182(1):131–138. doi: 10.1152/ajplegacy.1955.182.1.131. [DOI] [PubMed] [Google Scholar]
- OWEN E. E., ROBINSON R. R. Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride. J Clin Invest. 1963 Feb;42:263–276. doi: 10.1172/JCI104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLAK V. E., MATTENHEIMER H., DEBRUIN H., WEINMAN K. J. EXPERIMENTAL METABOLIC ACIDOSIS: THE ENZYMATIC BASIS OF AMMONIA PRODUCTION BY THE DOG KIDNEY. J Clin Invest. 1965 Feb;44:169–181. doi: 10.1172/JCI105132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkington L. A., O'Donovan D. J. Metabolism of glutamine in cortex slices from dog kidney during acid-base alterations. Am J Physiol. 1971 Jun;220(6):1634–1639. doi: 10.1152/ajplegacy.1971.220.6.1634. [DOI] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, ORLOFF J. The effect of the administration of sodium bicarbonate and ammonium chloride on the excretion and production of ammonia; the absence of alterations in the activity of renal ammonia-producing enzymes in the dog. J Clin Invest. 1959 Feb;38(2):366–372. doi: 10.1172/JCI103810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, SELDIN D. W., COPENHAVER J. H. The mechanism of ammonia excretion during ammonium chloride acidosis. J Clin Invest. 1955 Jan;34(1):20–26. doi: 10.1172/JCI103058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxe D. M. Renal gluconeogenesis after NH 4 Cl, NaHCO 3 , hypoglycemia, or pregnancy. Am J Physiol. 1972 Jan;222(1):55–60. doi: 10.1152/ajplegacy.1972.222.1.55. [DOI] [PubMed] [Google Scholar]
- Sherrard D. J., Simpson D. P. An improved method for the microdetermination of glutamine in plasma and urine. J Lab Clin Med. 1969 May;73(5):877–882. [PubMed] [Google Scholar]
- Simpson D. P. Control of hydrogen ion homeostasis and renal acidosis. Medicine (Baltimore) 1971 Nov;50(6):503–541. doi: 10.1097/00005792-197111000-00002. [DOI] [PubMed] [Google Scholar]
- Simpson D. P. Regulation of renal citrate metabolism by bicarbonate ion and pH: observations in tissue slices and mitochondria. J Clin Invest. 1967 Feb;46(2):225–238. doi: 10.1172/JCI105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. P., Sherrard D. J. Regulation of glutamine metabolism in vitro by bicarbonate ion and pH. J Clin Invest. 1969 Jun;48(6):1088–1096. doi: 10.1172/JCI106065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Goodman A. D., Treble D. H. Effect of metabolic acidosis on renal gluconeogenesis in vivo. Am J Physiol. 1968 Jul;215(1):211–217. doi: 10.1152/ajplegacy.1968.215.1.211. [DOI] [PubMed] [Google Scholar]
- Watson J. A., Lowenstein J. M. Citrate and the conversion of carbohydrate into fat. Fatty acid synthesis by a combination of cytoplasm and mitochondria. J Biol Chem. 1970 Nov 25;245(22):5993–6002. [PubMed] [Google Scholar]


